Influence of Removing or Leaving the Prosthesis after Regenerative Surgery in Peri-Implant Defects: Retrospective Study: 32 Clinical Cases with 2 to 8 Years of Follow-Up
Abstract
1. Introduction
2. Materials and Methods
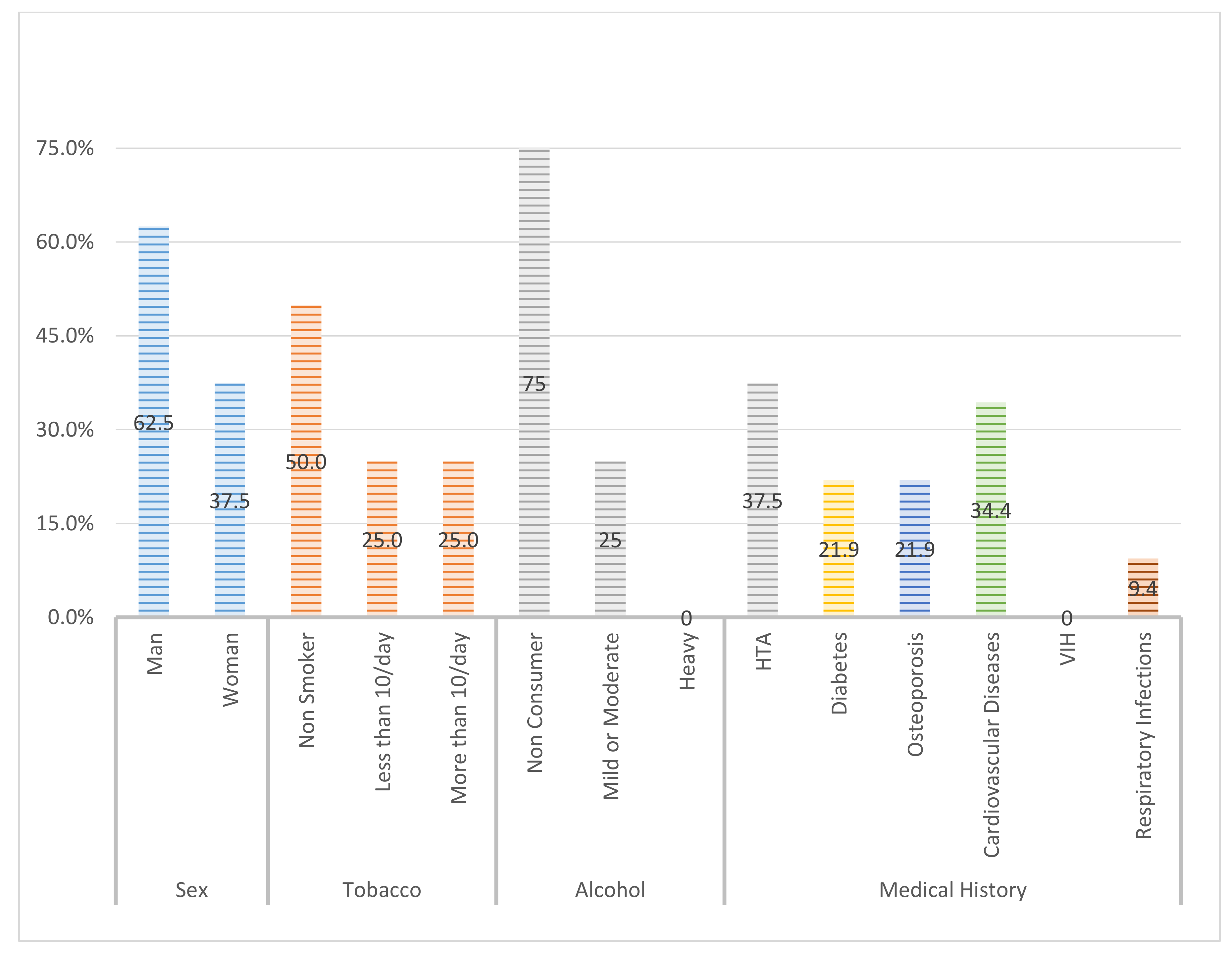
2.1. Study Sample
2.2. Clinical Assessment
- Probing depth (PD) (in mm): with a North Carolina periodontal probe applying a pressure of 0.15 Ncm.
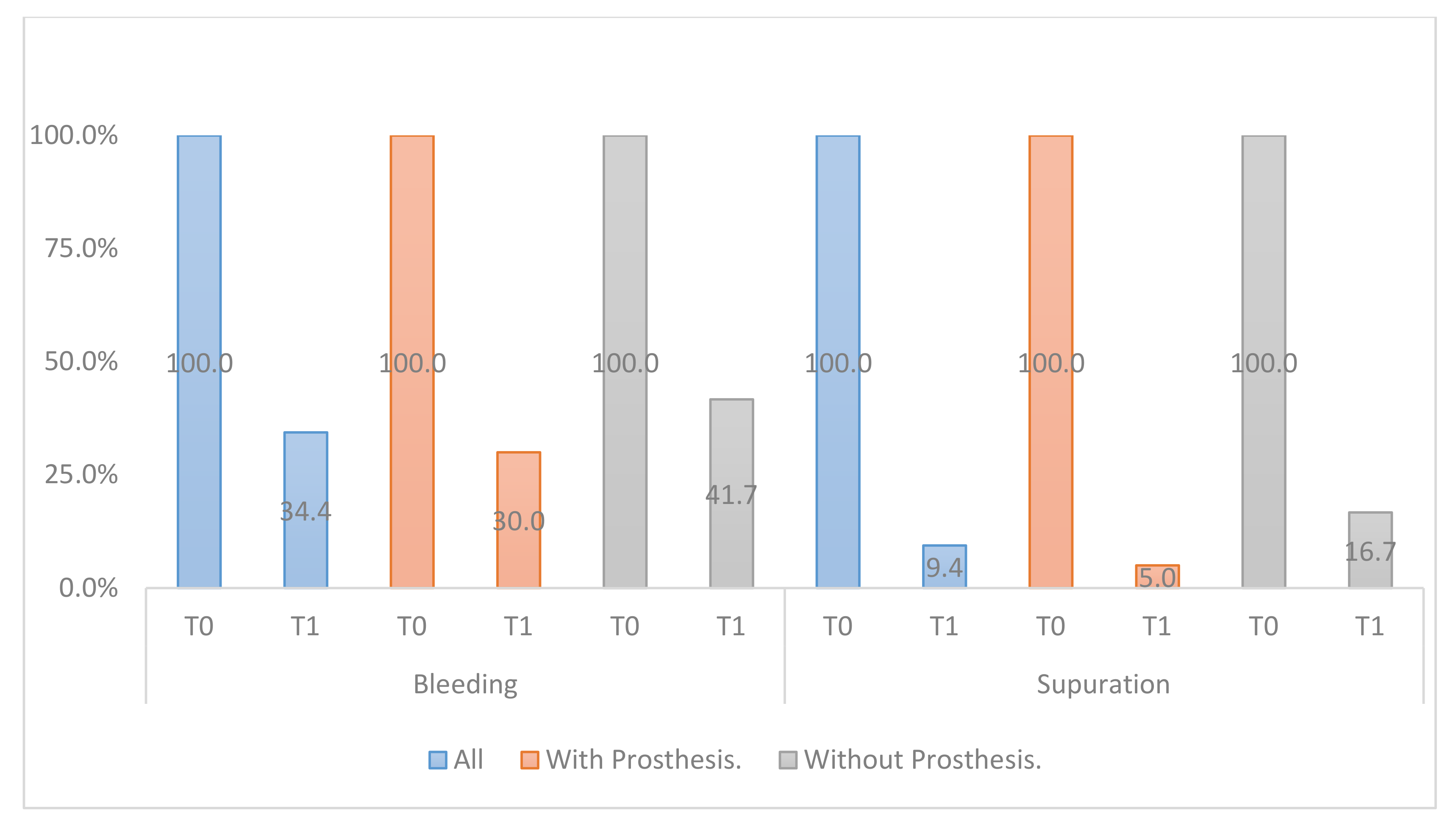
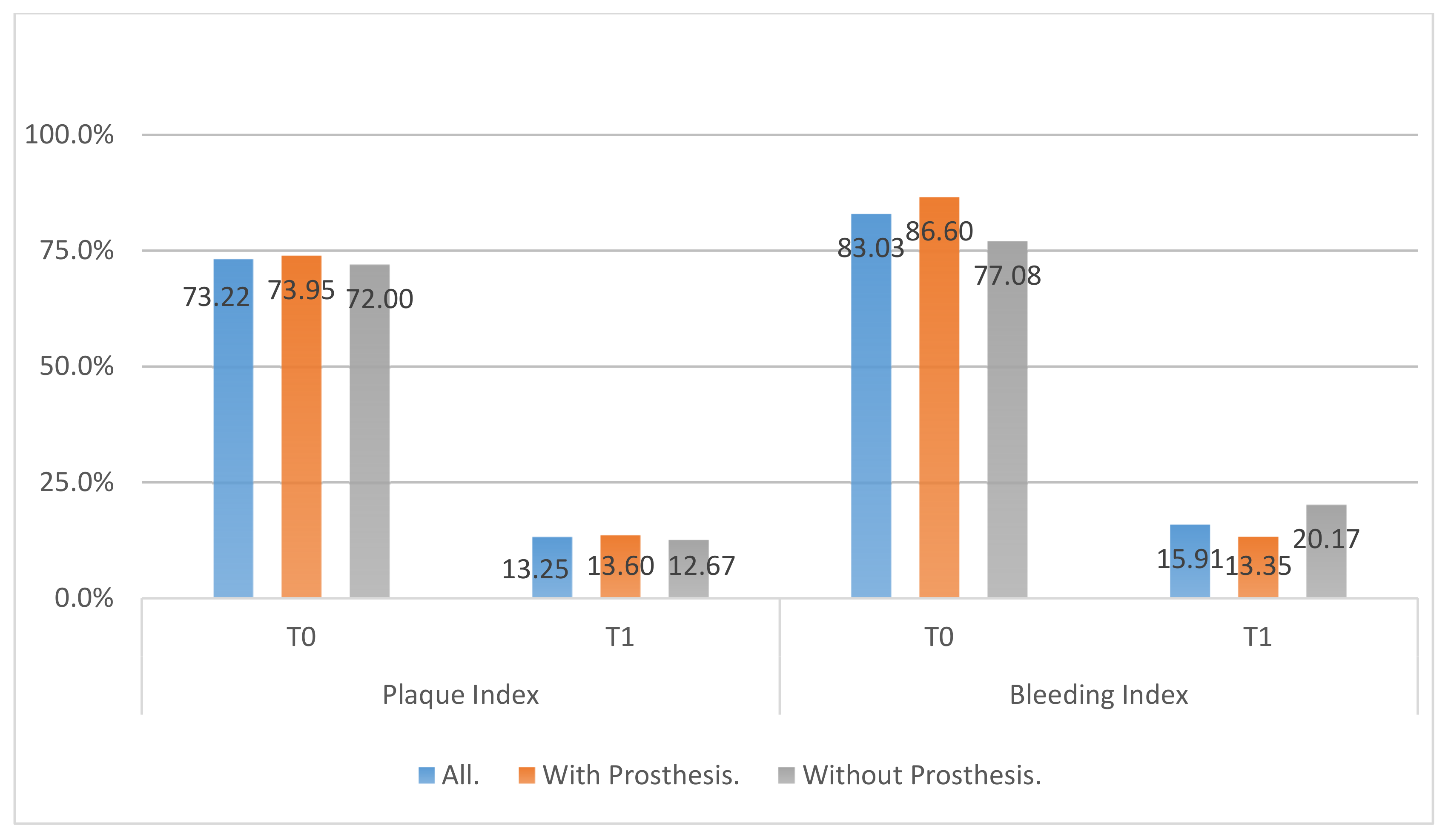
- Modified Sulcular Bleeding Index (mBI): registered from 0 to 3, according to the criteria of Mombelli et al. and the extension and severity of bleeding on probing (BOP) [22].
- Modified plaque index (mPI): registered according to the O’Leary index that represents the average percentage of dental surfaces affected by bacterial plaque [23].
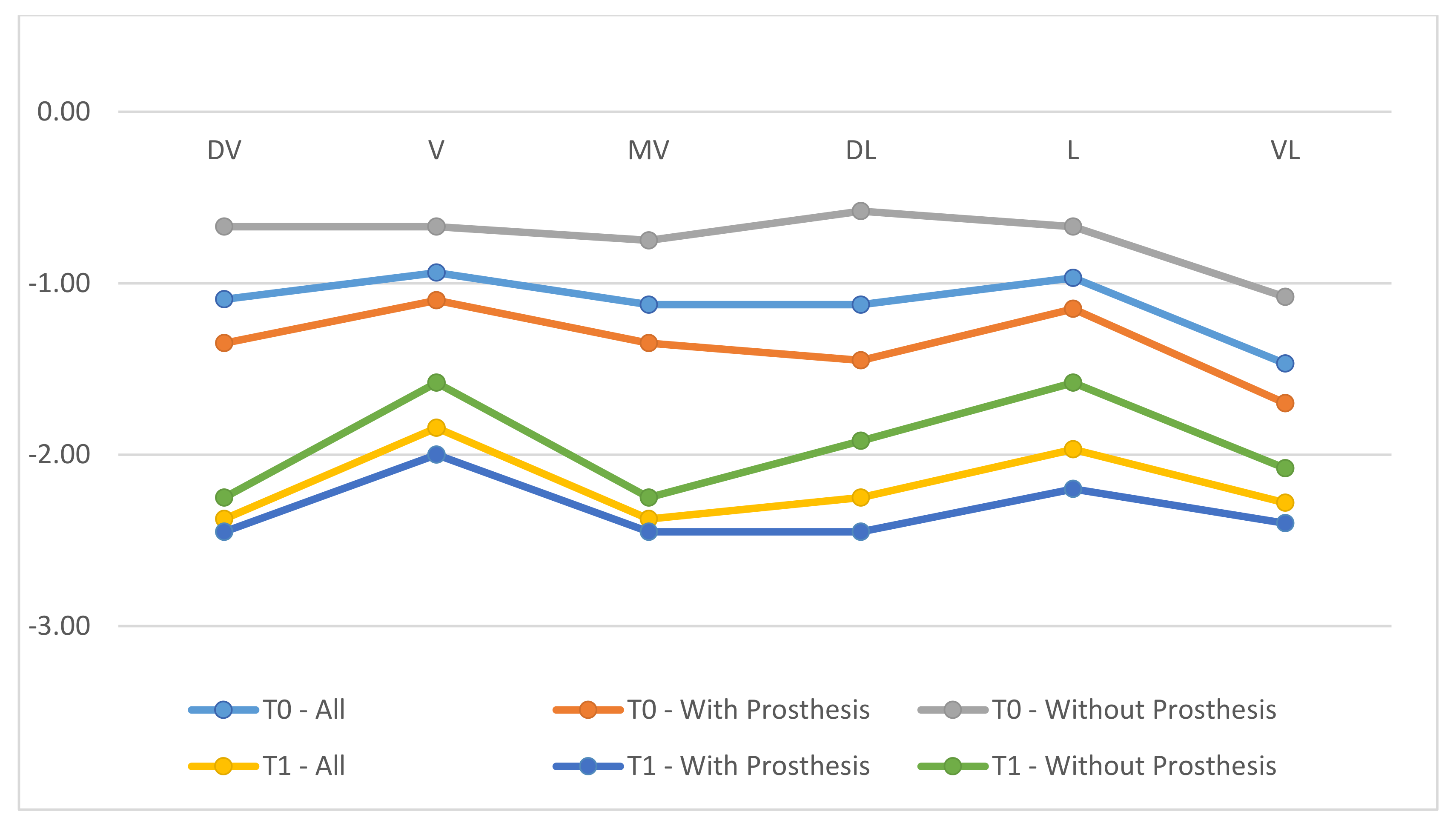
- Mucosal recession (REC): defined as the distance (in mm) from the implant platform as a stable mark and margin of the mucosa.
- Suppuration (SUP): around the implants according to the dichotomous scale (0/1), using the University of North Carolina probe 15, applying a force of 0.15 Ncm.
- Smoking habits: No, <10/day, Yes, >10/day.
- Systemic diseases: arterial hypertension, diabetes, osteoporosis, cardiovascular disease, HIV, respiratory infections.
- Periodontal disease: No, Mild (Clinical Attachment Level—CAL 1–2 mm), Moderate (CAL 3–4 mm), Advanced (>5 mm) [23].
- Maintenance: No, Every 4 months, Every 6 months, Every 12 months.
- Date of implant placement.
- Surface: 1: minimally rough (0.5–1 µm), 2: moderately rough (1–2 µm), 3: rough (> 2 µm), based on the Albrektsson and Wenneberg’s 2004 classification [24].
- Location: Following World Dental Federation (FDI) nomenclature.
- Type of prosthesis: screwed/cemented single crown, screwed/cemented fixed partial prosthesis, hybrid prosthesis, metal–ceramic fixed prosthesis, overdenture.
- Diameter and length of each implant.
- Type of connection: 1: bone level, 2: tissue level, 3: external connection.
- Date of prosthesis placement: 1: immediate loading (24 h–1 week), 2: early (1–8 weeks), 3: conventional (>2 months), according to the criteria of Esposito et al. [25].
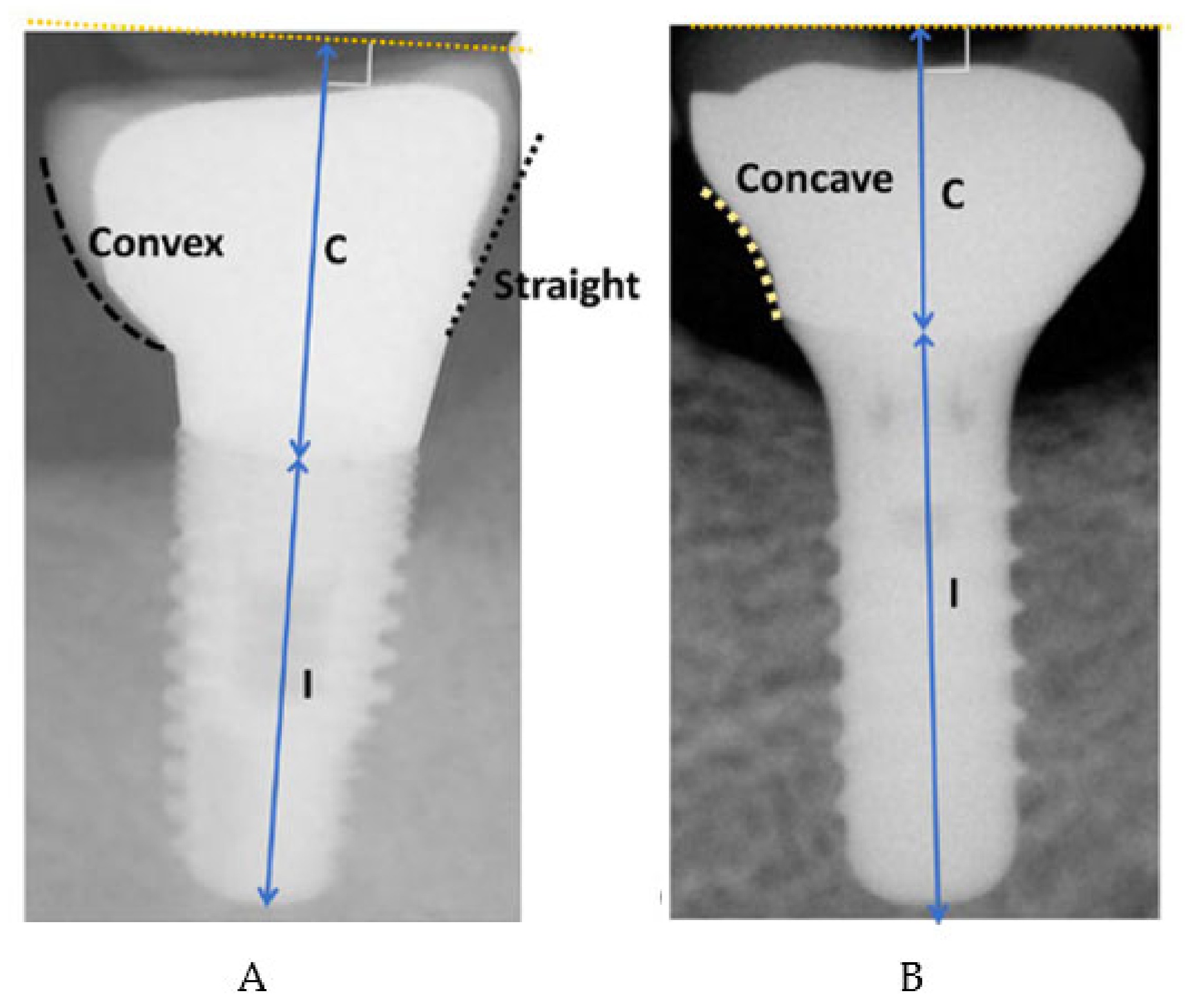
- Overcontoured or non-overcontoured prostheses: assessed by periapical radiographs and clinical evaluation by a calibrated examiner, where an overcontoured prosthesis is more associated with inflammation and the retention of bacterial plaque compared to prostheses with an adequate contour (Figure 1).
2.3. Satisfaction Questionnaire
2.4. Definition of Disease Resolution
- Absence of BOP after soft probing (0.15–0.2 Ncm).
- Probing Depth ≤5 mm.
- Progressive radiographic bone gain (±0.5 mm).
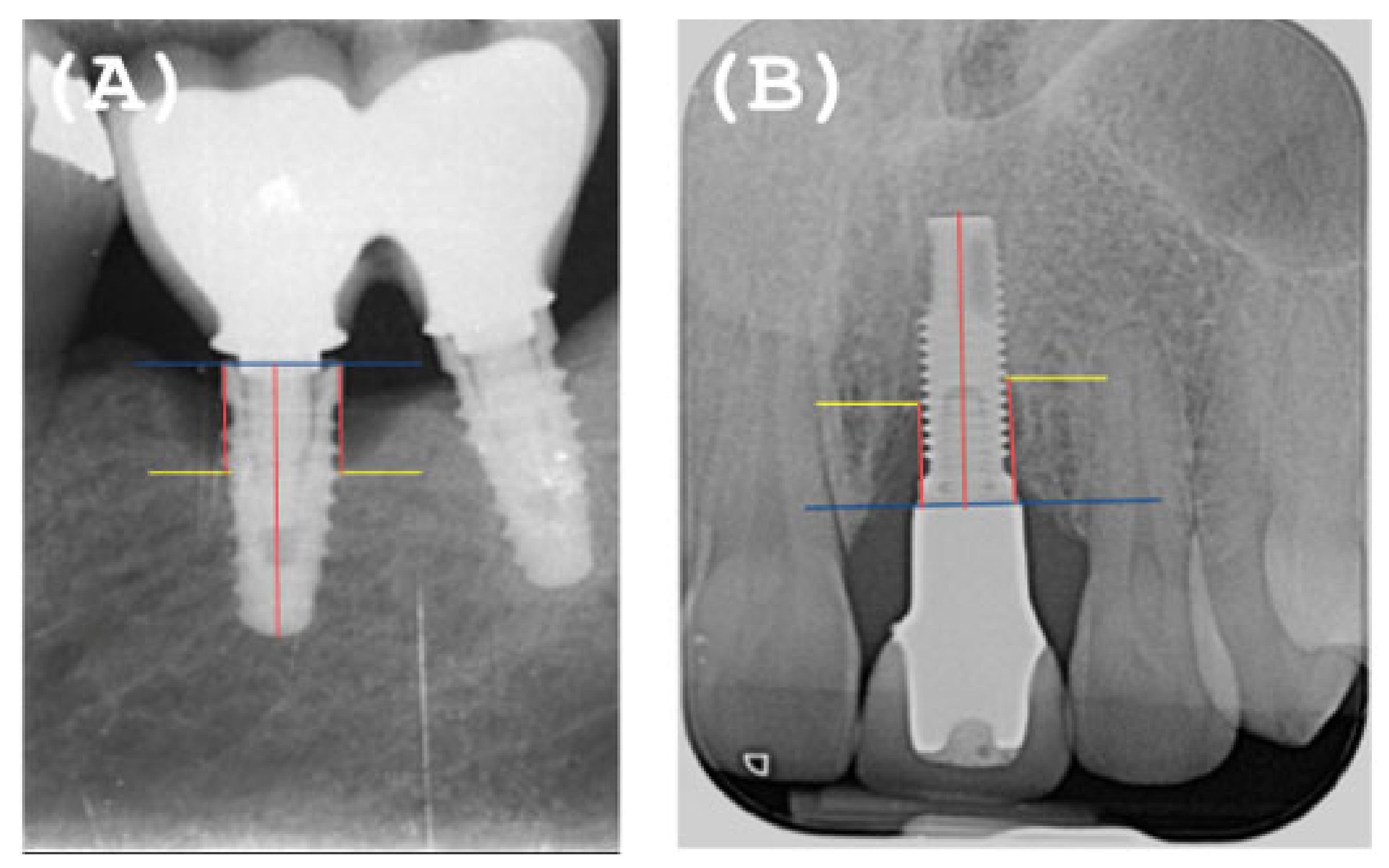
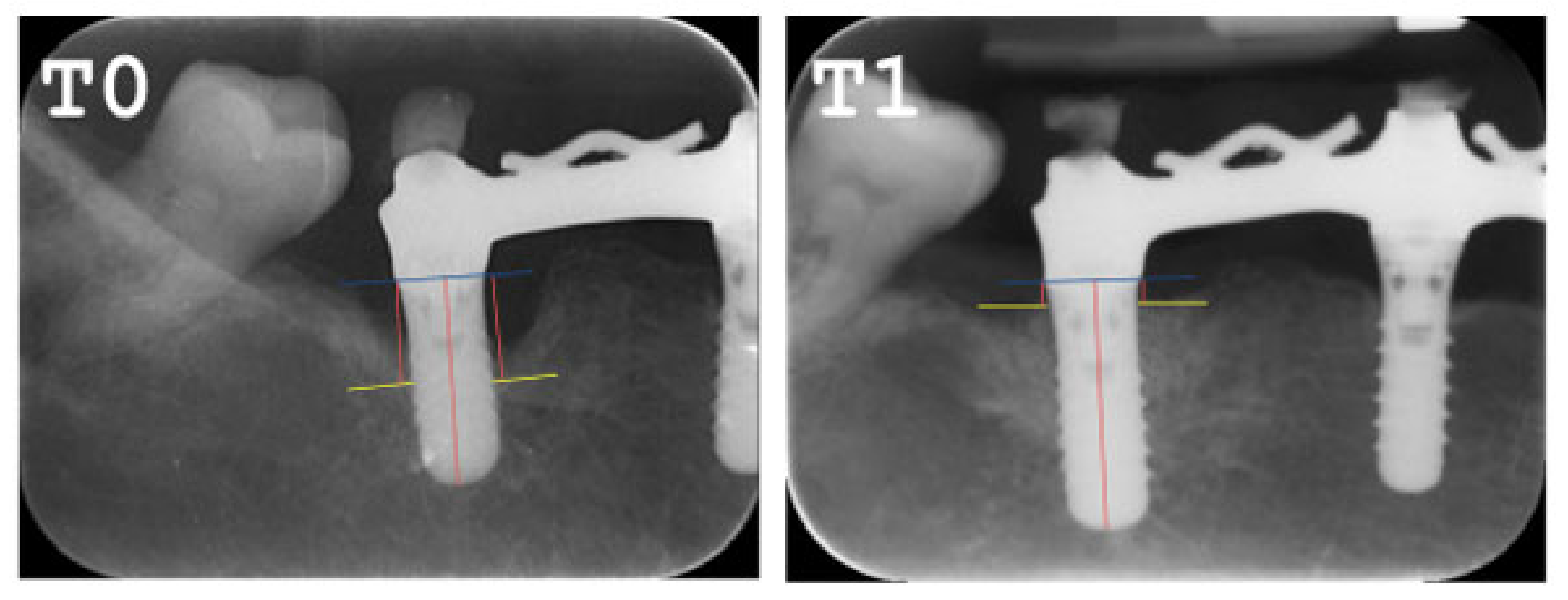
2.5. Radiographic Evaluation
2.6. Non-Surgical Therapy Phase
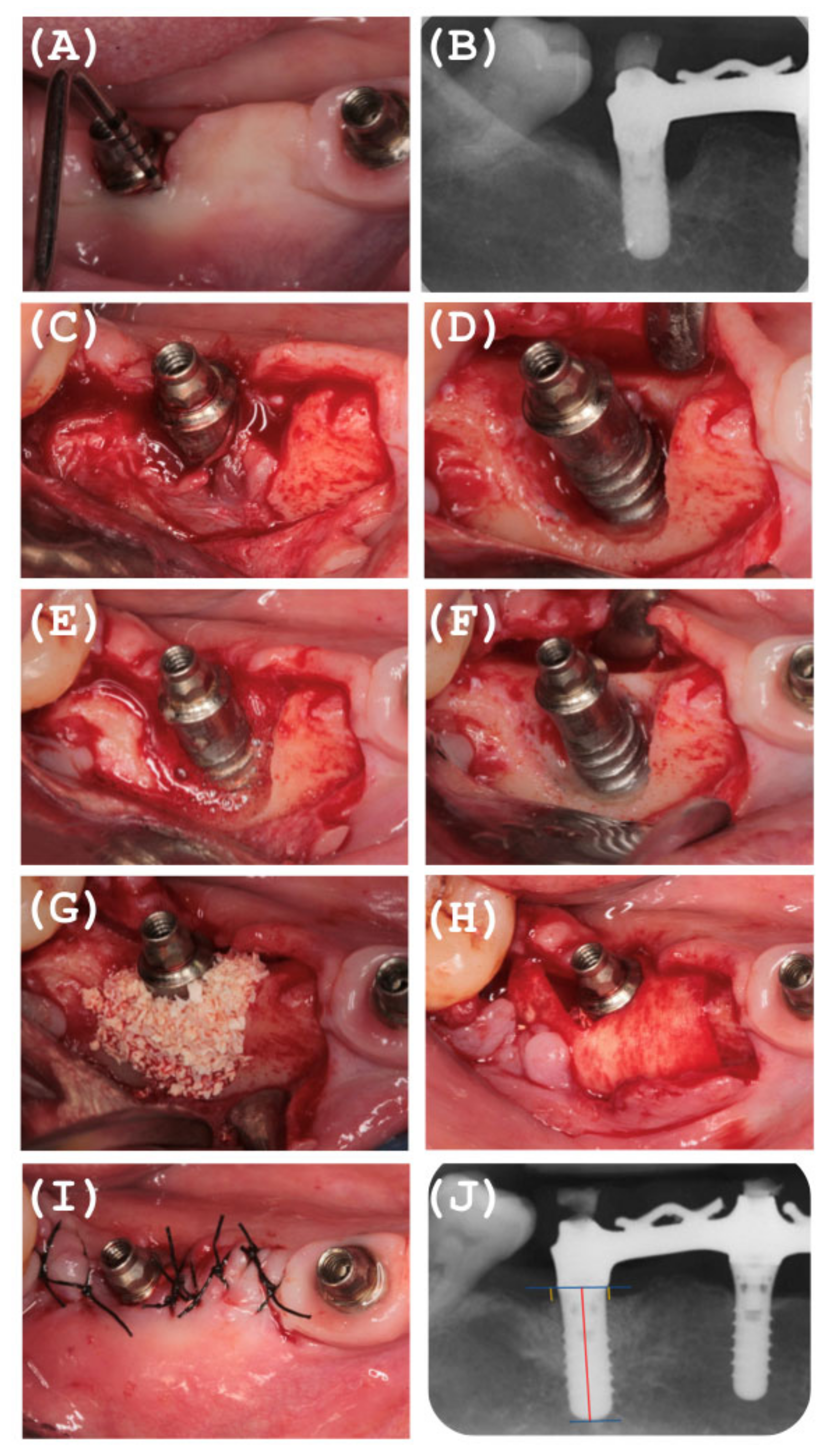
2.7. Surgical Reconstructive Phase
2.8. Postoperative Care
2.9. Recall Program
2.10. Statistical Analysis
- A complete descriptive analysis was made detailing all variables.
- The Chi square test was carried out. In order to determine the groups that made the difference, Haberman’s corrected standardized waste was used, which allowed us to obtain the significance of the cells independently. This significance implies that the percentage of the cell is different, statistically, from that corresponding to the total of the sample.
3. Results
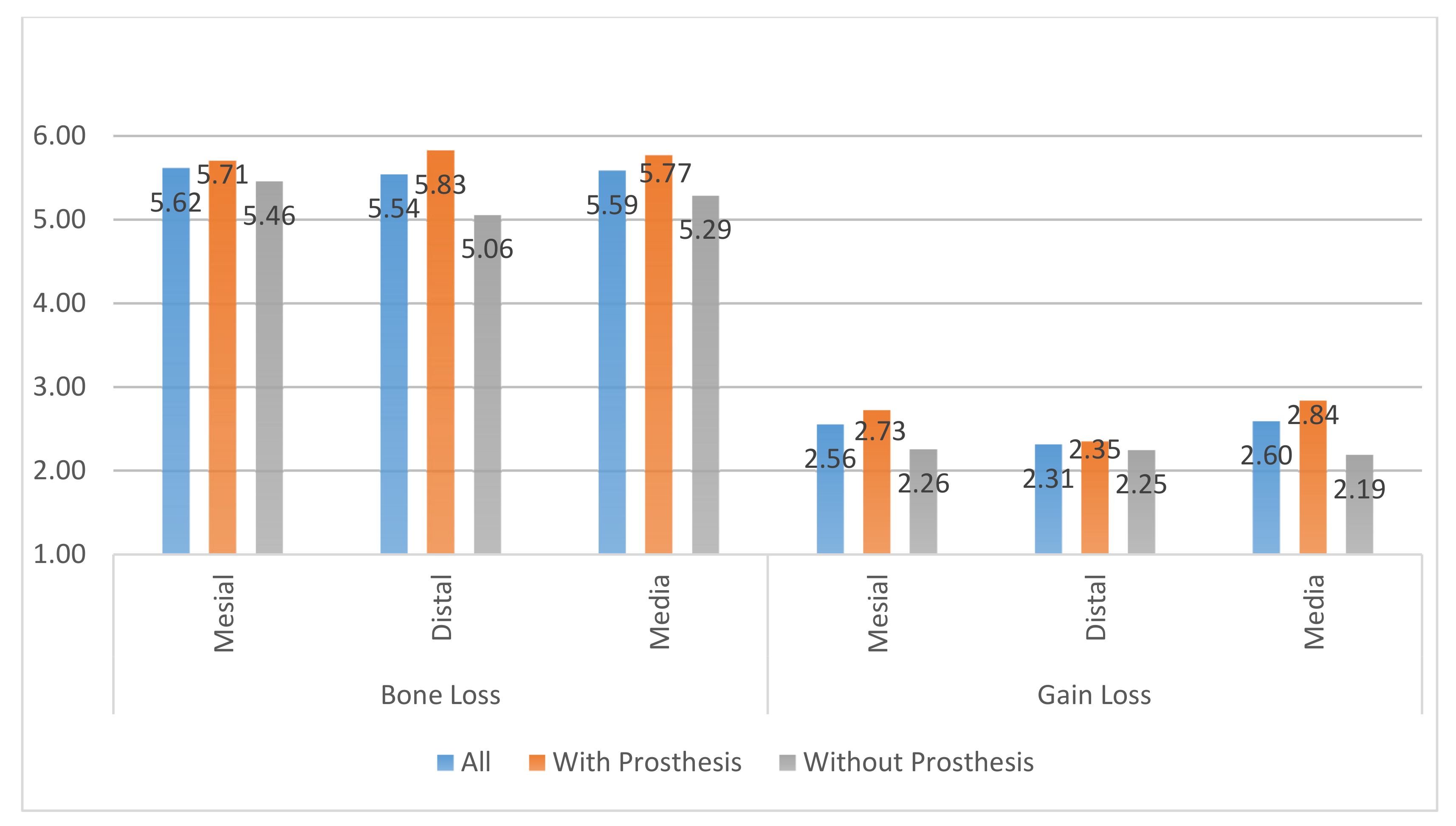
- All clinical variables changed substantially from T0 to T1.
- REC increased from T0 to T1.
- ▪
- The MBL decreased from T0 to T1.
- ▪
- A radiographic bone filling was obtained in most cases.
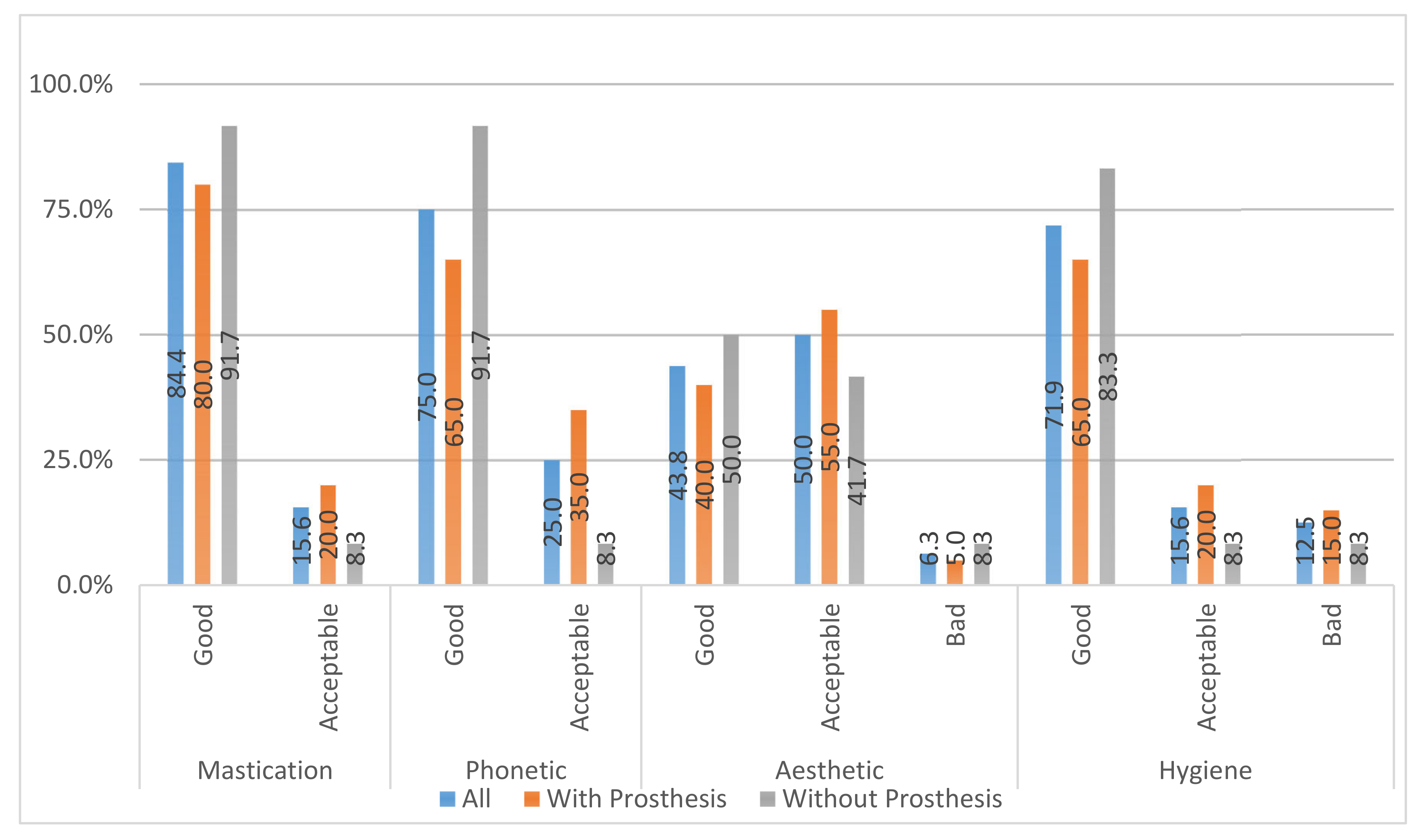
Satisfaction Questionnaire
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, M.; Grusovin, M.G.; Willings, M.; Coulthard, P.; Worthington, H.V. The effectiveness of immediate, early, and conventional loading of dental implants: A Cochrane systematic review of randomized controlled clinical trials. Int. J. Oral Maxillofac. Implants 2007, 22, 893–904. [Google Scholar] [PubMed]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89, S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Sánz-Sánchez, I.; Figuero, E.; Llodrá, J.C.; Bravo, M.; Caffesse, R.G.; Vallcorba, N.; Guerrero, A.; Herrera, D. Prevalence and Risk Indicators of Peri-implant Diseases in Spain. J. Clin. Periodontol. 2018, 45, 1510–1520. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, Peri-implant Mucositis, and Peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89, S304–S312. [Google Scholar] [CrossRef]
- Karlsson, K.; Derks, J.; Hakansson, J.; Wennstrom, J.L.; Petzold, M.; Berglundh, T. Interventions for peri-implantitis and their effects on further bone loss: A retrospective analysis of a registry-based cohort. J. Clin. Periodontol. 2019, 46, 872–879. [Google Scholar] [CrossRef]
- Persson, L.G.; Berglundh, T.; Lindhe, J.; Sennerby, L. Re-osseointegration after treatment of peri-implantitis at different implant surfaces. An experimental study in the dog. Clin. Oral Implants Res. 2001, 12, 595–603. [Google Scholar] [CrossRef]
- Carcuac, O.; Abrahamsson, I.; Charalampakis, G.; Berglundh, T. The effect of the local use of chlorhexidine in surgical treatment of experimentalperi-implantitis in dogs. J. Clin. Periodontol. 2015, 42, 196–203. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Daugela, P.; Juodzbalys, G. Treatment of peri-implantitis: Meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int. 2016, 47, 379–393. [Google Scholar]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of Peri-implantitis with Respective Surgery. A 3 year clinical trial on rough screw shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implants Res. 2007, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C. 1.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone Defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Schwarz, K.; Becker, J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Strom, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral Implants Res. 2009, 20, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Insua, A.; Nart, J.; Wang, H.L.; Schwarz, F. Morphology and severity of peri-implantitis bone defects. Clin. Implant Dent. Relat. Res. 2019, 21, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, R.; Tonetti, M.P.; Carnevale, G.; Mombelli, A.; Nyman, S.R.; Lang, N.P. Experimentally Induced Peri-implant mucositis. A clinical study in humans. Clin. Oral Implants Res. 1994, 5, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of peri-Implantitis. Periodontol. 2000 1998, 17, 63–76. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.M.; Persson, G.R.; Lindahl, C.; Renvert, S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: A 5-year follow-up. J. Clin. Periodontol. 2014, 41, 1108–1114. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.M.; Renvert, H.; Lindahl, C.; Renvert, S. Submerged healing following surgical treatment of peri-implantitis: A case series. J. Clin. Periodontol. 2007, 34, 723–727. [Google Scholar] [CrossRef]
- Schwarz, F.; Sager, M.; Ferrari, D.; Herten, M.; Wieland, M.; Becker, J. Bone regeneration in dehiscence-type defects at non-submerged and submerged chemically modified (SLActive) and conventional SLA titanium implants: An immunohistochemical study in dogs. J. Clin. Periodontol. 2008, 35, 64–75. [Google Scholar] [CrossRef]
- Canullo, L.; Signorini, L.; Pistilli, R.; Patini, R.; Pistilli, V.; Pesce, P. A prospective case series on surgical treatment of circumferential and semi-circumferential defects due to peri-implantitis. Braz. Oral Res. 2019, 33, e072. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 4, 145–151. [Google Scholar] [CrossRef] [PubMed]
- 1999 International Workshop for a Classification of Periodontal Diseases and Conditions. Papers. Oak Brook. Ilinnois. October 30–November 2, 1999. Ann. Periodontol. 1999, 4, 1–112.
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Esposito, M.; Grusovin, M.G.; Maghaireh, H.; Worthingyon, H. Interventions for replacing missing teeth: Differente times for loading dental implants. Cochrane Database Syst. Rev. 2013, 28. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Koo, K.T.; Schwarz, F.; Ben Amara, H.; Heo, S.J. Association of prosthetic features and peri-implantitis: A cross-sectional study. J. Clin. Periodontol. 2020, 47, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.; Iorio Siciliano, V.; Aglietta, M.; Andreuccetti, G.; Salvi, G.E. Clinical and radiographic outcomes of a combined resective and regenerative approach in the treatment of peri-implantitis: A prospective case series. Clin. Oral Implants Res. 2014, 25, 132–136. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin. Oral Implants Res. 2014, 25, 132–136. [Google Scholar] [CrossRef]
- Schwarz, F.; Jepsen, S.; Herten, M.; Sager, M.; Rothamel, D.; Becker, J. Influence of different approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: An experimental study in dogs. J. Clin. Periodontol. 2006, 33, 584–595. [Google Scholar] [CrossRef]
- Björn, H.; Hollender, L.; Lindhe, J. Tissue regeneration in patients with periodontal disease. Odontol. Revy. 1965, 16, 317–326. [Google Scholar]
- Sigurdsson, T.J.; Hardwick, R.; Bogle, G.C.; Wikesjo, U.M. Periodontal repair in dogs: Space provision by reinforced ePTFE membranes enhances bone and cementum regeneration in large supraalveolar defects. J. Periodontol. 1994, 65, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Buchmann, R. Surgical therapy of peri-implant disease: A 3-year follow-up study of cases treated with 3 different techniques of bone regeneration. J. Periodontol. 2001, 72, 1498–1508. [Google Scholar] [CrossRef]
- Wiltfang, J.; Zernial, O.; Behrens, E.; Schlegel, A.; Warnke, P.H.; Becker, S.T. Regenerative treatment of peri-implantitis bone defects with a combination of autologous bone and a demineralized xenogenic bone graft: A series of 36 defects. Clin. Implant Dent. Relat. Res. 2012, 14, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Lollobrigida, M.; Fortunato, L.; Lamazza, L.; Serafini, G.; De Biase, A. Reosseointegration after the surgical treatment of induced peri-implantitis: Systematic review on current evidence and translation from the animal to the human model. Minerva Stomatol. 2020, 69, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Daugela, P.; Cicciù, M.; Saulacic, N. Surgical Regenerative Treatments for Peri-Implantitis: Meta-analysis of Recent Findings in a Systematic Literature Review. J. Oral Maxillofac. Res. 2016, 7, e15. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Layton, D.M.; Roccuzzo, A.; Heitz-Mayfield, L.J. Clinical outcomes of peri-implantitis treatment and supportive care: A systematic review. Clin. Oral Implants Res. 2018, 29, 331–350. [Google Scholar] [CrossRef]
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis—Consensus report of working group 4. Int. Dent. J. 2019, 69, 18–22. [Google Scholar] [CrossRef] [PubMed]

| Variable | Rank | Yes | No | Sign. | ||
|---|---|---|---|---|---|---|
| Frec. | Porc. | Frec. | Porc. | |||
| Tobacco | Nonsmoker | 7 | 43.8 | 9 | 56.3 | 0 |
| Less than 10 cig/day | 6 | 75 | 2 | 25 | 0 | |
| More than 10 cig/day | 7 | 87.5 | 1 | 12.5 | 0 | |
| Alcohol | Non-Consumer | 14 | 58.3 | 10 | 41.7 | 0 |
| Mild or Moderate Consumer | 6 | 75 | 2 | 25 | 0 | |
| Heavy Consumer | 0 | --- | 0 | --- | 0 | |
| Variable | Rank | Yes | No | Sign. | ||
|---|---|---|---|---|---|---|
| Frec. | Porc. | Frec. | Porc. | |||
| Type of Prosthetic Restoration | Cemented Retained | 2 | 100 | 0 | 0 | <0.05 (s) |
| Screw Retained | 18 | 100 | 12 | 100 | 0 | |
| Passive Fit | Yes | 18 | 64.3 | 10 | 35.7 | 0 |
| No | 2 | 50 | 2 | 50 | 0 | |
| Prosthetic Contour | Yes | 6 | 60 | 4 | 40 | 0 |
| No | 14 | 63.6 | 8 | 36.4 | 0 | |
| Antagonistic Arcade | Natural Teeth | 13 | 56.5 | 10 | 43.5 | 0 |
| Fixed Dental Prostheses | 3 | 75 | 1 | 25 | 0 | |
| Fixed Implant Supported | 4 | 80 | 1 | 20 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astolfi, V.; Gómez-Menchero, A.; Ríos-Santos, J.V.; Bullón, P.; Galeote, F.; Ríos-Carrasco, B.; Bullón de la Fuente, B.; Herrero-Climent, M. Influence of Removing or Leaving the Prosthesis after Regenerative Surgery in Peri-Implant Defects: Retrospective Study: 32 Clinical Cases with 2 to 8 Years of Follow-Up. Int. J. Environ. Res. Public Health 2021, 18, 645. https://doi.org/10.3390/ijerph18020645
Astolfi V, Gómez-Menchero A, Ríos-Santos JV, Bullón P, Galeote F, Ríos-Carrasco B, Bullón de la Fuente B, Herrero-Climent M. Influence of Removing or Leaving the Prosthesis after Regenerative Surgery in Peri-Implant Defects: Retrospective Study: 32 Clinical Cases with 2 to 8 Years of Follow-Up. International Journal of Environmental Research and Public Health. 2021; 18(2):645. https://doi.org/10.3390/ijerph18020645
Chicago/Turabian StyleAstolfi, Víctor, Alberto Gómez-Menchero, José Vicente Ríos-Santos, Pedro Bullón, Francisco Galeote, Blanca Ríos-Carrasco, Beatriz Bullón de la Fuente, and Mariano Herrero-Climent. 2021. "Influence of Removing or Leaving the Prosthesis after Regenerative Surgery in Peri-Implant Defects: Retrospective Study: 32 Clinical Cases with 2 to 8 Years of Follow-Up" International Journal of Environmental Research and Public Health 18, no. 2: 645. https://doi.org/10.3390/ijerph18020645
APA StyleAstolfi, V., Gómez-Menchero, A., Ríos-Santos, J. V., Bullón, P., Galeote, F., Ríos-Carrasco, B., Bullón de la Fuente, B., & Herrero-Climent, M. (2021). Influence of Removing or Leaving the Prosthesis after Regenerative Surgery in Peri-Implant Defects: Retrospective Study: 32 Clinical Cases with 2 to 8 Years of Follow-Up. International Journal of Environmental Research and Public Health, 18(2), 645. https://doi.org/10.3390/ijerph18020645








