Increased Risk of Neurodegenerative Dementia after Benign Paroxysmal Positional Vertigo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Population and Data Collection
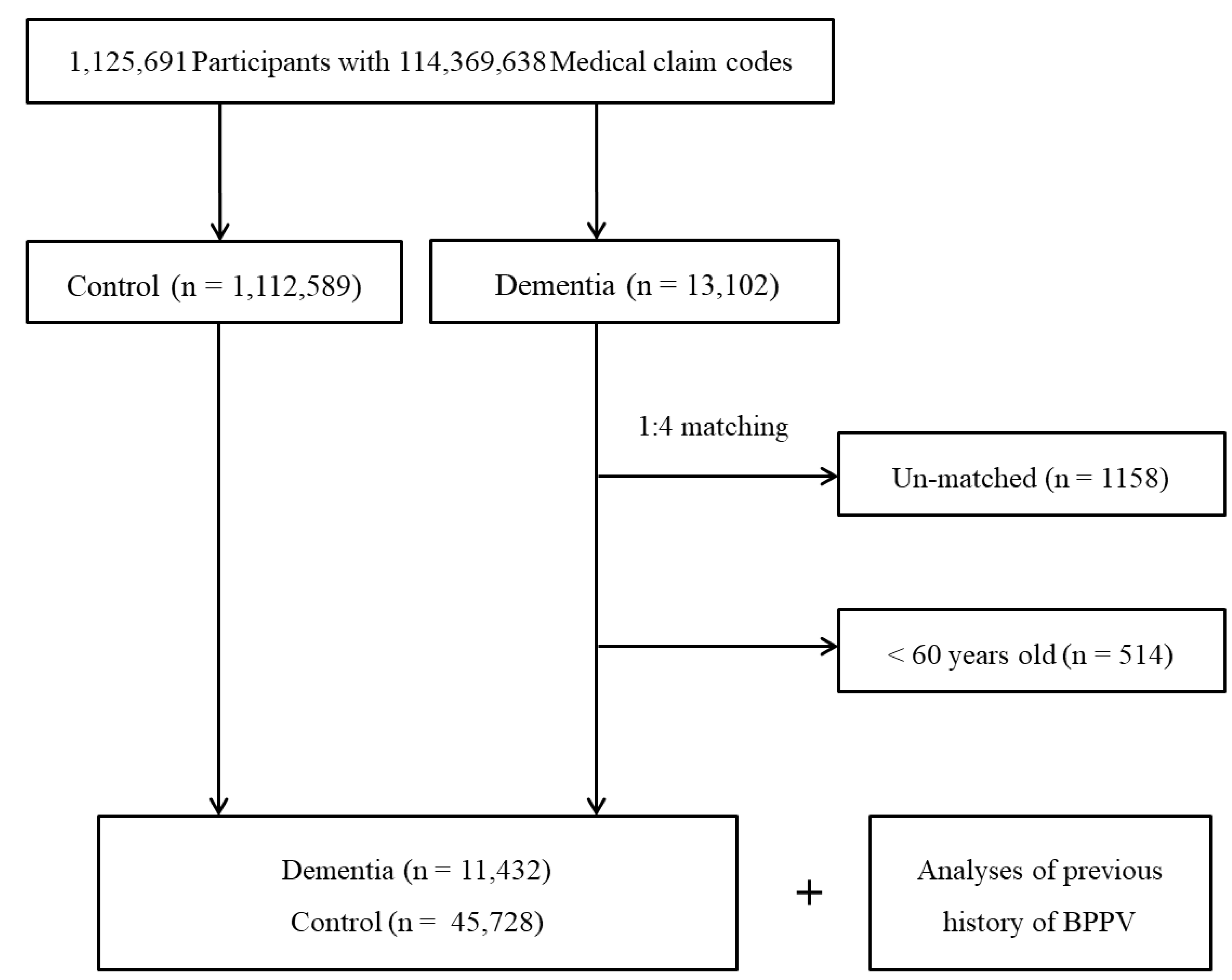
2.3. Participant Selection
2.4. Variables
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harun, A.; Oh, E.S.; Bigelow, R.T.; Studenski, S.; Agrawal, Y. Vestibular Impairment in Dementia. Otol. Neurotol. 2016, 37, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-W.; Lim, Y.-H.; Kim, S.-H. Dizziness in patients with cognitive impairment. J. Vestib. Res. 2020, 30, 17–23. [Google Scholar] [CrossRef]
- Smith, P.F. Why dizziness is likely to increase the risk of cognitive dysfunction and dementia in elderly adults. N. Z. Med. J. 2020, 133, 112–127. [Google Scholar]
- Liu, P.; Zheng, Y.; King, J.; Darlington, C.; Smith, P. Long-term changes in hippocampal n-methyl-d-aspartate receptor subunits following unilateral vestibular damage in rat. Neuroscience 2003, 117, 965–970. [Google Scholar] [CrossRef]
- Brandt, T.; Schautzer, F.; Hamilton, D.A.; Brüning, R.; Markowitsch, H.J.; Kalla, R.; Darlington, C.; Smith, P.; Strupp, M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 2005, 128, 2732–2741. [Google Scholar] [CrossRef]
- Batuecas-Caletrío, Á.; Trinidad-Ruiz, G.; Zschaeck, C.; del Pozo de Dios, J.C.; Gil, L.D.T.; Martín-Sánchez, V.; Martin-Sanz, E. Benign Paroxysmal Positional Vertigo in the Elderly. Gerontology 2013, 59, 408–412. [Google Scholar] [CrossRef]
- Cho, E.I.; White, J.A. Positional Vertigo: As Occurs Across All Age Groups. Otolaryngol. Clin. North Am. 2011, 44, 347–360. [Google Scholar] [CrossRef]
- Shim, D.B. Treatment of Benign Paroxysmal Positional Vertigo: An Approach Considering Patients’ Convenience. Clin. Exp. Otorhinolaryngol. 2020, 13, 320–321. [Google Scholar] [CrossRef]
- Ribeiro, K.F.; Oliveira, B.S.; Freitas, R.V.; Ferreira, L.M.; Deshpande, N.; Guerra, R. Effectiveness of Otolith Repositioning Maneuvers and Vestibular Rehabilitation exercises in elderly people with Benign Paroxysmal Positional Vertigo: A systematic review. Braz. J. Otorhinolaryngol. 2018, 84, 109–118. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeon, E.-J.; Lee, D.-H.; Seo, J.-H. Therapeutic Efficacy of the Modified Epley Maneuver with a Pillow Under the Shoulders. Clin. Exp. Otorhinolaryngol. 2020, 13, 376–380. [Google Scholar] [CrossRef]
- Lo, M.-H.; Lin, C.-L.; Chuang, E.; Chuang, T.-Y.; Kao, C.-H. Association of dementia in patients with benign paroxysmal positional vertigo. Acta Neurol. Scand. 2017, 135, 197–203. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Speechley, M. Falls in Cognitively Impaired Older Adults: Implications for Risk Assessment and Prevention. J. Am. Geriatr. Soc. 2018, 66, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Ganança, F.F.; Gazzola, J.M.; Ganança, C.F.; Caovilla, H.H.; Ganança, M.M.; Cruz, O.L.M. Elderly falls associated with benign paroxysmal positional vertigo. Braz. J. Otorhinolaryngol. 2010, 76, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population based study. J. Neurol. Neurosurg. Psychiatry 2006, 78, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Ekao, C.-L.; Cheng, Y.-Y.; Eleu, H.-B.; Echen, T.-J.; Ema, H.-I.; Echen, J.-W.; Elin, S.-J.; Echan, R.-C. Increased Risk of Ischemic Stroke in Patients with Benign Paroxysmal Positional Vertigo: A 9-Year Follow-Up Nationwide Population Study in Taiwan. Front. Aging Neurosci. 2014, 6, 108. [Google Scholar] [CrossRef]
- Wolters, F.J.; Segufa, R.A.; Darweesh, S.K.; Bos, D.; Ikram, M.A.; Sabayan, B.; Hofman, A.; Sedaghat, S. Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimer’s Dement. 2018, 14, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, Y.-H.; Han, K.; Lee, B.-W.; Kang, E.S.; Kim, J.; Cha, B.-S. Impact of diabetes mellitus and chronic liver disease on the incidence of dementia and all-cause mortality among patients with dementia. Medicine 2017, 96, e8753. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, J.; Serrano, A.; Santos-Silva, A.; Gomes, M.; Afonso, C.; Freitas, P.; Paul, C.; Costa, E. Cardiovascular Risk Factors Are Correlated with Low Cognitive Function among Older Adults Across Europe Based on The SHARE Database. Aging Dis. 2018, 9, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.F. Early-Onset Alzheimer Disease. Neurol. Clin. 2017, 35, 263–281. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Min, C.; Yoo, D.M.; Chang, J.; Lee, H.-J.; Park, B.; Choi, H.G. Hearing Impairment Increases Economic Inequality. Clin. Exp. Otorhinolaryngol. 2021, 14, 278–286. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.-H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service–National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2016, 46, 319. [Google Scholar] [CrossRef]
- National Health Insurance Sharing Service. Available online: http://nhiss.nhis.or.kr/ (accessed on 1 January 2016).
- Kim, S.Y.; Sim, S.; Kim, H.-J.; Choi, H.G. Sudden sensory neural hearing loss is not predictive of myocardial infarction: A longitudinal follow-up study using a national sample cohort. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eryaman, E.; Oz, I.D.; Ozker, B.Y.; Erbek, S.S. Evaluation of vestibular evoked myogenic potentials during benign paroxysmal positional vertigo attacks; neuroepithelial degeneration? B-ENT 2012, 8, 247–250. [Google Scholar]
- Yang, W.S.; Kim, S.H.; Lee, J.D.; Lee, W.-S. Clinical Significance of Vestibular Evoked Myogenic Potentials in Benign Paroxysmal Positional Vertigo. Otol. Neurotol. 2008, 29, 1162–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnes, L.S.; McClure, J.A. Free-Floating Endolymph Particles: A new operative finding during posterior semicircular canal occlusion. Laryngoscope 1992, 102, 988–992. [Google Scholar] [CrossRef]
- Gacek, R.R. Pathology of Benign Paroxysmal Positional Vertigo Revisited. Ann. Otol. Rhinol. Laryngol. 2003, 112, 574–582. [Google Scholar] [CrossRef]
- Lyness, S.; Zarow, C.; Chui, H.C. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: A meta-analysis. Neurobiol. Aging 2002, 24, 1–23. [Google Scholar] [CrossRef]
- Nandi, R.; Luxon, L.M. Development and assessment of the vestibular system. Int. J. Audiol. 2008, 47, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.; Tomassen, S.; Lane, L.; Bishop, K.; Thomas, N. Assessment for benign paroxysmal positional vertigo in medical patients admitted with falls in a district general hospital. Clin. Med. 2016, 16, 335–338. [Google Scholar] [CrossRef] [Green Version]
- Buchner, D.M.; Larson, E.B. Falls and fractures in patients with Alzheimer-type dementia. JAMA 1987, 257, 1492–1495. [Google Scholar] [CrossRef]
- Peluso, É.T.P.; Quintana, M.I.; Ganança, F.F. Anxiety and depressive disorders in elderly with chronic dizziness of vestibular origin. Braz. J. Otorhinolaryngol. 2016, 82, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacqmin-Gadda, H.; Guillet, F.; Mathieu, C.; Helmer, C.; Pariente, A.; Joly, P. Impact of benzodiazepine consumption reduction on future burden of dementia. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Osler, M.; Jørgensen, M.B. Associations of Benzodiazepines, Z-Drugs, and Other Anxiolytics with Subsequent Dementia in Patients With Affective Disorders: A Nationwide Cohort and Nested Case-Control Study. Am. J. Psychiatry 2020, 177, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Liu, C.-J.; Lin, L.-Y.; Chen, T.-J.; Wang, S.-J. Migraine is associated with an increased risk for benign paroxysmal positional vertigo: A nationwide population-based study. J. Headache Pain 2015, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, S.; Zhang, H.; Xu, Y.; Fu, S.; Yu, M.; Ji, P. Evaluation of vertebrobasilar artery changes in patients with benign paroxysmal positional vertigo. NeuroReport 2013, 24, 741–745. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Reitz, C.; Honig, L.S.; Tang, M.X.; Shea, S.; Mayeux, R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005, 65, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Park, J.-M. Factors affecting cognitive function according to gender in community-dwelling elderly individuals. Epidemiol. Health 2017, 39, e2017054. [Google Scholar] [CrossRef] [Green Version]
- Priola, R.S.F.; Lorusso, F.; Immordino, A.; Dispenza, F. Complex forms of benign paroxysmal positional vertigo. In Dizziness: Prevalence, Risk Factors and Management; Nova Publisher: Hauppauge, NY, USA, 2021; pp. 117–149. [Google Scholar]
- Balatsouras, D.G.; Fassolis, G.K.; Moukos, A.; Apris, A. Benign paroxysmal positional vertigo in the elderly: Current insights. Clin. Interv. Aging 2018, 13, 2251–2266. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, N.K.; Kaski, D.; Saman, Y.; Sulaiman, A.A.-S.; Anwer, A.; Bamiou, D.-E. Central Positional Nystagmus: A Systematic Literature Review. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Total Participants | ||
|---|---|---|---|
| Dementia (n, %) | Control Group (n, %) | Standardized Differences | |
| Age (years old) | 0.00 | ||
| 60–64 | 580 (5.1) | 2320 (5.1) | |
| 65–69 | 1289 (11.3) | 5156 (11.3) | |
| 70–74 | 2325 (20.3) | 9300 (20.3) | |
| 75–79 | 2978 (26.1) | 11,912 (26.1) | |
| 80–84 | 2705 (23.7) | 10,820 (23.7) | |
| 85+ | 1555 (13.6) | 6220 (13.6) | |
| Sex | 0.00 | ||
| Male | 3659 (32.0) | 14,636 (32.0) | |
| Female | 7773 (68.0) | 31,092 (68.0) | |
| Income | |||
| 1 (lowest) | 2858 (25.0) | 11,432 (25.0) | 0.00 |
| 2 | 1037 (9.1) | 4148 (9.1) | |
| 3 | 1371 (12.0) | 5484 (12.0) | |
| 4 | 1886 (16.5) | 7544 (16.5) | |
| 5 (highest) | 4280 (37.4) | 17,120 (37.4) | |
| Region of residence | 0.00 | ||
| Urban | 4617 (40.4) | 18,468 (40.4) | |
| Rural | 6815 (59.6) | 27,260 (59.6) | |
| Hypertension | 0.00 | ||
| Yes | 8314 (72.7) | 33,256 (72.7) | |
| No | 3118 (27.3) | 12,472 (27.3) | |
| Diabetes Mellitus | 0.00 | ||
| Yes | 4060 (35.5) | 16,240 (35.5) | |
| No | 7372 (64.5) | 29488 (64.5) | |
| Dyslipidemia | 0.00 | ||
| Yes | 3558 (31.1) | 14,232 (31.1) | |
| No | 7874 (68.9) | 31,496 (68.9) | |
| Ischemic heart disease | 0.05 | ||
| Yes | 1703 (15.0) | 5992 (13.1) | |
| No | 9729 (85.1) | 39,736 (86.9) | |
| Cerebral stroke | 0.50 | ||
| Yes | 5524 (48.3) | 11,475 (25.1) | |
| No | 5908 (51.7) | 34,253 (74.9) | |
| Meniere’s disease | 0.03 | ||
| Yes | 651 (5.7) | 2319 (5.1) | |
| No | 10,781 (94.3) | 43,409 (94.9) | |
| Head trauma | 0.03 | ||
| Yes | 886 (7.8) | 1887 (4.1) | |
| No | 10,546 (92.3) | 43,841 (95.9) | |
| BPPV (Benging Paroxysmal Positional Vertigo) | 0.05 | ||
| Yes | 609 (5.3) | 1914(2.6) | |
| No | 10,823 (94.7) | 43,814 (95.8) | |
| Characteristics | No. of BPPV/ | No. of BPPV/ | ORs for Dementia | p for Interaction | |||
|---|---|---|---|---|---|---|---|
| No. of Dementia (%) | No. of Control (%) | Crude† | p-Value | Adjusted †,‡ | p-Value | ||
| Total participants (n = 57,160) | |||||||
| BPPV | 609/11,432 (5.3) | 1914/45,728 (4.2) | 1.29 (1.18–1.42) | <0.001 * | 1.14 (1.03–1.26) | 0.009 * | |
| Age < 75 years old (n = 20,970) | 0.245 | ||||||
| BPPV | 216/4194 (5.2) | 624/16,776 (3.7) | 1.41 (1.20–1.65) | <0.001 * | 1.18 (0.99–1.39) | 0.062 | |
| Age ≥ 75 years old (n = 36,190) | |||||||
| BPPV | 393/7238 (5.4) | 1290/28,952 (4.5) | 1.23 (1.10–1.39) | <0.001 * | 1.12 (0.99–1.26) | 0.066 | |
| Men (n = 18,295) | 0.063 | ||||||
| BPPV | 158/3659 (4.3) | 428/14,636 (2.9) | 1.50 (1.25–1.81) | <0.001 * | 1.32 (1.09–1.61) | 0.005 * | |
| Women (n = 38,865) | |||||||
| BPPV | 451/7773 (5.8) | 1486/31,092 (4.8) | 1.23 (1.10–1.37) | <0.001 * | 1.09 (0.97–1.22) | 0.148 | |
| Low income (n = 26,330) | 0.651 | ||||||
| BPPV | 262/5266 (5.0) | 795/21,064 (3.8) | 1.34 (1.16–1.55) | <0.001 * | 1.19 (1.02–1.38) | 0.026 * | |
| High income (n = 30,830) | |||||||
| BPPV | 347/6166 (5.6) | 1119/24,664 (4.5) | 1.26 (1.11–1.42) | <0.001 * | 1.10 (0.97–1.26) | 0.134 | |
| Urban (n = 23,085) | 0.920 | ||||||
| BPPV | 242/4617 (5.2) | 762/18,468 (4.1) | 1.29 (1.11–1.50) | 0.001 * | 1.14 (0.98–1.33) | 0.096 | |
| Rural (n = 34,075) | |||||||
| BPPV | 367/6815 (5.4) | 1152/27,260 (4.2) | 1.29 (1.15–1.46) | <0.001 * | 1.14 (1.00–1.29) | 0.046 * | |
| Characteristics | No. of BPPV/ | No. of BPPV/ | ORs for Dementia | p for Interaction | |||
|---|---|---|---|---|---|---|---|
| No. of Dementia (%) | No. of Control (%) | Crude † | p-Value | Adjusted †,‡ | p-Value | ||
| Non-hypertension (n = 15,590) | 0.978 | ||||||
| BPPV | 112/3118 (3.6) | 346/12,472 (2.8) | 1.31 (1.05–1.63) | 0.016 | 1.12 (0.89–1.41) | 0.327 | |
| Hypertension (n = 41,570) | |||||||
| BPPV | 497/8314 (6.0) | 1568/33,256 (4.7) | 1.29 (1.16–1.43) | <0.001 * | 1.14 (1.03–1.27) | 0.016 * | |
| Non-diabetes mellitus (n = 36,860) | 0.099 | ||||||
| BPPV | 343/7372 (4.7) | 1142/29,488 (3.9) | 1.21 (1.07–1.37) | 0.002 * | 1.07 (0.94–1.22) | 0.288 | |
| Diabetes mellitus (n = 20,300) | |||||||
| BPPV | 266/4060 (6.6) | 772/16,240 (4.8) | 1.41 (1.22–1.63) | <0.001 * | 1.24 (1.07–1.44) | 0.005* | |
| Non-dyslipidemia (n = 39,370) | 0.500 | ||||||
| BPPV | 323/7874 (4.1) | 1051/31,496 (3.3) | 1.24 (1.09–1.41) | 0.001 * | 1.11 (0.97–1.27) | 0.125 | |
| Dyslipidemia (n = 17,790) | |||||||
| BPPV | 286/3558 (8.0) | 863/14,232 (6.1) | 1.36 (1.18–1.56) | <0.001 * | 1.17 (1.02–1.36) | 0.031 * | |
| Non-ischemic heart disease (n = 49,465) | 0.107 | ||||||
| BPPV | 500/9729 (5.1) | 1546/39,736 (3.9) | 1.34 (1.21–1.48) | <0.001 * | 1.18 (1.06–1.31) | 0.003* | |
| Ischemic heart disease (n = 7695) | |||||||
| BPPV | 109/1703 (6.4) | 368/5992 (6.1) | 1.05 (0.84–1.30) | 0.693 | 0.97 (0.77–1.22) | 0.781 | |
| Non-cerebral stroke (n = 40,161) | <0.001* | ||||||
| BPPV | 287/5908 (4.9) | 1156/34,253 (3.4) | 1.46 (1.28–1.67) | <0.001 * | 1.46 (1.27–1.67) | <0.001 * | |
| Cerebral stroke (n = 16,999) | |||||||
| BPPV | 322/5524 (5.8) | 758/11,475 (6.6) | 0.88 (0.77–1.00) | 0.052 | 0.90 (0.79–1.04) | 0.146 | |
| Non-Meniere’s disease (n = 54,190) | 0.934 | ||||||
| BPPV | 496/10,781 (4.6) | 1564/43,409 (3.6) | 1.29 (1.16–1.43) | <0.001 * | 1.14 (1.02–1.26) | 0.020 * | |
| Meniere’s disease (n = 2970) | |||||||
| BPPV | 113/651 (17.4) | 350/2319 (15.1) | 1.18 (0.94–1.49) | 0.160 | 1.13 (0.89–1.43) | 0.318 | |
| Non-head trauma (n = 54,387) | 0.163 | ||||||
| BPPV | 560/10,546 (5.3) | 1804/43,841 (4.1) | 1.31 (1.19–1.44) | <0.001 * | 1.16 (1.05–1.28) | 0.005 * | |
| Head trauma (n = 2773) | |||||||
| BPPV | 49/886 (5.5) | 110/1887 (5.8) | 0.95 (0.67–1.34) | 0.754 | 0.90 (0.63–1.29) | 0.571 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Yoo, D.M.; Min, C.; Choi, H.G. Increased Risk of Neurodegenerative Dementia after Benign Paroxysmal Positional Vertigo. Int. J. Environ. Res. Public Health 2021, 18, 10553. https://doi.org/10.3390/ijerph181910553
Kim SY, Yoo DM, Min C, Choi HG. Increased Risk of Neurodegenerative Dementia after Benign Paroxysmal Positional Vertigo. International Journal of Environmental Research and Public Health. 2021; 18(19):10553. https://doi.org/10.3390/ijerph181910553
Chicago/Turabian StyleKim, So Young, Dae Myoung Yoo, Chanyang Min, and Hyo Geun Choi. 2021. "Increased Risk of Neurodegenerative Dementia after Benign Paroxysmal Positional Vertigo" International Journal of Environmental Research and Public Health 18, no. 19: 10553. https://doi.org/10.3390/ijerph181910553
APA StyleKim, S. Y., Yoo, D. M., Min, C., & Choi, H. G. (2021). Increased Risk of Neurodegenerative Dementia after Benign Paroxysmal Positional Vertigo. International Journal of Environmental Research and Public Health, 18(19), 10553. https://doi.org/10.3390/ijerph181910553






