Secondary Dentin Formation Mechanism: The Effect of Attrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
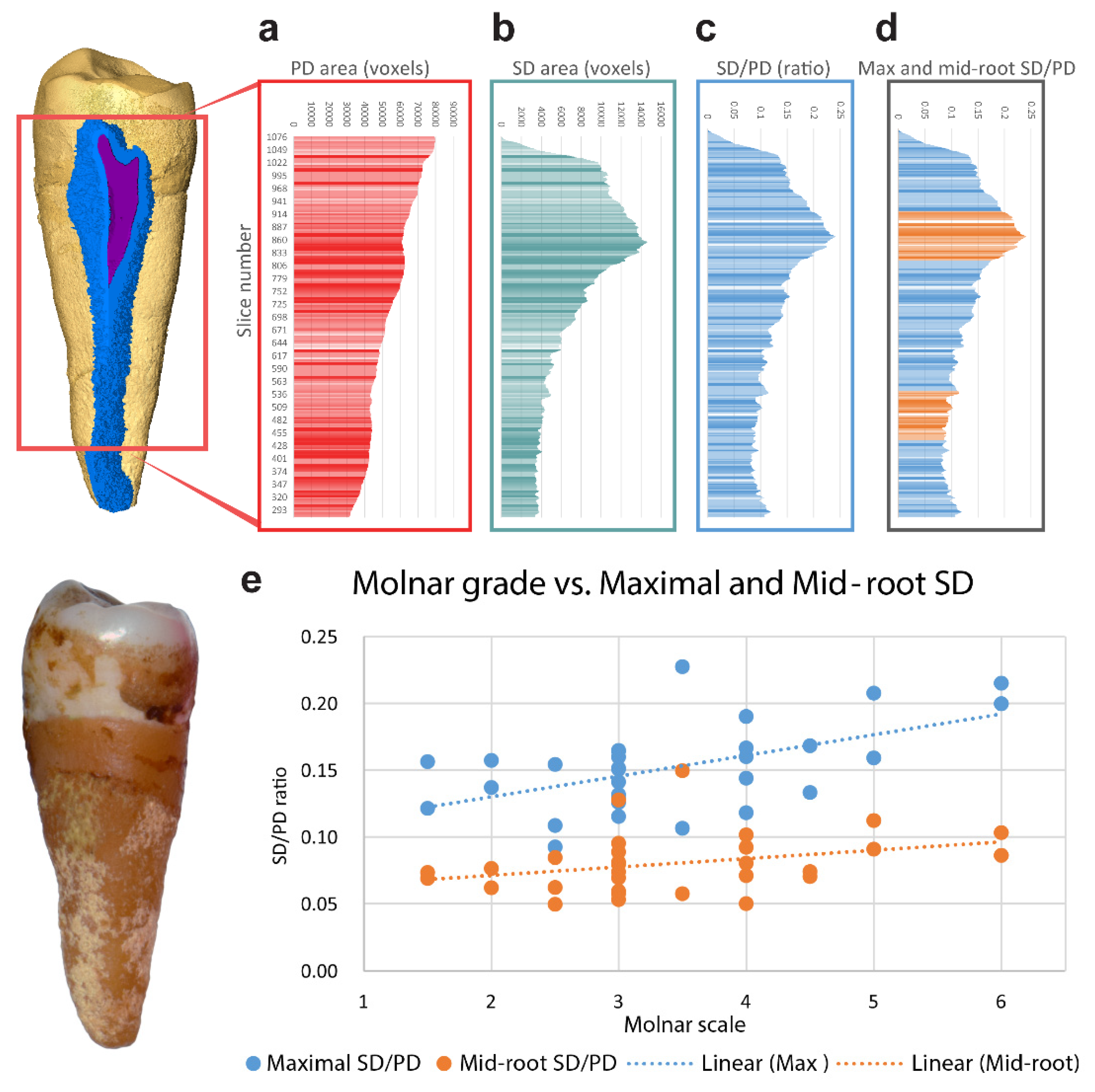
2.2.1. Micro CT Scan
2.2.2. Micro CT Analysis
2.2.3. SD Analysis
2.2.4. Attrition
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solheim, T. Amount of secondary dentin as an indicator of age. Eur. J. Oral Sci. 1992, 100, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kvaal, S.I.; Kolltveit, K.M.; Thomsen, I.O.; Solheim, T. Age estimation of adults from dental radiographs. Forensic Sci. Int. 1995, 74, 175–185. [Google Scholar] [CrossRef]
- Philippas, G.G.; Applebaum, E. Age Factor in Secondary Dentin Formation. J. Dent. Res. 1966, 45, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Arana-Chavez, V.E.; Massa, L.F. Odontoblasts: The cells forming and maintaining dentine. Int. J. Biochem. Cell Biol. 2004, 36, 1367–1373. [Google Scholar] [CrossRef]
- Burke, F.M.; Samarawickrama, D.Y.D. Progressive changes in the pulpo-dentinal complex and their clinical consequences. Gerodontology 1995, 12, 57–66. [Google Scholar] [CrossRef]
- Burstone, M.S. The ground substance of abnormal dentin, secondary dentin, and pulp calcifications. J. Dent. Res. 1953, 32, 269–279. [Google Scholar] [CrossRef]
- Oi, T.; Saka, H.; Ide, Y. Three-dimensional observation of pulp cavities in the maxillary first premolar tooth using micro-CT. Int. Endod. J. 2004, 37, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Star, H.; Thevissen, P.; Jacobs, R.; Fieuws, S.; Solheim, T.; Willems, G. Human dental age estimation by calculation of pulp-tooth volume ratios yielded on clinically acquired cone beam computed tomography images of monoradicular teeth. J. Forensic Sci. 2011, 56, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Molnar, S. Human tooth wear, tooth function and cultural variability. Am. J. Phys. Anthropol. 1971, 34, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.G.; Knight, J.K. An index for measuring the wear of teeth. Br. Dent. J. 1984, 156, 435–438. [Google Scholar] [CrossRef]
- Sarig, R.; Hershkovitz, I.; Shvalb, N.; Sella-Tunis, T.; May, H.; Vardimon, A.D. Proximal attrition facets: Morphometric, demographic, and aging characteristics. Eur. J. Oral Sci. 2014, 122, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sarig, R.; Hershkovitz, I.; Shpack, N.; May, H.; Vardimon, A.D. Rate and pattern of interproximal dental attrition. Eur. J. Oral Sci. 2015, 123, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Senawongse, P.; Otsuki, M.; Tagami, J.; Mjör, I. Age-related changes in hardness and modulus of elasticity of dentine. Arch. Oral Biol. 2006, 51, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tagami, J.; Hosoda, H.; Burrow, M.F.; Nakajima, M. Effect of aging and caries on dentin permeability. Proc. Finn. Dent. Soc. 1992, 88, 149–154. [Google Scholar]
- Stanley, H.R.; Pereira, J.C.; Spiegel, E.; Broom, C.; Schultz, M. The detection and prevalence of reactive and physiologic sclerotic dentin, reparative dentin and dead tracts beneath various types of dental lesions according to tooth surface and age. J. Oral Pathol. Med. 1983, 12, 257–289. [Google Scholar] [CrossRef]
- Tronstad, L.; Langeland, K. Effect of attrition on subjacent dentin and pulp. J. Dent. Res. 1971, 50, 17–30. [Google Scholar] [CrossRef]
- Marroquin, T.Y.; Karkhanis, S.; Kvaal, S.I.; Vasudavan, S.; Kruger, E.; Tennant, M. Age estimation in adults by dental imaging assessment systematic review. Forensic Sci. Int. 2017, 275, 203–211. [Google Scholar] [CrossRef]
- Cameriere, R.; Ferrante, L.; Cingolani, M. Variations in pulp/tooth area ratio as an indicator of age: A preliminary study. J. Forensic Sci. 2004, 49, 1–3. [Google Scholar] [CrossRef]
- Nudel, I.; Pokhojaev, A.; Hausman, B.S.; Bitterman, Y.; Shpack, N.; May, H.; Sarig, R. Age estimation of fragmented human dental remains by secondary dentin virtual analysis. Int. J. Legal Med. 2020, 134, 1853–1860. [Google Scholar] [CrossRef]
- Benazzi, S.; Fornai, C.; Buti, L.; Toussaint, M.; Mallegni, F.; Ricci, S.; Gruppioni, G.; Weber, G.W.; Condemi, S.; Ronchitelli, A. Cervical and crown outline analysis of worn Neanderthal and modern human lower second deciduous molars. Am. J. Phys. Anthropol. 2012, 149, 537–546. [Google Scholar] [CrossRef]
- Grippo, J.O.; Simring, M.S.; Schreiner, S.S. Attrition, abrasion, corrosion and abfraction revisited: A new perspective on tooth surface lesions. J. Am. Dent. Assoc. 2004, 135, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Cicchetti, D.V. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Penaloza, T.Y.M.; Karkhanis, S.; Kvaal, S.I.; Nurul, F.; Kanagasingam, S.; Franklin, D.; Vasudavan, S.; Kruger, E.; Tennant, M. Application of the Kvaal method for adult dental age estimation using Cone Beam Computed Tomography (CBCT). J. Forensic Leg. Med. 2016, 44, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Naito, T.; Yokota, M.; Kohno, M. Finite element analysis of the possible mechanism of cervical lesion formation by occlusal force. J. Oral Rehabil. 2003, 30, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Benazzi, S.; Kullmer, O.; Grosse, I.R.; Weber, G.W. Using occlusal wear information and finite element analysis to investigate stress distributions in human molars. J. Anat. 2011, 219, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Benazzi, S.; Grosse, I.R.; Gruppioni, G.; Weber, G.W.; Kullmer, O. Comparison of occlusal loading conditions in a lower second premolar using three-dimensional finite element analysis. Clin. Oral Investig. 2014, 18, 369–375. [Google Scholar] [CrossRef]
- Lee, W.; Alom, G.; Kim, M.S.; Park, Y.S.; Lim, Y.J.; Kim, M.J.; Kwon, H.B. Comparison of the outcomes of three-dimensional finite element analysis under arbitrary and realistic occlusal loading conditions in mandibular posterior region. J. Dent. Rehabil. Appl. Sci. 2020, 36, 112–120. [Google Scholar] [CrossRef]
- Soares, P.V.; Souza, L.V.; Veríssimo, C.; Zeola, L.F.; Pereira, A.G.; Santos-Filho, P.C.F.; Fernandes-Neto, A.J. Effect of root morphology on biomechanical behaviour of premolars associated with abfraction lesions and different loading types. J. Oral Rehabil. 2014, 41, 108–114. [Google Scholar] [CrossRef]
- Benazzi, S.; Nguyen, H.N.; Schulz, D.; Grosse, I.R.; Gruppioni, G.; Hublin, J.J.; Kullmer, O. The Evolutionary Paradox of Tooth Wear: Simply Destruction or Inevitable Adaptation? PLoS ONE 2013, 8, e62263. [Google Scholar]
- Zeola, L.F.; Pereira, F.A.; Machado, A.C.; Reis, B.R.; Kaidonis, J.; Xie, Z.; Townsend, G.C.; Ranjitkar, S.; Soares, P.V. Effects of non-carious cervical lesion size, occlusal loading and restoration on biomechanical behaviour of premolar teeth. Aust. Dent. J. 2016, 61, 408–417. [Google Scholar] [CrossRef] [Green Version]
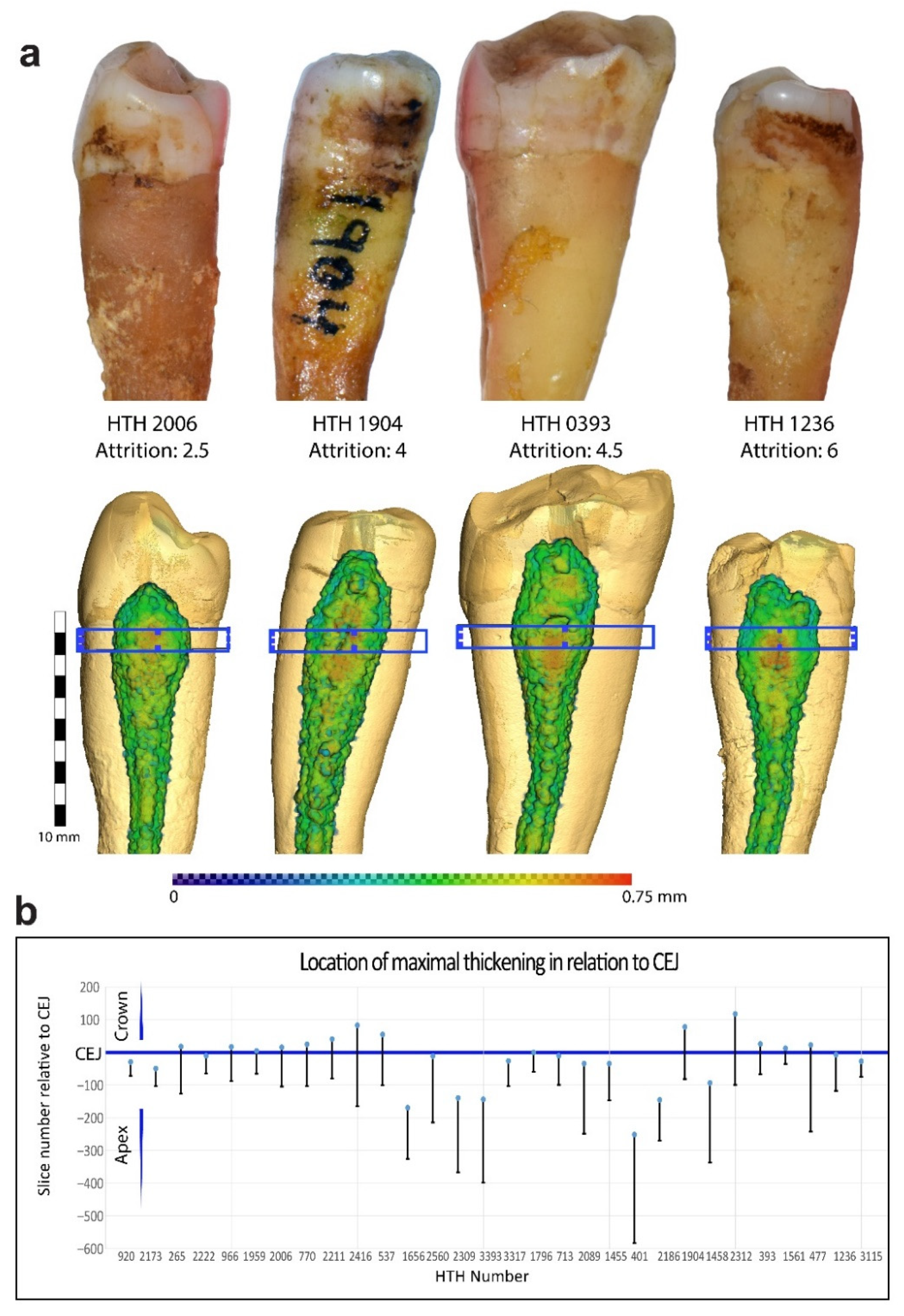
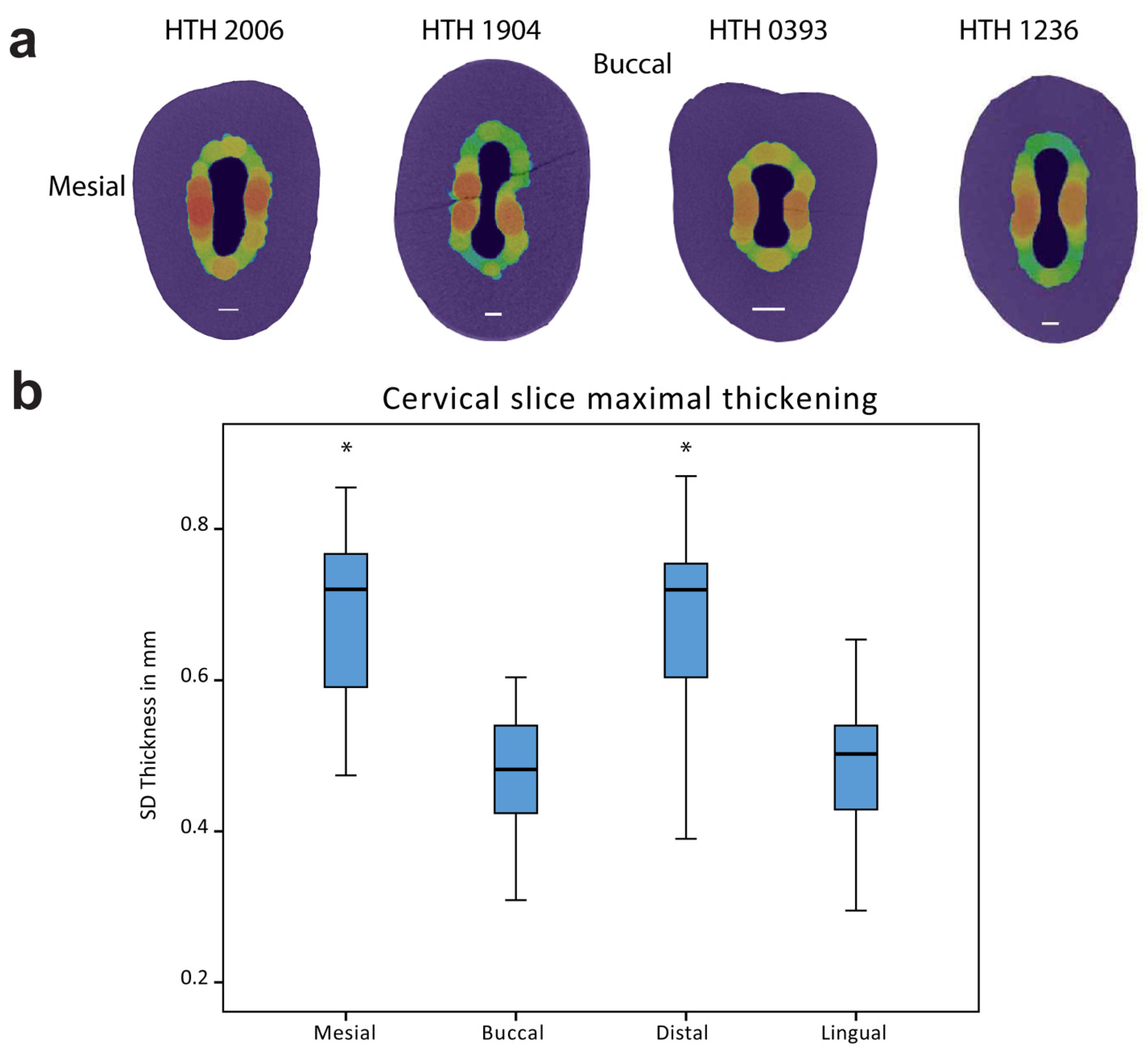
| HTH | Age | Attrition | Tooth No. | Mid-Root SD/PD | Maximal SD/PD | Mesial | Buccal | Distal | Lingual |
|---|---|---|---|---|---|---|---|---|---|
| HTH 0920 | 60 | 1.5 | 45 | 0.07 | 0.16 | 0.59 | 0.42 | 0.60 | 0.44 |
| HTH 2173 | 60 | 1.5 | 45 | 0.07 | 0.12 | 0.53 | 0.32 | 0.39 | 0.30 |
| HTH 0265 | 50 | 2 | 35 | 0.08 | 0.14 | 0.73 | 0.49 | 0.74 | 0.47 |
| HTH 2222 | 54 | 2 | 35 | 0.06 | 0.16 | 0.69 | 0.55 | 0.66 | 0.43 |
| HTH 0966 | 50 | 2.5 | 35 | 0.08 | 0.15 | 0.79 | 0.59 | 0.79 | 0.54 |
| HTH 1959 | 50 | 2.5 | 35 | 0.06 | 0.09 | 0.77 | 0.60 | 0.70 | 0.54 |
| HTH 2006 | 59 | 2.5 | 35 | 0.05 | 0.11 | 0.69 | 0.51 | 0.74 | 0.61 |
| HTH 0770 | 50 | 3 | 35 | 0.05 | 0.13 | 0.83 | 0.59 | 0.75 | 0.64 |
| HTH 2211 | 50 | 3 | 45 | 0.08 | 0.14 | 0.47 | 0.31 | 0.49 | 0.38 |
| HTH 2416 | 53 | 3 | 45 | 0.06 | 0.12 | 0.71 | 0.46 | 0.58 | 0.53 |
| HTH 0537 | 55 | 3 | 45 | 0.07 | 0.16 | 0.75 | 0.59 | 0.81 | 0.64 |
| HTH 1656 | 50 | 3 | 35 | 0.13 | 0.15 | 0.58 | 0.41 | 0.58 | 0.51 |
| HTH 2560 | 52 | 3 | 34 | 0.06 | 0.13 | 0.63 | 0.51 | 0.65 | 0.40 |
| HTH 2309 | 54 | 3 | 35 | 0.07 | 0.13 | 0.57 | 0.44 | 0.60 | 0.40 |
| HTH 3393 | 54 | 3 | 45 | 0.09 | 0.15 | 0.74 | 0.46 | 0.69 | 0.50 |
| HTH 3317 | 58 | 3 | 35 | 0.08 | 0.16 | 0.67 | 0.52 | 0.74 | 0.51 |
| HTH 1796 | 60 | 3 | 45 | 0.10 | 0.16 | 0.58 | 0.42 | 0.60 | 0.43 |
| HTH 0713 | 59 | 3.5 | 35 | 0.15 | 0.23 | 0.56 | 0.44 | 0.60 | 0.43 |
| HTH 2089 | 59 | 3.5 | 45 | 0.06 | 0.11 | 0.77 | 0.58 | 0.75 | 0.53 |
| HTH 1455 | 53 | 4 | 45 | 0.05 | 0.19 | 0.73 | 0.49 | 0.82 | 0.57 |
| HTH 0401 | 54 | 4 | 34 | 0.07 | 0.12 | 0.63 | 0.52 | 0.63 | 0.41 |
| HTH 2186 | 54 | 4 | 35 | 0.08 | 0.17 | 0.78 | 0.54 | 0.81 | 0.54 |
| HTH 1904 | 55 | 4 | 35 | 0.10 | 0.16 | 0.74 | 0.46 | 0.72 | 0.48 |
| HTH 1458 | 56 | 4 | 35 | 0.09 | 0.14 | 0.86 | 0.47 | 0.87 | 0.50 |
| HTH 2312 | 53 | 4.5 | 35 | 0.07 | 0.17 | 0.82 | 0.57 | 0.80 | 0.57 |
| HTH 0393 | 56 | 4.5 | 45 | 0.07 | 0.13 | 0.75 | 0.51 | 0.75 | 0.65 |
| HTH 1561 | 52 | 5 | 35 | 0.11 | 0.21 | 0.67 | 0.38 | 0.55 | 0.34 |
| HTH 0477 | 55 | 5 | 45 | 0.09 | 0.16 | 0.81 | 0.43 | 0.75 | 0.54 |
| HTH 1236 | 55 | 6 | 35 | 0.09 | 0.22 | 0.74 | 0.36 | 0.72 | 0.46 |
| HTH 3115 | 57 | 6 | 35 | 0.10 | 0.20 | 0.59 | 0.41 | 0.82 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nudel, I.; Pokhojaev, A.; Bitterman, Y.; Shpack, N.; Fiorenza, L.; Benazzi, S.; Sarig, R. Secondary Dentin Formation Mechanism: The Effect of Attrition. Int. J. Environ. Res. Public Health 2021, 18, 9961. https://doi.org/10.3390/ijerph18199961
Nudel I, Pokhojaev A, Bitterman Y, Shpack N, Fiorenza L, Benazzi S, Sarig R. Secondary Dentin Formation Mechanism: The Effect of Attrition. International Journal of Environmental Research and Public Health. 2021; 18(19):9961. https://doi.org/10.3390/ijerph18199961
Chicago/Turabian StyleNudel, Itay, Ariel Pokhojaev, Yoli Bitterman, Nir Shpack, Luca Fiorenza, Stefano Benazzi, and Rachel Sarig. 2021. "Secondary Dentin Formation Mechanism: The Effect of Attrition" International Journal of Environmental Research and Public Health 18, no. 19: 9961. https://doi.org/10.3390/ijerph18199961
APA StyleNudel, I., Pokhojaev, A., Bitterman, Y., Shpack, N., Fiorenza, L., Benazzi, S., & Sarig, R. (2021). Secondary Dentin Formation Mechanism: The Effect of Attrition. International Journal of Environmental Research and Public Health, 18(19), 9961. https://doi.org/10.3390/ijerph18199961






