Fate of Emerging Contaminants in High-Rate Activated Sludge Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
| EDCs | PhACs | ||||||
|---|---|---|---|---|---|---|---|
| NP | TCS | BPA | IBF | NPX | DCF | KFN | |
| Structure | 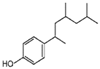 | 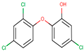 |  |  | 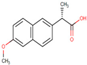 | 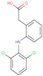 | 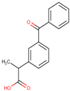 |
| Chem. Formula | C15H24O | C12H7Cl3O2 | C15H16O2 | C13H18O2 | C14H14O3 | C14Η11Cl2ΝΟ2 | C16H14O3 |
| Mol. Weight (g/mol) | 220.3505 | 289.5418 | 228.2863 | 206.2808 | 230.2592 | 296.1486 | 254.2896 |
| Water Solubility (mg/L at 25 °C) | 4.9–6.4 | 10 | 300 | 21 | 15.90 | 2.37 | 51 |
| pKa | 10.25 | 7.90 | 9.60 | 4.91–5.3 | 4.15 | 4.15 | 4.45 |
| log Kow | 5.76 | 4.76 | 3.32 | 3.97 | 3.18 | 4.51 | 3.12 |
| log Koc | 4–4.7 | 3.38–4.2 | 2.90 | 2.20 | 2.52 | 2.39 | 0.20 |
| Henry’s Law constant (atm-cu m/mol at 25 °C) | 5.6 × 10−6 | 2.1 × 10−8 | 4.0 × 10−11 | 1.4 × 10−7 | 3.4 × 10−10 | 4.73 × 10−12 | 2.1 × 10−11 |
2.2. HRAS Units Description and Sampling Strategy
2.3. Sample Preparation and Analytical Determination
2.4. Data Calculation
3. Results and Discussion
3.1. Emerging Contaminants in Influent Wastewater Samples
3.2. Emerging Contaminants in Sewage Sludge
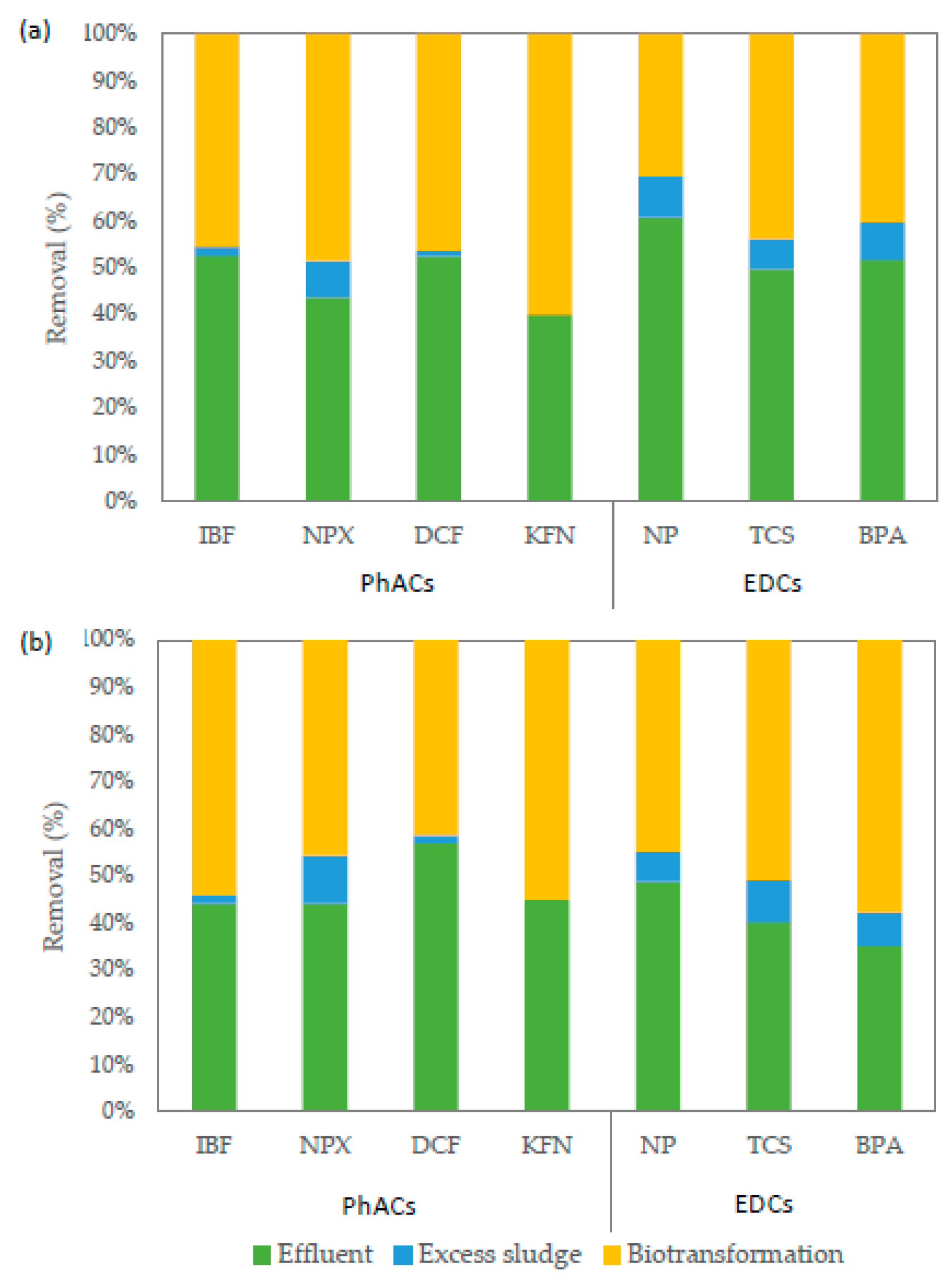
3.3. Removal of Emerging Contaminants in HRAS Systems
3.4. Fate of Emerging Contaminants during HRAS Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aymerich, I.; Rieger, L.; Sobhani, R.; Rosso, D.; Corominas, L. The difference between energy consumption and energy cost: Modelling energy tariff structures for water resource recovery facilities. Water Res. 2015, 81, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Mamais, D.; Noutsopoulos, C.; Dimopoulou, A.; Stasinakis, A.; Lekkas, T.D. Wastewater treatment process impact on energy savings and greenhouse gas emissions. Water Sci. Technol. 2015, 71, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Gu, J.; Zhao, Q.; Liu, Y. COD capture: A feasible option towards energy self-sufficient domestic wastewater treatment. Sci. Rep. 2016, 6, 25054. [Google Scholar] [CrossRef]
- Gikas, P. Towards energy positive wastewater treatment plants. J. Environ. Manag. 2017, 203, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Xu, X.; Yang, F. High-Rate Contact Stabilization Process-Coupled Membrane Bioreactor for Maximal Recovery of Organics from Municipal Wastewater. Water 2018, 10, 878. [Google Scholar] [CrossRef]
- Guven, H.; Dereli, R.K.; Ozgun, H.; Ersahin, M.E.; Ozturk, I. Towards sustainable and energy efficient municipal wastewater treatment by up-concentration of organics. Prog. Energy Combust. Sci. 2019, 70, 145–168. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef]
- Silvestre, G.; Fernández, B.; Bonmatí, A. Significance of anaerobic digestion as a source of clean energy in wastewater treatment plants. Energy Convers. Manag. 2015, 101, 255–262. [Google Scholar] [CrossRef]
- Taboada-Santos, A.; Rivadulla, E.; Paredes, L.; Carballa, M.; Romalde, J.; Lema, J.M. Comprehensive comparison of chemically enhanced primary treatment and high-rate activated sludge in novel wastewater treatment plant configurations. Water Res. 2020, 169, 115258. [Google Scholar] [CrossRef]
- Sancho, I.; Lopez-Palau, S.; Arespacochaga, N.; Cortina, J.L. New concepts on carbon redirection in wastewater treatment plants: A review. Sci. Total Environ. 2019, 647, 1373–1384. [Google Scholar] [CrossRef]
- Rahman, A.; Yapuwa, H.; Baserba, M.G.; Rosso, D.; Jimenez, J.A.; Bott, C.; Al-Omari, A.; Murthy, S.; Riffat, R.; De Clippeleir, H. Methods for quantification of biosorption in high-rate activated sludge systems. Biochem. Eng. J. 2017, 128, 33–44. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, J.; Zhang, M. Chapter 2: Approaches to energy and resource recovery from municipal wastewater. In A-B Processes: Towards Energy Self-Sufficient Municipal Wastewater Treatment; IWA Publishing: London, UK, 2020; ISBN 9781789060089. [Google Scholar] [CrossRef][Green Version]
- Smith, A.L.; Stadler, L.B.; Cao, L.; Love, N.G.; Raskin, L.; Skerlos, S.J. Navigating wastewater energy recovery strategies: A life cycle comparison of anaerobic membrane bioreactor and conventional treatment systems with anaerobic digestion. Environ. Sci. Technol. 2014, 48, 5972–5981. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Miller, M.; Bott, C.; Murthy, S.; De Clippeleir, H.; Wett, B. High-rate activated sludge system for carbon management—Evaluation of crucial process mechanisms and design parameters. Water Res. 2015, 87, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2020, 124413. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bečanová, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Klánová, J.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic ingredients as emerging pollutants of environmental and health concern. A mini-review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 558, 750–767. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence and treatment of contaminants of emerging concern in the African aquatic environment: Literature review and a look ahead. J. Environ. Manag. 2020, 254, 109752. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Koumaki, E.; Sarantopoulos, V.; Mamais, D. Analytical and mathematical assessment of emerging pollutants fate in a river system. J. Hazard. Mater. 2019, 364, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Y.; Carvalho, G.; Reis, M.A.M.; Oehmen, A. A review of the biotransformations of priority pharmaceuticals in biological wastewater treatment processes. Water Res. 2020, 188, 116446. [Google Scholar] [CrossRef] [PubMed]
- McGettigan, P.; Henry, D. Use of Non-Steroidal Anti-Inflammatory Drugs that Elevate Cardiovascular Risk: An Examination of Sales and Essential Medicines Lists in Low-, Middle-, and High-Income Countries. PLoS Med. 2013, 10, 1001388. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Mentor, A.; Brunström, B.; Mattsson, A.; Jönsson, M. Developmental exposure to a human relevant mixture of endocrine disruptors alters metabolism and adipogenesis in zebrafish (Danio rerio). Chemosphere 2020, 238, 124584. [Google Scholar] [CrossRef]
- Mathias, F.T.; Fockink, D.H.; Disner, G.R.; Prodocimo, V.; Ribas, J.L.C.; Ramos, L.P.; Cestari, M.M.; de Assis, H.C.S. Effects of low concentrations of ibuprofen on freshwater fish Rhamdia quelen. Environ. Toxicol. Pharmacol. 2018, 59, 105–113. [Google Scholar] [CrossRef]
- Rangasamy, B.; Hemalatha, D.; Shobana, C.; Nataraj, B.; Ramesh, M. Developmental toxicity and biological responses of zebrafish (Danio rerio) exposed to anti-inflammatory drug ketoprofen. Chemosphere 2018, 213, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Charalambous, V.; Koumaki, E. Evaluating the fate of emerging contaminants in wastewater treatment plants through plant-wide mathematical modelling. Environ. Process. 2020, 7, 1065–1094. [Google Scholar] [CrossRef]
- Koumaki, E.; Mamais, D.; Noutsopoulos, C. Environmental fate of non-steroidal anti-inflammatory drugs in river water/sediment systems. J. Hazard. Mater. 2017, 323, 233–241. [Google Scholar] [CrossRef]
- The PubChem Project USA: National Center for Biotechnology Information, Online PubChem Compound Database. Available online: http://www.ncbi.nlm.nih.gov/pccompound (accessed on 3 November 2020).
- Toxicology Data Network (TOXNET) U.S. National Library of Medicine, National Institutes of Health, Health & Human Services. Available online: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB (accessed on 3 November 2020).
- Rahman, A.; Hasan, M.; Meerburg, F.; Jimenez, J.A.; Miller, M.W.; Bott, C.B.; Al-Omari, A.; Murthy, S.; Shaw, A.; De Clippeleir, H.; et al. Moving forward with A-stage and high-rate contact-stabilization for energy efficient water resource recovery facility: Mechanisms, factors, practical approach, and guidelines. J. Water Process Eng. 2020, 36, 101329. [Google Scholar] [CrossRef]
- Samaras, V.G.; Thomaidis, N.S.; Stasinakis, A.S.; Lekkas, T.D. An analytical method for the simultaneous trace determination of acidic pharmaceuticals and phenolic endocrine disrupting chemicals in wastewater and sewage sludge by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2011, 99, 2549–2561. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for Examination of Waters and Wastewaters, 21st ed.; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Samaras, V.G.; Stasinakis, A.S.; Mamais, D.; Thomaidis, N.S.; Lekkas, T.D. Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J. Hazard. Mater. 2013, 244–245, 259–267. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Thomaidis, N.S.; Arvaniti, O.S.; Asimakopoulos, A.G.; Samaras, V.G.; Ajibola, A.; Mamais, D.; Lekkas, T.D. Contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in a sewage treatment plant. Sci. Total Environ. 2013, 463–464, 1067–1075. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Sci. Total Environ. 2013, 442, 310–316. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Ahkola, H.S.J.; Knuutinen, J.S.; Herve, S.H. Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in central Finland. Environ. Sci. Pollut. Res. Int. 2016, 23, 7985–7997. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Thalla, A.K.; Vannarath, A.S. Occurrence and environmental risks of nonsteroidal anti-inflammatory drugs in urban wastewater in the southwest monsoon region of India. Environ. Monit. Assess. 2020, 192, 193. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, N.K.; Konstantinou, I.K. Occurrence and removal of emerging pharmaceutical, personal care compounds and caffeine tracer in municipal sewage treatment plant in Western Greece. J. Environ. Sci. Health B 2013, 48, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Pothitou, P.; Voutsa, D. Endocrine disrupting compounds in municipal and industrial wastewater treatment plants in Northern Greece. Chemosphere 2008, 73, 1716–1723. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.-H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef]
- Birkett, J.W.; Lester, J.N. Endocrine Disrupters in Wastewater and Sludge Treatment Processes; Lewis Publishers: London, UK, 2003; ISBN 9781566706018. [Google Scholar]
- Nakada, N.; Shinohar, H.; Murata, A.; Kiri, K.; Managaki, S.; Sato, N.; Takada, H. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 2007, 41, 4373–4382. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Gatidou, G.; Mamais, D.; Thomaidis, N.S.; Lekkas, T.D. Occurrence and fate of endocrine disrupters in Greek sewage treatment plants. Water Res. 2008, 42, 1796–1804. [Google Scholar] [CrossRef]
- Nie, Y.; Qiang, Ζ.; Zhang, Η.; Ben, W. Fate and seasonal variation of endocrine-disrupting chemicals in a sewage treatment plant with A/A/O process. Sep. Purif. Technol. 2012, 84, 9–15. [Google Scholar] [CrossRef]
- González, M.M.; Martin, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and risk assessment of nonylphenols and nonylphenol ethoxylates in sewage sludge from different conventional treatment processes. Sci. Total Environ. 2010, 408, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, X.; Sun, W.; Ren, D.; Li, X.; Li, X.; Liu, Y.; Li, Q.; Pan, X. Occurrence, removal, and fate of progestogens, androgens, estrogens, and phenols in six sewage treatment plants around Dianchi Lake in China. Environ. Sci. Pollut. Res. Int. 2014, 21, 12898–12908. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Distribution and temporal evolution of pharmaceutically active compounds alongside sewage sludge treatment. Risk assessment of sludge application onto soils. J. Environ. Manag. 2012, 102, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239–240, 40–47. [Google Scholar] [CrossRef]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Pharmaceutically active compounds in sludge stabilization treatments: Anaerobic and aerobic digestion, wastewater stabilization ponds and composting. Sci. Total Environ. 2015, 503–504, 97–104. [Google Scholar] [CrossRef]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.P. Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Maeng, S.K.; Choi, B.G.; Lee, K.T.; Song, K.G. Influences of solid retention time, nitrification and microbial activity on the attenuation of pharmaceuticals and estrogens in membrane bioreactors. Water Res. 2013, 47, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fontaina, E.; Carballa, M.; Omil, F.; Lema, J.M. Modelling cometabolic biotransformation of organic micropollutants in nitrifying reactors. Water Res. 2014, 65, 371–383. [Google Scholar] [CrossRef]
- Goswami, L.; Kumar, R.V.; Borah, S.N.; Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: A review. J. Water Process Eng. 2018, 26, 314–328. [Google Scholar] [CrossRef]
- Fernandez-Fontaina, E.; Pinho, I.; Carballa, M.; Omil, F.; Lema, J.M. Biodegradation kinetic constants and sorption coefficients of micropollutants in membrane bioreactors. Biodegradation 2013, 24, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Wu, C.; Xiao, K.; Huang, X.; Zhou, H.; Tsuno, H.; Tanaka, H. Elimination and fate of selected micro-organic pollutants in a full-scale anaerobic/anoxic/aerobic process combined with membrane bioreactor for municipal wastewater reclamation. Water Res. 2010, 44, 5999–6010. [Google Scholar] [CrossRef] [PubMed]
- Hyland, K.C.; Dickenson, E.R.V.; Drewes, J.E.; Higgins, C.P. Sorption of ionized and neutral emerging trace organic compounds onto activated sludge from different wastewater treatment configurations. Water Res. 2012, 46, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the sorption and biotransformation of organic micropollutants in innovative biological wastewater treatment technologies. Sci. Total Environ. 2018, 615, 297–306. [Google Scholar] [CrossRef]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387e394. [Google Scholar] [CrossRef]
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A review of the fate of micropollutants in wastewater treatment plants. Compr. Anal. Chem. 2013, 62, 453–557. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebrenda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Collado, N.; Buttiglieri, G.; Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D.; Comas, J.; Rodriguez-Roda, I. Removal of ibuprofen and its transformation products: Experimental and simulation studies. Sci. Total Environ. 2012, 433, 296–301. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Kordoutis, C.I.; Tsiouma, V.C.; Gatidou, G.; Thomaidis, N.S. Removal of selected endocrine disrupters in activated sludge systems: Effect of sludge retention time on their sorption and biodegradation. Bioresour. Technol. 2010, 101, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Koenig, B.G.; Bennie, D.T.; Servos, M.; Ternes, T.A.; Hirsch, R. Occurrence of neutral and acidic drugs in the effluents of Canadian sewage treatment plants. Environ. Toxicol. Chem. 2003, 22, 2872–2880. [Google Scholar] [CrossRef] [PubMed]


| HiCAS | HiCS | |
|---|---|---|
| Influent flowrate (L/day) | 720 | 580 |
| Volume (L) | 15 | 15/22.2 1 |
| Excess sludge (WAS) (L/day) | 48 | 38 |
| Return Activated Sludge (RAS) % | 100 | 150 |
| HRT (h) | 0.5 | 0.625/0.625 1 |
| SRT (days) | 0.25 | 1.1 |
| DO (mgO2/L) | >2 | >2 |
| T (°C) | 20 ± 2 | 20 ± 2 |
| MLSS (g/L) | 2.3 ± 0.24 | 3.2 ± 0.15/4.9 ± 0.35 1 |
| F/M (kgCOD/kgVSS/day) | 14 | 9.5 |
| tCODin (mg/L) | 675 ± 50 | 675 ± 50 |
| TSSeff (mg/L) | 90 ± 10 | 45 ± 8 |
| tCODeff (mg/L) | 220 ± 50 | 70 ± 20 |
| tCOD removal (%) | 68 ± 8 | 90 ± 4 |
| Compound | Influent (ng/L) | HiCAS | HiCS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Effluent (ng/L) | Sludge (ng/gSS) | Removal Efficiency (%) | Effluent (ng/L) | Stabilization Tank (ng/L) | Sludge (ng/gSS) | Removal Efficiency (%) | |||
| EDCs | NP | 10,896 ± 1051 | 7103 ± 1226 | 6297 ± 2000 | 35% ± 9% | 5685 ± 1093 | 21,860 ± 919 | 3293 ± 314 | 48% ± 8% |
| TCS | 886 ± 164 | 473 ± 232 | 373 ± 106 | 47% ± 19% | 381 ± 202 | 2345 ± 221 | 379 ± 35 | 57% ± 18% | |
| BPA | 3578 ± 479 | 1983 ± 856 | 1916 ± 883 | 45% ± 25% | 1347 ± 652 | 7962 ± 1739 | 1223 ± 179 | 62% ± 21% | |
| PhACs | IBF | 5085 ± 1247 | 2869 ± 665 | 607 ± 175 | 44% ± 17% | 2405 ± 512 | 4474 ± 657 | 552 ± 198 | 53% ± 22% |
| NPX | 1572 ± 483 | 735 ± 237 | 811 ± 128 | 53% ± 10% | 743 ± 136 | 4498 ± 432 | 767 ± 97 | 53% ± 20% | |
| DCF | 1920 ± 432 | 1081 ± 223 | 142 ± 91 | 44% ± 14% | 1171 ± 165 | 1890 ± 135 | 149 ± 92 | 39% ± 18% | |
| KFN | 822 ± 336 | 353 ± 61 | <LOD 1 | 57% ± 19% | 396 ± 34 | 385 ± 19 | <LOD | 52% ± 23% | |
| Compound | HiCAS | HiCS | |
|---|---|---|---|
| EDCs | NP | 1081 ± 116 | 669 ± 85 |
| TCS | 1260 ± 843 | 1322 ± 596 | |
| BPA | 1409 ± 813 | 648 ± 115 | |
| PhACs | IBF | 243 ± 97 | 211 ± 106 |
| NPX | 1485 ± 465 | 952 ± 196 | |
| DCF | 152 ± 124 | 124 ± 82 | |
| KFN | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koumaki, E.; Noutsopoulos, C.; Mamais, D.; Fragkiskatos, G.; Andreadakis, A. Fate of Emerging Contaminants in High-Rate Activated Sludge Systems. Int. J. Environ. Res. Public Health 2021, 18, 400. https://doi.org/10.3390/ijerph18020400
Koumaki E, Noutsopoulos C, Mamais D, Fragkiskatos G, Andreadakis A. Fate of Emerging Contaminants in High-Rate Activated Sludge Systems. International Journal of Environmental Research and Public Health. 2021; 18(2):400. https://doi.org/10.3390/ijerph18020400
Chicago/Turabian StyleKoumaki, Elena, Constantinos Noutsopoulos, Daniel Mamais, Gerasimos Fragkiskatos, and Andreas Andreadakis. 2021. "Fate of Emerging Contaminants in High-Rate Activated Sludge Systems" International Journal of Environmental Research and Public Health 18, no. 2: 400. https://doi.org/10.3390/ijerph18020400
APA StyleKoumaki, E., Noutsopoulos, C., Mamais, D., Fragkiskatos, G., & Andreadakis, A. (2021). Fate of Emerging Contaminants in High-Rate Activated Sludge Systems. International Journal of Environmental Research and Public Health, 18(2), 400. https://doi.org/10.3390/ijerph18020400








