Effect of Processing on Residual Buprofezin Levels in Ginseng Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trials
2.2. Processing Procedures and Sample Preparation
2.3. Reagents and Materials
2.4. Extraction and Purification
2.5. Optimization of Instrumental Analysis
2.6. Matrix Effect
2.7. Limits of Quantitation and Recovery
2.8. Processing and Reduction Factors
2.9. Statistical Analysis
3. Results and Discussion
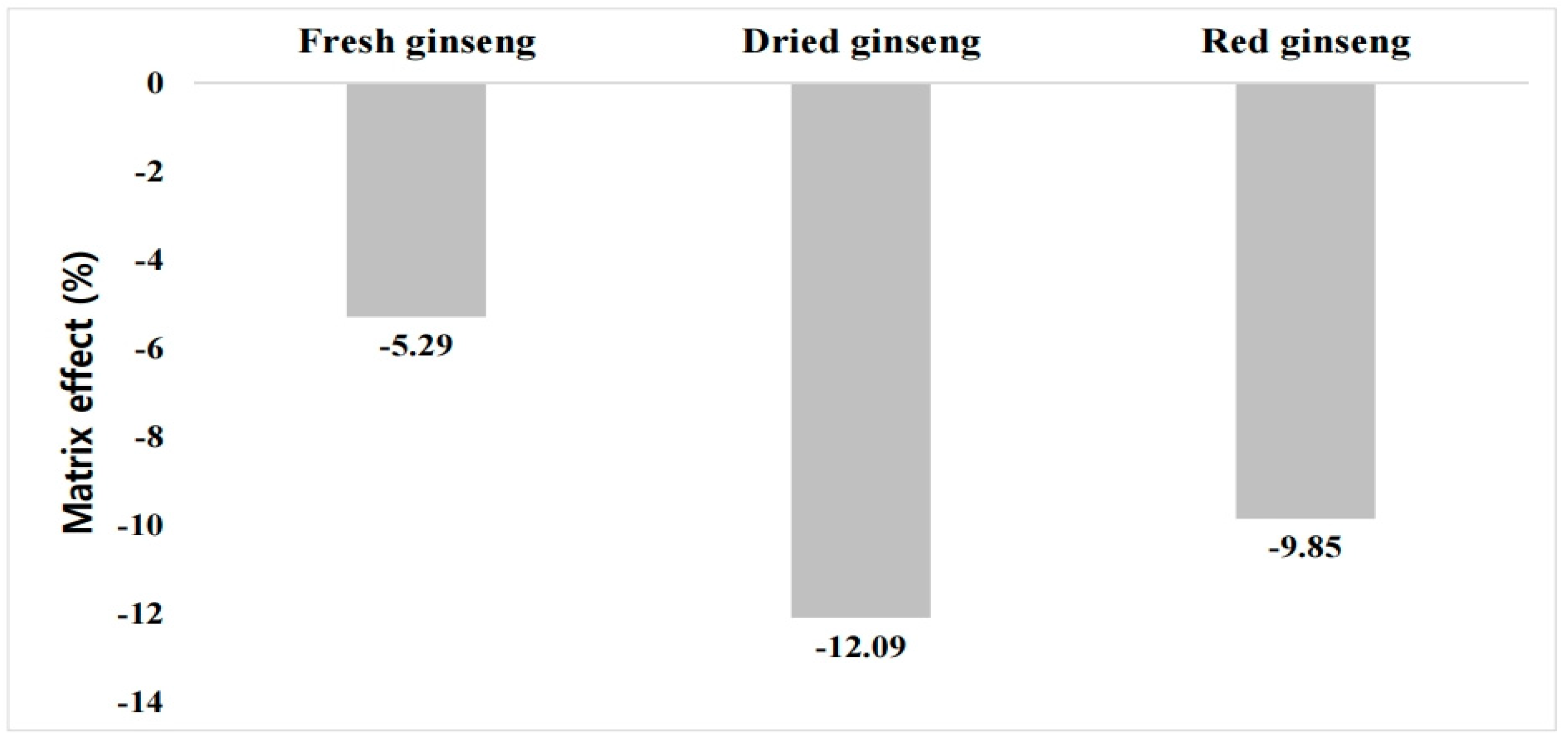
3.1. Calibration Curve and ME
3.2. Recovery
3.3. Residues in Fresh Ginseng
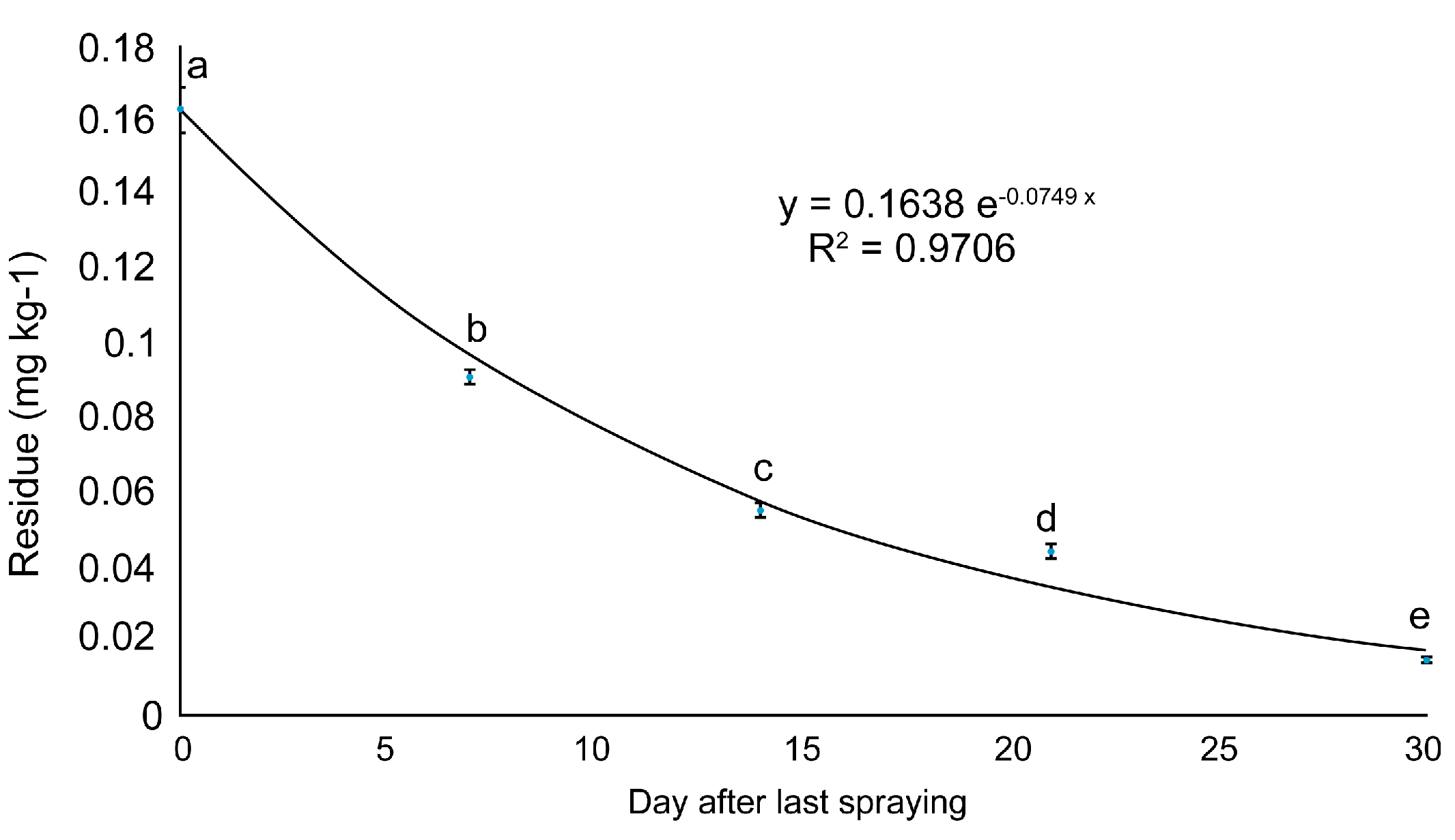
3.4. Half-Lives and Prediction of the Residual Reduction Period
3.5. Processing and Reduction Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitts, D.D.; Hu, C. Efficacy and safety of ginseng. Public Health Nutr. 2000, 3, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-H.; Lee, K.-H.; Lee, J.-Y.; Lee, E.-Y.; Park, Y.-S.; Park, H.-K.; Oh, J.-H.; Im, M.-H.; Lee, Y.-J.; Baeg, I.-H.; et al. Residual Characteristics and Processing Factors of Difenoconazole in Fresh Ginseng and Processed Ginseng Products. Korean J. Pestic. Sci. 2012, 16, 35–42. [Google Scholar] [CrossRef]
- Zhang, W.J. Constructing ecological interaction networks by correlation analysis: Hints from community sampling. Netw. Biol. 2011, 1, 81–98. [Google Scholar]
- Peshin, R.; Zhang, W. Integrated Pest Management and Pesticide Use. In Integrated Pest Management: Pesticide Problems; Pimentel, D., Peshin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 3, pp. 1–46. [Google Scholar]
- Cai, D.W. Understand the role of chemical pesticides and prevent misuses of pesticides. Bull. Agric. Sci. Technol. 2008, 1, 36–38. [Google Scholar]
- Guo, X.F.; Zhang, H.F.; Li, J.G. The importance of fungicides/bactericides in American agriculture. World Pest. 2007, 9, 21–25. [Google Scholar]
- Lee, J.-Y.; Noh, H.-H.; Lee, K.-H.; Park, H.-K.; Oh, J.-H.; Im, M.-H.; Kwon, C.-H.; Lee, J.-K.; Woo, H.-D.; Kwon, K.-S.; et al. Processing factors of azoxystrobin in processed ginseng products. Korean J. Pestic. Sci. 2012, 16, 222–229. [Google Scholar] [CrossRef][Green Version]
- Lee, S.M.; Bae, B.-S.; Park, H.-W.; Ahn, N.-G.; Cho, B.-G.; Cho, Y.-L.; Kwak, Y.-S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Cabitza, F.; Cubeddu, M. Pesticide residues in raisin processing. J. Agric. Food Chem. 1998, 46, 2309–2311. [Google Scholar] [CrossRef]
- Athanasopoulos, P.E.; Pappas, C.; Kyriakidis, N.V.; Thanos, A. Degradation of methamidophos on soultanina grapes on the vines and during refrigerated storage. Food Chem. 2005, 91, 235–240. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Cabitza, F.; Cubeddu, M. Pesticide residues on field-sprayed apricots and in apricot drying processes. J. Agric. Food Chem. 1998, 46, 2306–2308. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, F.; Ge, J.; Ma, L.; Wu, L.; Xue, X. Changes in eleven pesticide residues in jujube (Ziziphus jujuba Mill.) during drying processing. Dry. Technol. 2017, 36, 965–972. [Google Scholar] [CrossRef]
- Noh, H.-H.; Lee, J.-Y.; Park, S.-H.; Lee, K.-H.; Park, H.-K.; Oh, J.-H.; Im, M.-H.; Kwon, C.-H.; Lee, J.-K.; Woo, H.-D.; et al. Residual characteristics of tolclofos-methyl treated by seed dressing in ginseng. Korean J. Pestic. Sci. 2012, 16, 217–221. [Google Scholar] [CrossRef]
- MacBean, C. (Ed.) The Pesticide Manual: A World Compendium; British Crop Production Council (BCPC): Alton, Hampshire, UK, 2012; pp. 138–140. [Google Scholar]
- Kittlaus, S.; Schimanke, J.; Kempe, G.; Speer, K. Assessment of sample cleanup and matrix effects in the pesticide residue analysis of foods using postcolumn infusion in liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 8399–8410. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.; Botitsi, H.; Antoniou, S.; Tsipi, D. Determination of multi-class pesticides in wines by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 5856–5867. [Google Scholar] [CrossRef] [PubMed]
- Sood, C.; Jaggi, S.; Kumar, V.; Ravindranath, S.; Shanker, A. How manufacturing processes affect the level of pesticide residues in tea. J. Sci. Food Agric. 2004, 84, 2123–2127. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Submission and evaluation of pesticide residues data for the esti-mation of maximum residue levels in food and feed. In Pesticide Residues; FAO: Rome, Italy, 2016; pp. 27–32. [Google Scholar]
- Sur, R.; Stork, A. Uptake, translocation and metabolism of imidacloprid in plants. Bull. Insectol. 2003, 56, 35–40. [Google Scholar]
- Utture, S.C.; Banerjee, K.; Kolekar, S.S.; Dasgupta, S.; Oulkar, D.P.; Patil, S.H.; Wagh, S.S.; Adsule, P.G.; Anuse, M.A. Food safety evaluation of buprofezin, dimethoate and imidacloprid residues in pomegranate. Food Chem. 2012, 131, 787–795. [Google Scholar] [CrossRef]
- Lee, J.Y.; Noh, H.H.; Park, H.K.; Kim, J.C.; Jeong, H.R.; Jin, M.J.; Kyung, K.S. Residual Characteristics and Behavior of Azoxystrobin in Ginseng by Cultivation Conditions. Korean J. Pestic. Sci. 2015, 19, 14–21. [Google Scholar] [CrossRef][Green Version]
- Valverde-Garcia, A.; González-Pradas, E.; Real, A.A.-D. Analysis of buprofezin residues in vegetables. Application to the degradation study on eggplant grown in a greenhouse. J. Agric. Food Chem. 1993, 41, 2319–2323. [Google Scholar] [CrossRef]
- Mohapatra, S.; Siddamallaiah, L.; Matadha, N.Y.; Gadigeppa, S.; Raja, D.P.; Udupi, V.R. Persistence and dissipation study of azoxystrobin, buprofezin, dinocap and hexaconazole on mango (Mangifera indica L.). Environ. Sci. Pollut. Res. 2020, 27, 32820–32828. [Google Scholar] [CrossRef]
- Noh, H.H.; Lee, J.Y.; Park, H.K.; Lee, J.W.; Jo, S.H.; Lim, J.B.; Shin, H.G.; Kwon, H.; Kyung, K.S. Dissipation, persistence, and risk assessment of fluxapyroxad and penthiopyrad residues in perilla leaf (Perilla frutescens var. japonica Hara). PLoS ONE 2019, 14, e0212209. [Google Scholar] [CrossRef] [PubMed]
- Dong, F. The Pesticide Residue Changes during Food Processing and Storage. Ph.D. Thesis, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China, 2012. [Google Scholar]

| Site | Application No. | Application | ||||
|---|---|---|---|---|---|---|
| Method | Volume (L ha−1) | Dose (kg a.i. ha−1) | Re-Treatment Interval (d) | Total Applied (kg a.i. ha−1) | ||
| I (Yeongju) | 1 | Foliar application | 2016 | 0.252 | - | 0.774 |
| 2 | Foliar application | 2070 | 0.259 | 10 | ||
| 3 | Foliar application | 2103 | 0.263 | 10 | ||
| II (Geumsan) | 1 | Foliar application | 1646 | 0.206 | - | 0.608 |
| 2 | Foliar application | 1580 | 0.198 | 10 | ||
| 3 | Foliar application | 1634 | 0.204 | 10 | ||
| III (Goesan) | 1 | Foliar application | 1951 | 0.244 | - | 0.724 |
| 2 | Foliar application | 1901 | 0.238 | 10 | ||
| 3 | Foliar application | 1938 | 0.242 | 10 | ||
| <LC Condition> | ||||
|---|---|---|---|---|
| Instrument | Acquity UPLC H Class System, Waters (Milford, MA, USA) | |||
| Column | Acquity UHPLC BEH C18 | |||
| Flow rate | 0.3 mL min−1 | |||
| Mobile phase | 0.1% (v/v) formic acid in water:0.1% (v/v) formic acid in acetonitrile (1:9, v/v) | |||
| Injection volume | 1 μL | |||
| <Mass condition> | ||||
| Instrument | Triple-quadruple spectrometer, Xevo TQD, Waters (USA) | |||
| Source temperature | 150 °C | Cone gas flow rate | 50 L h−1 | |
| Capillary voltage | 3.0 kV | Ion source | ESI(+) | |
| Desolvation gas | Temperature 200 °C; flow rate 650 L h−1 | Scan type | MRM mode | |
| <MRM condition> | ||||
| Precursor ion (m/z) | Quantitation ion | Confirmation ion | ||
| m/z | Collision energy | m/z | Collision energy | |
| 306.2 | 116.1 | 15 | 201.1 | 10 |
| Ginseng | Fortification Level (mg kg−1) | Mean Value (%, n = 5) | Standard Deviation | Coefficient of Variation (%) |
|---|---|---|---|---|
| Fresh ginseng | 0.005 | 91.09 | 1.87 | 2.05 |
| 0.05 | 88.98 | 2.43 | 2.74 | |
| 0.07 | 88.86 | 2.43 | 2.73 | |
| Dried ginseng | 0.005 | 83.85 | 3.15 | 3.75 |
| 0.05 | 90.98 | 1.51 | 1.66 | |
| 0.07 | 89.24 | 1.27 | 1.42 | |
| Red ginseng | 0.005 | 90.66 | 2.87 | 3.16 |
| 0.05 | 96.04 | 1.83 | 1.91 | |
| 0.07 | 97.58 | 1.96 | 2.01 |
| Site | Mean Residue (mg kg−1, n = 3) | ||||
|---|---|---|---|---|---|
| Days after Last Application | |||||
| 0 | 7 | 14 | 21 | 30 | |
| 1 | 0.076 b ± 0.005 | 0.018 b ± 0.001 | <LOQ | <LOQ | <LOQ |
| 2 | 0.163 a ± 0.006 | 0.091 a ± 0.002 | 0.055 ± 0.002 | 0.044 ± 0.002 | 0.014 ± 0.002 |
| 3 | 0.078 b ± 0.002 | 0.012 b ± 0.000 | <LOQ | <LOQ | <LOQ |
| Site | Dried Ginseng | Red Ginseng | ||||
|---|---|---|---|---|---|---|
| Residue (mg kg−1, n = 3) | SD | CV (%) | Residue (mg kg−1, n = 3) | SD | CV (%) | |
| 1 | 0.046 b | 0.001 | 1.87 | 0.049 b | 0.002 | 4.69 |
| 2 | 0.189 a | 0.002 | 0.81 | 0.171 a | 0.003 | 1.74 |
| 3 | 0.013 c | 0.000 | 2.08 | 0.006 c | 0.000 | 0.90 |
| Rep. | Residue (mg kg−1) | Water Content (%) | Yield (%) | Residue Based on Dry Weight (mg kg−1) | Processing Factor | Reduction Factor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | D | R | F | D | R | D | R | F | D | R | D | R | D | R | |
| 1 | 0.053 | 0.190 | 0.175 | 70.1 | 13.2 | 13.7 | 27.8 | 30.5 | 0.177 | 0.158 | 0.141 | 3.58 | 3.30 | 0.89 | 0.80 |
| 2 | 0.053 | 0.190 | 0.169 | 70.5 | 12.9 | 13.0 | 28.1 | 31.8 | 0.180 | 0.157 | 0.132 | 3.58 | 3.19 | 0.87 | 0.74 |
| 3 | 0.057 | 0.187 | 0.170 | 71.1 | 13.8 | 13.5 | 29.0 | 30.0 | 0.197 | 0.154 | 0.138 | 3.28 | 2.98 | 0.78 | 0.70 |
| Mean | 0.054 | 0.189 | 0.171 | 70.6 | 13.3 | 13.4 | 28.3 | 30.8 | 0.185 | 0.156 | 0.137 | 3.48 | 3.16 | 0.85 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, H.H.; Shin, H.W.; Kim, D.J.; Lee, J.W.; Jo, S.H.; Kim, D.; Kyung, K.S. Effect of Processing on Residual Buprofezin Levels in Ginseng Products. Int. J. Environ. Res. Public Health 2021, 18, 471. https://doi.org/10.3390/ijerph18020471
Noh HH, Shin HW, Kim DJ, Lee JW, Jo SH, Kim D, Kyung KS. Effect of Processing on Residual Buprofezin Levels in Ginseng Products. International Journal of Environmental Research and Public Health. 2021; 18(2):471. https://doi.org/10.3390/ijerph18020471
Chicago/Turabian StyleNoh, Hyun Ho, Hyeon Woo Shin, Dong Ju Kim, Jeong Woo Lee, Seung Hyeon Jo, Danbi Kim, and Kee Sung Kyung. 2021. "Effect of Processing on Residual Buprofezin Levels in Ginseng Products" International Journal of Environmental Research and Public Health 18, no. 2: 471. https://doi.org/10.3390/ijerph18020471
APA StyleNoh, H. H., Shin, H. W., Kim, D. J., Lee, J. W., Jo, S. H., Kim, D., & Kyung, K. S. (2021). Effect of Processing on Residual Buprofezin Levels in Ginseng Products. International Journal of Environmental Research and Public Health, 18(2), 471. https://doi.org/10.3390/ijerph18020471





