Phytoecdysteroids Do Not Have Anabolic Effects in Skeletal Muscle in Sedentary Aging Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Crude Extraction
2.2. Animals
2.2.1. Chronic Treatment
2.2.2. Acute Treatment
2.3. Myosin Heavy Chain Fiber Typing Immunofluorescence Analysis
2.4. Western Blotting
2.5. RNA Isolation and Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Body, Muscle, and Organ Mass
3.2. Muscle Fiber Cross-Sectional Area and Fiber Type
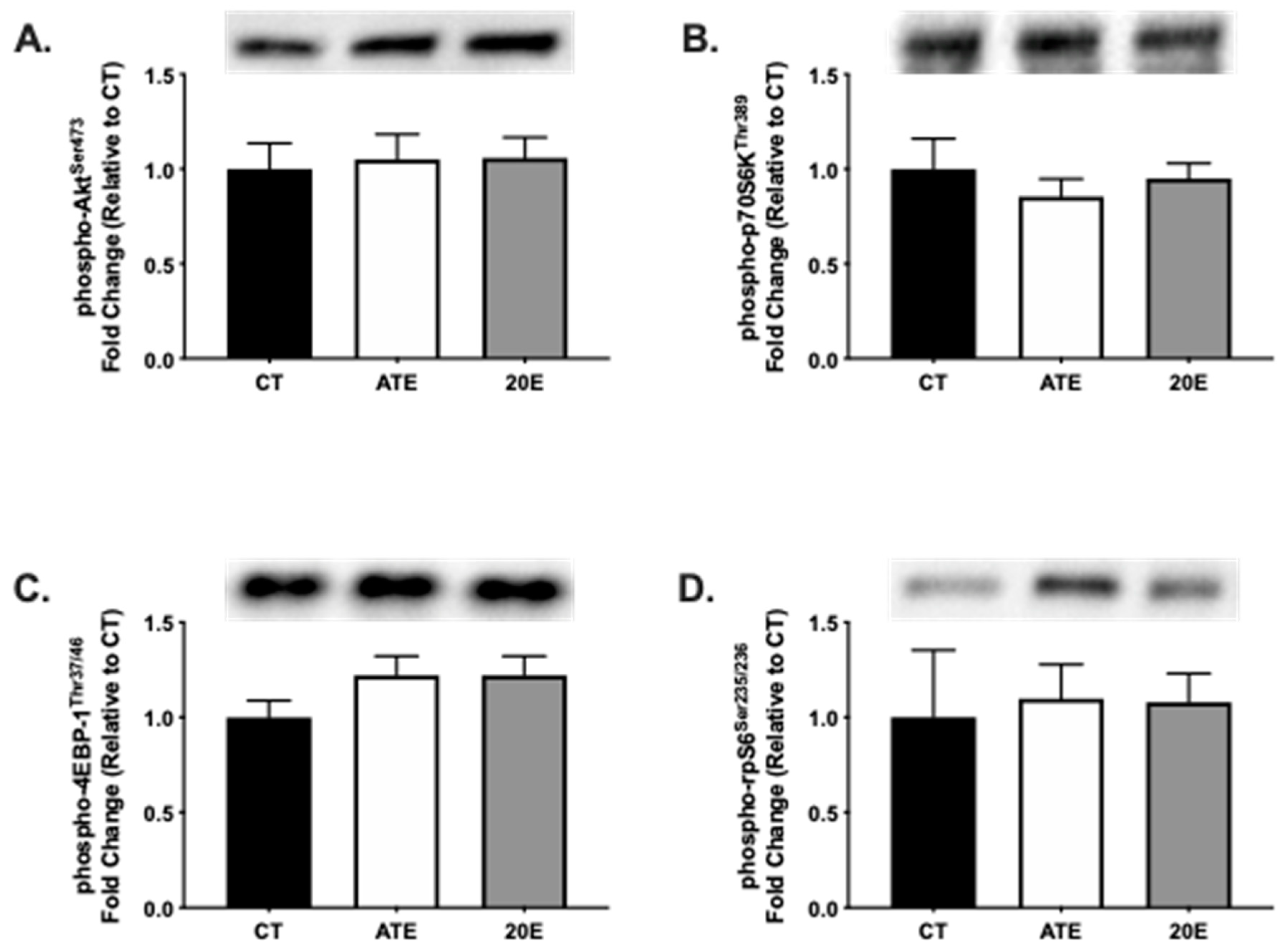
3.3. Protein Synthesis Signaling Pathway
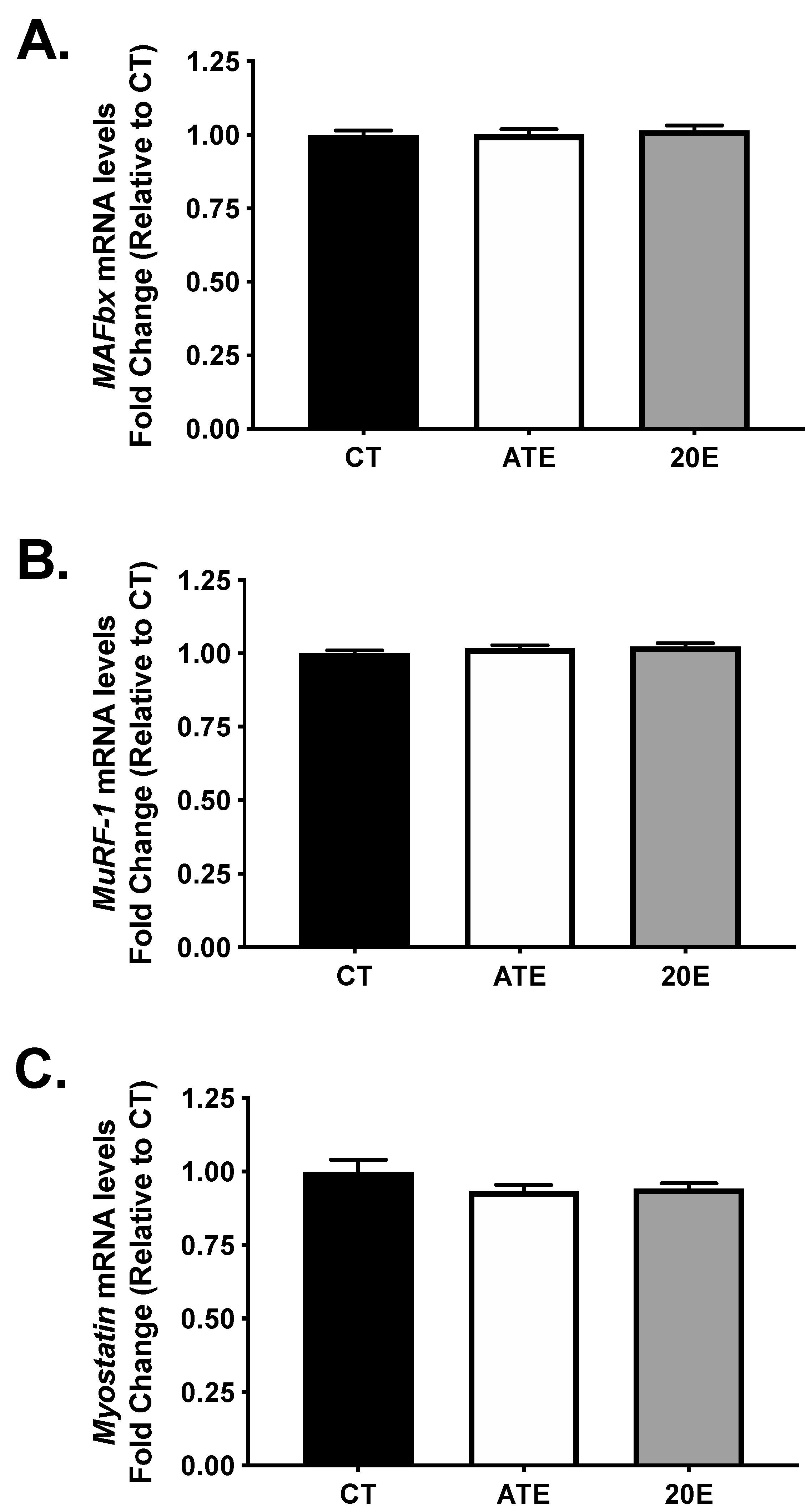
3.4. Atrogene and Myostatin mRNA Levels
4. Discussion
4.1. Phytoecdysteroids in Aging Muscle
4.2. Method for Dietary Phytoecdysteroid Supplementation
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Talbot, L.A.; Morrell, C.H.; Fleg, J.L.; Metter, E.J. Changes in leisure time physical activity and risk of all-cause mortality in men and women, the Baltimore Longitudinal Study of Aging. Prev. Med. 2007, 45, 169–176. [Google Scholar] [CrossRef]
- Phillips, S.M.; Glover, E.I.; Rennie, M.J. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J. Appl. Physiol. 2009, 107, 645–654. [Google Scholar] [CrossRef]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet. Muscle 2011, 1, 11. [Google Scholar] [CrossRef]
- Fry, C.S.; Rasmussen, B.B. Skeletal Muscle Protein Balance and Metabolism in the Elderly. Curr. Aging Sci. 2011, 4, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Amirouche, A.; Durieux, A.C.; Banzet, S.; Koulmann, N.; Bonnefoy, R.; Mouret, C.; Bigard, X.; Peinnequin, A.; Freyssenet, D. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 2009, 150, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.C.; Hadj Sassi, A.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Leger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008, 11, 163B–175B. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, P.A.; Coyne, E.S.; Wing, S.S. The ubiquitin proteasome system in atrophying skeletal muscle, roles and regulation. Am. J. Physiol. Cell Physiol. 2016, 311, C392–C403. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Edward, F. Adolph Distinguished Lecture. Skeletal muscle atrophy, Multiple pathways leading to a common outcome. J. Appl. Physiol. 2020, 129, 272–282. [Google Scholar] [CrossRef]
- Clavel, S.; Coldefy, A.S.; Kurkdjian, E.; Salles, J.; Margaritis, I.; Derijard, B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech. Ageing Dev. 2006, 127, 794–801. [Google Scholar] [CrossRef]
- Arden, K.C. FoxO, linking new signaling pathways. Mol. Cell. 2004, 14, 416–418. [Google Scholar] [CrossRef]
- Wang, X.; Hu, S.; Liu, L. Phosphorylation and acetylation modifications of FOXO3a, Independently or synergistically? Oncol. Lett. 2017, 13, 2867–2872. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Murphy, K.T.; Koopman, R.; Naim, T.; Leger, B.; Trieu, J.; Ibebunjo, C.; Lynch, G.S. Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. FASEB J. 2010, 24, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Yousef, G.G.; Grace, M.H.; Rogers, R.B.; Gorelick-Feldman, J.; Raskin, I.; Lila, M.A. In vitro production of metabolism-enhancing phytoecdysteroids from Ajuga turkestanica. Plant Cell Tiss Organ Cult. 2008, 93, 73–83. [Google Scholar] [CrossRef]
- Dinan, L. Phytoecdysteroids, biological aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Bathori, M.; Toth, N.; Hunyadi, A.; Marki, A.; Zador, E. Phytoecdysteroids and anabolic-androgenic steroids—structure and effects on humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Israili, Z.H.; Lyoussi, B. Ethnopharmacology of the plants of genus Ajuga. Pak. J. Pharm. Sci. 2009, 22, 425–462. [Google Scholar] [PubMed]
- Gorelick-Feldman, J.; MacLean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids Increase Protein Synthesis in Skeletal Muscle Cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef]
- Zubeldia, J.; Santana, A.; Jimenez-del-Rio, M.; Lopez, V.; Machin, R.; Castellanos, J. In vitro characterization of the efficacy and safety profile of a proprietary Ajuga turkestanica extract. Chin. Med. 2012, 3, 215–222. [Google Scholar] [CrossRef]
- Cheng, D.M.; Kutzler, L.W.; Boler, D.D.; Drnevich, J.; Killefer, J.; Lila, M.A. Continuous infusion of 20-hydroxyecdysone increased mass of triceps brachii in C57BL/6 mice. Phytother. Res. 2013, 27, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G. The Mouse in Biomedical Research, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2007. [Google Scholar]
- Lexell, J.; Taylor, C.C.; Sjostrom, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; Van Kranenburg, J.; Verdijk, L.B.; Van Loon, L.J.C. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R.; Gaertner, J.R.; OReilly, S. The effects of sarcopenia on muscles with different recruitment patterns and myofiber profiles. Curr. Aging Sci. 2013, 6, 266–272. [Google Scholar] [CrossRef]
- Sayed, R.K.; De Leonardis, E.C.; Guerrero-Martinez, J.A.; Rahim, I.; Mokhtar, D.M.; Saleh, A.M.; Abdalla, K.E.H.; Pozo, M.J.; Escames, G.; Lopez, L.C.; et al. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Exp. Gerontol. 2016, 83, 22–30. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Elashry, M.I.; Eldaey, A.; Glenske, K.; Matsakas, A.; Wenisch, S.; Arnhold, S.; Patel, K. The effect of high-fat diet on the morphological properties of the forelimb musculature in hypertrophic myostatin null mice. J. Anat. 2019, 235, 825–835. [Google Scholar] [CrossRef]
- Ballak, S.B.; Degens, H.; Buse-Pot, T.; De Haan, A.; Jaspers, R.T. Plantaris muscle weakness in old mice, relative contributions of changes in specific force, muscle mass, myofiber cross-sectional area, and number. Age 2014, 36, 9726. [Google Scholar] [CrossRef][Green Version]
- Smith, L.R.; Barton, E.R. SMASH—semi-automatic muscle analysis using segmentation of histology, a MATLAB application. Skelet. Muscle 2014, 4, 21. [Google Scholar] [CrossRef]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, T.A.; Mateja, R.D.; Chin, E.R.; Andrews, J.L.; Esser, K.A. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J. Appl. Physiol. 2005, 98, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Gorelick-Feldman, J.; Cohick, W.; Raskin, I. Ecdysteroids elicit a rapid Ca2+ flux leading to Akt activation and increased protein synthesis in skeletal muscle cells. Steroids 2010, 75, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, N.S. Phytoecdysteroids and other biologically active compounds from plants of the genus Ajuga. Chem. Nat. Comp. 2005, 41, 361–369. [Google Scholar] [CrossRef]
- Toth, N.; Szabo, A.; Kacsala, P.; Heger, J.; Zador, E. 20-Hydroxyecdysone increases fiber size in a muscle-specific fashion in rat. Phytomedicine. Int. J. Phytother. Phytopharmacol. 2008, 15, 691–698. [Google Scholar]
- Gao, L.; Cai, G.; Shi, X. Beta-ecdysterone induces osteogenic differentiation in mouse mesenchymal stem cells and relieves osteoporosis. Biol. Pharm. Bull. 2008, 31, 2245–2249. [Google Scholar] [CrossRef]
- Kumpun, S.; Girault, J.P.; Dinan, L.; Blais, C.; Maria, A.; Dauphin-Villemant, C.; Yingyongnarongkul, B.; Suksamrarn, A.; Lafont, R. The metabolism of 20-hydroxyecdysone in mice, relevance to pharmacological effects and gene switch applications of ecdysteroids. J. Steroid Biochem. Mol. Biol. 2011, 126, 1–9. [Google Scholar] [CrossRef]
- Ramazanov, N.S.; Saatov, Z.; Syrov, B.N. Study of ecdysterone metabolites isolated from rat urine. Chem. Nat. Compd. 1996, 32, 545–549. [Google Scholar] [CrossRef]
- Csabi, J.; Rafai, T.; Hunyadi, A.; Zador, E. Poststerone increases muscle fibre size partly similar to its metabolically parent compound, 20-hydroxyecdysone. Fitoterapia 2019, 134, 459–464. [Google Scholar] [CrossRef]
- Norton, L.E.; Layman, D.K.; Bunpo, P.; Anthony, T.G.; Brana, D.V.; Garlick, P.J. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J. Nutr. 2009, 139, 1103–1109. [Google Scholar] [CrossRef]
- Dzhukharova MKh Sakhibov, A.D.; Kasymov, B.; Syrov, V.N.; Takanaev, A.A.; Saatov, Z. Pharmacokinetics of Ecdysterone in Experiments. Khimiko Farmatsevticheskii Zhurnal 1987, 21, 1163–1167. [Google Scholar] [CrossRef]
- Anthony, T.G.; Mirek, E.T.; Bargoud, A.R.; Phillipson-Weiner, L.; DeOliveira, C.M.; Wetstein, B.; Graf, B.L.; Kuhn, P.E.; Raskin, I. Evaluating the effect of 20-hydroxyecdysone (20HE) on mechanistic target of rapamycin complex 1 (mTORC1) signaling in the skeletal muscle and liver of rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Body Mass D1 (g) | Body Mass D28 (g) | p-Value |
|---|---|---|---|
| CT | 32.7 ± 1.0 | 32.4 ± 1.1 | 0.44 |
| ATE | 33.0 ± 0.9 | 32.8 ± 0.7 | 0.40 |
| 20E | 33.4 ± 0.4 | 32.5 ± 0.6 | 0.12 |
| Muscle (mg/g BM) | CT | ATE | 20E | p-Value |
|---|---|---|---|---|
| Soleus | 0.27 ± 0.00 | 0.27 ± 0.01 | 0.28 ± 0.00 | 0.36 |
| Plantaris | 0.62 ± 0.01 | 0.60 ± 0.01 | 0.65 ± 0.02 | 0.27 |
| Gastrocnemius | 4.13 ± 0.13 | 3.96 ± 0.11 | 4.07 ± 0.11 | 0.60 |
| Tibialis Anterior | 1.74 ± 0.08 | 1.69 ± 0.03 | 1.74 ± 0.03 | 0.72 |
| Extensor Digitorum Longus | 0.39 ± 0.02 | 0.36 ± 0.01 | 0.36 ± 0.10 | 0.50 |
| Organ (mg/g BM) | CT | ATE | 20E | p-Value |
|---|---|---|---|---|
| Heart | 5.0 ± 0.2 | 4.8 ± 0.2 | 4.9 ± 0.1 | 0.66 |
| Liver | 46.4 ± 3.6 | 40.4 ± 0.9 | 41.6 ± 1.2 | 0.16 |
| Spleen | 3.8 ± 1.4 | 2.4 ± 0.2 | 2.6 ± 0.1 | 0.47 |
| Kidneys | 13.6 ± 0.4 | 14.1 ± 0.5 | 14.5 ± 0.5 | 0.44 |
| Testes | 5.7 ± 0.2 | 5.5 ± 0.1 | 5.4 ± 0.1 | 0.57 |
| Plantaris | Triceps | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT | ATE | 20E | p-Value | CT | ATE | 20E | p-Value | ||
| Fiber CSA (μm) | IIa | 1000 ± 77 | 928 ± 56 | 1163 ± 149 | 0.720 | 926 ± 62 | 832 ± 46 | 837 ± 31 | 0.711 |
| IIx | 1840 ± 126 | 1875 ± 88 | 1751 ± 137 | 1416 ± 76 | 1305 ± 74 | 1387 ± 68 | |||
| IIb | 2407 ± 149 | 2482 ± 29 | 2365 ± 208 | 2506 ± 84 | 2554 ± 88 | 2572 ± 77 | |||
| Min. Feret Dia. | IIa | 30.2 ± 1.17 | 29.4 ± 0.96 | 32.1 ± 2.07 | 0.656 | 28.9 ± 0.94 | 27.7 ± 0.74 | 28.1 ± 0.52 | 0.848 |
| IIx | 41.1 ± 1.50 | 42.2 ± 1.02 | 40.1 ± 1.71 | 36.2 ± 0.94 | 35.1 ± 1.02 | 36.2 ± 0.81 | |||
| IIb | 48.0 ± 1.56 | 48.9 ± 0.30 | 46.8 ± 2.42 | 49.0 ± 0.96 | 49.4 ± 0.87 | 49.5 ± 0.68 | |||
| Fiber Type (%) | IIa | 32.2 ± 1.99 | 32.3 ± 2.87 | 27.9 ± 3.88 | 0.507 | 8.66 ± 1.29 | 10.67 ± 4.21 | 7.84 ± 1.1 | 0.787 |
| IIx | 20.7 ± 1.68 | 18.7 ± 2.55 | 23.9 ± 1.93 | 14.2 ± 2.10 | 17.7 ± 5.48 | 15.5 ± 2.97 | |||
| IIb | 47.2 ± 1.51 | 49.0 ± 1.76 | 48.1 ± 1.51 | 77.1 ± 2.22 | 71.6 ± 9.57 | 76.7 ± 3.70 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, M.M.; Zwetsloot, K.A.; Arthur, S.T.; Sherman, C.A.; Huot, J.R.; Badmaev, V.; Grace, M.; Lila, M.A.; Nieman, D.C.; Shanely, R.A. Phytoecdysteroids Do Not Have Anabolic Effects in Skeletal Muscle in Sedentary Aging Mice. Int. J. Environ. Res. Public Health 2021, 18, 370. https://doi.org/10.3390/ijerph18020370
Lawrence MM, Zwetsloot KA, Arthur ST, Sherman CA, Huot JR, Badmaev V, Grace M, Lila MA, Nieman DC, Shanely RA. Phytoecdysteroids Do Not Have Anabolic Effects in Skeletal Muscle in Sedentary Aging Mice. International Journal of Environmental Research and Public Health. 2021; 18(2):370. https://doi.org/10.3390/ijerph18020370
Chicago/Turabian StyleLawrence, Marcus M., Kevin A. Zwetsloot, Susan T. Arthur, Chase A. Sherman, Joshua R. Huot, Vladimir Badmaev, Mary Grace, Mary Ann Lila, David C. Nieman, and R. Andrew Shanely. 2021. "Phytoecdysteroids Do Not Have Anabolic Effects in Skeletal Muscle in Sedentary Aging Mice" International Journal of Environmental Research and Public Health 18, no. 2: 370. https://doi.org/10.3390/ijerph18020370
APA StyleLawrence, M. M., Zwetsloot, K. A., Arthur, S. T., Sherman, C. A., Huot, J. R., Badmaev, V., Grace, M., Lila, M. A., Nieman, D. C., & Shanely, R. A. (2021). Phytoecdysteroids Do Not Have Anabolic Effects in Skeletal Muscle in Sedentary Aging Mice. International Journal of Environmental Research and Public Health, 18(2), 370. https://doi.org/10.3390/ijerph18020370








