Environmental/Occupational Exposure to Radon and Non-Pulmonary Neoplasm Risk: A Review of Epidemiologic Evidence
Abstract
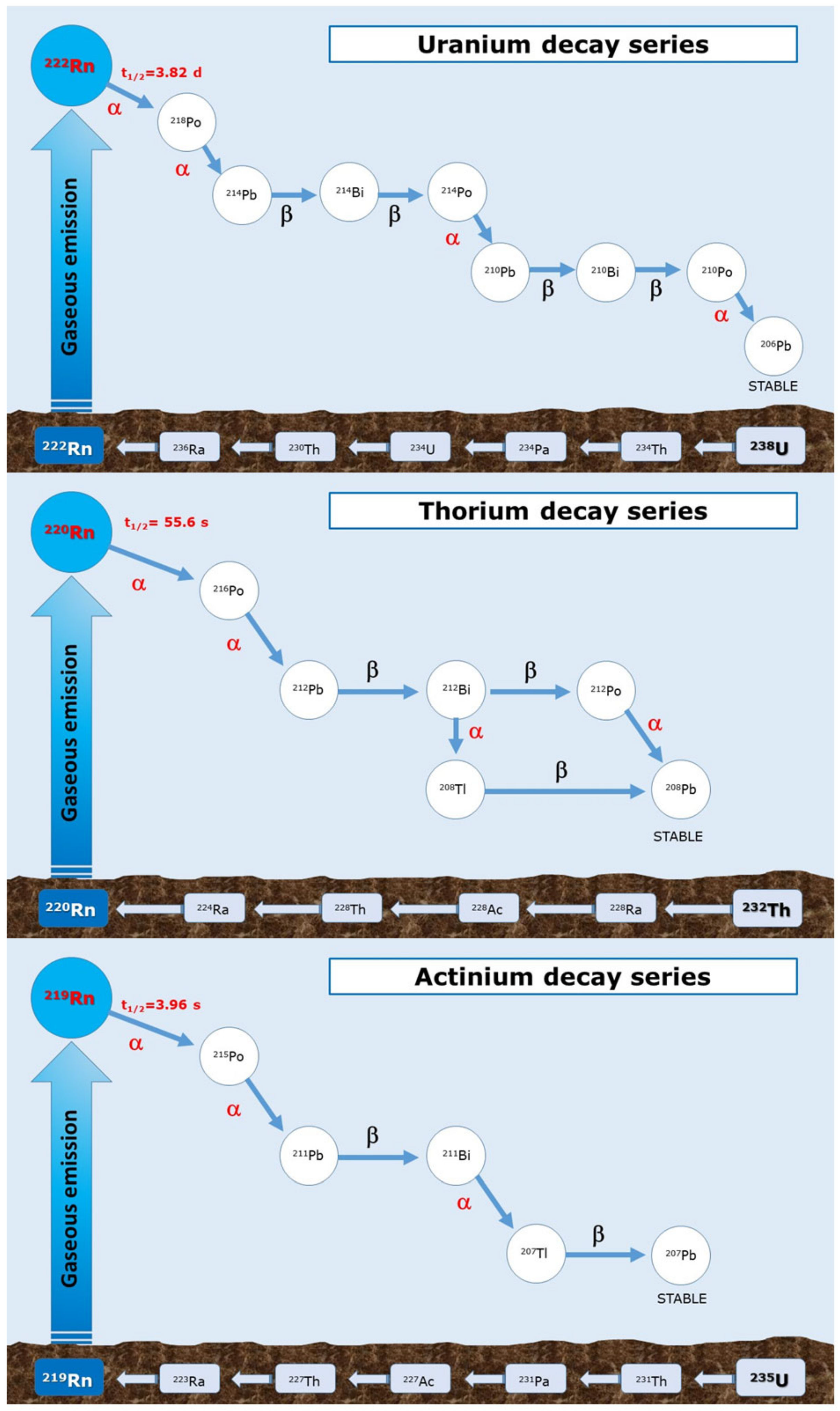
1. Introduction
2. Materials and Methods
3. Results
3.1. Brain and Central Nervous System (CNS) Cancer
3.1.1. Workers
3.1.2. General Population
3.1.3. Pediatric Population
3.2. Leukemia
3.2.1. Workers
3.2.2. General Population
3.2.3. Pediatric Population
3.3. Skin Cancer
General Population
3.4. Stomach Cancer
3.4.1. Workers
3.4.2. General Population
3.5. Kidney Cancer
Workers and General Population
3.6. Breast Cancer
Workers and General Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Radon Measurement Units
- Bequerel (Bq) = one radioactive disintegration per second. It is a measure of radioactivity;
- Bq/m3 = number of decays of Rn per second in one cubic meter of air;
- Curie (Ci) = the activity of one gram of Rn in radioactive equilibrium;
- pCi = 10−12 Curie;
- pCi/l = 37 Bq/m3;
- Sievert (Sv) = absorbed dose of any ionizing radiation having the same biological efficacy as 1 gray of X-rays;
- mSv = 10−3 Sv;
- Working Level (WL) = any combination of short-lived radon daughters in 1 L of air with the potential of emitting 1.3 × 105 MeV of alpha particle energy during decay;
- Working Level Month (WLM) = 1 WL exposure for 170 h.
References
- Polpong, P.; Bovornkitti, S. Indoor radon. J. Med. Assoc. Thai. 1998, 81, 47–57. [Google Scholar]
- Friedmann, H.; Baumgartner, A.; Bernreiter, M.; Gräser, J.; Gruber, V.; Kabrt, F.; Kaineder, H.; Maringer, F.J.; Ringer, W.; Seidel, C.; et al. Indoor radon, geogenic radon surrogates and geology—Investigations on their correlation. J. Environ. Radioact. 2017, 166, 382–389. [Google Scholar] [CrossRef]
- Robertson, A.; Allen, J.; Laney, R.; Curnow, A. The cellular and molecular carcinogenic effects of radon exposure: A review. Int. J. Mol. Sci. 2013, 5, 14024–14063. [Google Scholar] [CrossRef]
- Akiba, S.; Tokonami, S.; Bochicchio, F.; McLaughlin, J.; Tommasino, L.; Harley, N. Thoron: Its metrology, health effects and implications for radon epidemiology: A summary of roundtable discussions. Radiat. Prot. Dosim. 2010, 141, 477–481. [Google Scholar] [CrossRef]
- Steinhausler, F.; Hofmann, W.; Lettner, H. Thoron exposure of man: A negligible issue? Radiat. Prot. Dosim. 1994, 56, 1–4. [Google Scholar] [CrossRef]
- Amrane, M.; Oufni, L.; Misdaq, M.A. Attached and unattached fractions of short-lived radon decay products in outdoor environments: Effect on the human respiratory system. Radiat. Prot. Dosim. 2014, 162, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Porstendörfer, J.; Pagelkopf, P.; Gründel, M. Fraction of the positive 218Po and 214Pb clusters in indoor air. Radiat. Prot. Dosim. 2005, 113, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J.D. Radon: Sources, health risks, and hazard mapping. Ambio 2007, 36, 85–89. [Google Scholar] [CrossRef]
- Daniels, R.D.; Schubauer-Berigan, M.K. Radon in us workplaces: A review. Radiat. Prot. Dosim. 2017, 176, 278–286. [Google Scholar] [CrossRef]
- Craft, B.F.; Oser, J.L.; Norris, F.W. A method for determining relative amounts of combined and uncombined radon daughter activity in underground uranium mines. Am. Ind. Hyg. Assoc. J. 1966, 27, 154–159. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Flak, E.; Wesolowski, J.; Liu, K.S. Relation between residential radon concentrations and housing characteristics. The Cracow Study. Cent. Eur. J. Public Health 1995, 3, 158–160. [Google Scholar]
- Stanley, F.K.; Zarezadeh, S.; Dumais, C.D.; Dumais, K.; MacQueen, R.; Clement, F.; Goodarzi, A.A. Comprehensive survey of household radon gas levels and risk factors in southern Alberta. CMAJ Open 2017, 28, E255–E264. [Google Scholar] [CrossRef]
- Lee, C.; Lee, D. Review of domestic and international methods of measuring radon in residential buildings. Ann. Occup. Environ. Med. 2016, 28, 14. [Google Scholar] [CrossRef]
- Noh, J.; Sohn, J.; Cho, J.; Kang, D.R.; Joo, S.; Kim, C.; Shin, D.C. Residential radon and environmental burden of disease among Non-smokers. Ann. Occup. Environ. Med. 2016, 28, 12. [Google Scholar] [CrossRef]
- Bard, D.; Tirmarche, M.; Pirard, P. Exposition au radon et risques pour la santé publique [Radon exposure and risks for public health]. Ann. Pharm. Fr. 2000, 58, 373–382. (In French) [Google Scholar]
- Nazaroff, W.M. Entry by pressure-driven flow or molecular diffusion? A reassessment of 222Rn concentrations measured in an energy-efficient house. Health Phys. 1988, 55, 1005–1011. [Google Scholar] [CrossRef]
- Villalba, L.; Colmenero Sujo, L.; Montero Cabrera, M.E.; Cano Jiménez, A.; Rentería Villalobos, M.; Delgado Mendoza, C.J.; Jurado Tenorio, L.A.; Dávila Rangel, I.; Herrera Peraza, E.F. Radon concentrations in ground and drinking water in the state of Chihuahua, Mexico. J. Environ. Radioact. 2005, 80, 139–151. [Google Scholar] [CrossRef]
- Chen, J. A discussion on issues with radon in drinking water. Radiat. Prot. Dosim. 2019, 185, 526–531. [Google Scholar] [CrossRef]
- Cho, B.W.; Kim, H.K.; Kim, M.S.; Hwang, J.H.; Yoon, U.; Cho, S.Y.; Choo, C.O. Radon concentrations in the community groundwater system of South Korea. Environ. Monit. Assess. 2019, 191, 189. [Google Scholar] [CrossRef]
- Official Journal on European Community, N° L 80/26, 27.3.90. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31990H0143&from=IT (accessed on 28 September 2021).
- Official Journal on European Community, N° L 159/1, 29.6.96. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31996L0029&from=EN (accessed on 28 September 2021).
- Official Journal on European Community, N° 13/1, 17.1.2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013L0059&qid=1630058541624&from=EN (accessed on 28 September 2021).
- Gordon, K.; Terry, P.D.; Liu, X.; Harris, T.; Vowell, D.; Yard, B.; Chen, J. Radon in Schools: A Brief Review of State Laws and Regulations in the United States. Int. J. Environ. Res. Public Health 2018, 15, 2149. [Google Scholar] [CrossRef]
- Western Australian Legislation, Radiation Safety Act 1975. Available online: https://www.legislation.wa.gov.au/legislation/statutes.nsf/main_mrtitle_784_homepage.html (accessed on 28 September 2021).
- Western Australian Legislation, Mines Safety and Inspection Act 1994. Available online: https://www.legislation.wa.gov.au/legislation/statutes.nsf/main_mrtitle_599_homepage.html (accessed on 28 September 2021).
- Western Australian Legislation, Mines Safety and Inspection Regulations 1995. Available online: https://www.legislation.wa.gov.au/legislation/statutes.nsf/main_mrtitle_1819_homepage.html (accessed on 28 September 2021).
- Bolck, F. Man-Made Mineral Fibres and Radon IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 1990; Volume 43. [Google Scholar]
- WHO. Indoor Radon a Public Health Perspective. A Public Health Perspective; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Puskin, J.S.; James, A.C. Radon exposure assessment and dosimetry applied to epidemiology and risk estimation. Radiat. Res. 2006, 166, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Lubin, J.H.; Zielinski, J.M.; Alavanja, M.; Catalan, V.S.; Field, R.W.; Klotz, J.B.; Létourneau, E.G.; Lynch, C.F.; Lyon, J.L.; et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J. Toxicol. Environ. Health Part A 2006, 69, 533–597. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 28 September 2021).
- Elsevier. Available online: https://scopus.com (accessed on 28 September 2021).
- Web of Science. Available online: https://webofscience.com (accessed on 28 September 2021).
- U.S. Environmental Protection Agency–Radon. Available online: https://www.epa.gov/radon (accessed on 28 September 2021).
- Cool, D.A.; Kase, K.R.; Boice, J.D. NCRP Report no.180-management of exposure to ionizing radiation: NCRP radiation protection guidance for the United States. J. Radiol. Prot. 2019, 39, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Beir, V.I.; National Research Council. Committee on Health Risks of Exposure to Radon (BEIR VI). Health Effects of Exposure to Radon: BEIR VI; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- IARC-WHO. Available online: https://www.iarc.who.int/ (accessed on 28 September 2021).
- Center for Disease Control and Prevention - The National Institute for Occupational Safety and Health (NIOSH). Available online: https://www.cdc.gov/niosh/ (accessed on 28 September 2021).
- Tirmarche, M.; Raphalen, A.; Allin, F.; Chameaud, J.; Bredon, P. Mortality of a cohort of French uranium miners exposed to relatively low radon concentrations. Br. J. Cancer 1993, 67, 1090–1097. [Google Scholar] [CrossRef]
- Darby, S.C.; Whitley, E.; Howe, G.R.; Hutchings, S.J.; Kusiak, R.A.; Lubin, J.H.; Morrison, H.I.; Tirmarche, M.; Tomásek, L.; Radford, E.P.; et al. Radon and cancers other than lung cancer in underground miners: A collaborative analysis of 11 studies. J. Natl. Cancer Inst. 1995, 87, 378–384. [Google Scholar] [CrossRef]
- Darby, S.C.; Radford, E.P.; Whitley, E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ. Health Perspect. 1995, 103 (Suppl. 2), 45–47. [Google Scholar] [CrossRef]
- Vacquier, B.; Caer, S.; Rogel, A.; Feurprier, M.; Tirmarche, M.; Luccioni, C.; Quesne, B.; Acker, A.; Laurier, D. Mortality risk in the French cohort of uranium miners: Extended follow-up 1946-1999. Occup. Environ. Med. 2008, 65, 597–604. [Google Scholar] [CrossRef]
- Kreuzer, M.; Walsh, L.; Schnelzer, M.; Tschense, A.; Grosche, B. Radon and risk of extrapulmonary cancers: Results of the German uranium miners’ cohort study, 1960–2003. Br. J. Cancer 2008, 99, 1946–1953. [Google Scholar] [CrossRef]
- Schubauer-Berigan, M.K.; Daniels, R.D.; Pinkerton, L.E. Radon exposure and mortality among white and American Indian uranium miners: An update of the Colorado Plateau cohort. Am. J. Epidemiol. 2009, 169, 718–730. [Google Scholar] [CrossRef]
- Vacquier, B.; Rage, E.; Leuraud, K.; Caër-Lorho, S.; Houot, J.; Acker, A.; Laurier, D. The influence of multiple types of occupational exposure to radon, gamma rays and long-lived radionuclides on mortality risk in the French “post-55” sub-cohort of uranium miners: 1956–1999. Radiat. Res. 2011, 176, 796–806. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Chen, Y.; Pope, C.A., 3rd; Gapstur, S.M.; Thun, M.J. Radon and nonrespiratory mortality in the American Cancer Society cohort. Am. J. Epidemiol. 2012, 176, 808–814. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Andersen, Z.J.; Andersen, C.E.; Pedersen, C.; Gravesen, P.; Ulbak, K.; Hertel, O.; Loft, S.; Raaschou-Nielsen, O. Residential radon and brain tumour incidence in a Danish cohort. PLoS ONE. 2013, 8, e74435. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Aragonés, N.; Kelsey, K.T.; Pérez-Ríos, M.; Piñeiro-Lamas, M.; López-Abente, G.; Barros-Dios, J.M. Residential radon exposure and brain cancer: An ecological study in a radon prone area (Galicia, Spain). Sci. Rep. 2017, 7, 3595. [Google Scholar] [CrossRef]
- López-Abente, G.; Núñez, O.; Fernández-Navarro, P.; Barros-Dios, J.M.; Martín-Méndez, I.; Bel-Lan, A.; Locutura, J.; Quindós, L.; Sainz, C.; Ruano-Ravina, A. Residential radon and cancer mortality in Galicia, Spain. Sci. Total Environ. 2018, 610, 1125–1132. [Google Scholar] [CrossRef]
- Monastero, R.N.; Meliker, J.R. Incidence of brain and spinal cord cancer and county-level radon levels in New Jersey, Wisconsin, Minnesota, Pennsylvania, and Iowa, USA. Environ. Geochem. Health 2020, 42, 389–395. [Google Scholar] [CrossRef]
- Collman, G.W.; Loomis, D.P.; Sandler, D.P. Childhood cancer mortality and radon concentration in drinking water in North Carolina. Br. J. Cancer 1991, 63, 626–629. [Google Scholar] [CrossRef]
- Kaletsch, U.; Kaatsch, P.; Meinert, R.; Schüz, J.; Czarwinski, R.; Michaelis, J. Childhood cancer and residential radon exposure–results of a population-based case-control study in Lower Saxony (Germany). Radiat. Environ. Biophys. 1999, 38, 211–215. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, C.E.; Andersen, H.P.; Gravesen, P.; Lind, M.; Schüz, J.; Ulbak, K. Domestic radon and childhood cancer in Denmark. Epidemiology 2008, 19, 536–543. [Google Scholar] [CrossRef]
- Hauri, D.; Spycher, B.; Huss, A.; Zimmermann, F.; Grotzer, M.; von der Weid, N.; Weber, D.; Spoerri, A.; Kuehni, C.E.; Röösli, M.; et al. Domestic radon exposure and risk of childhood cancer: A prospective census-based cohort study. Environ. Health Perspect. 2013, 121, 1239–1244. [Google Scholar] [CrossRef]
- Kendall, G.M.; Little, M.P.; Wakeford, R.; Bunch, K.J.; Miles, J.C.; Vincent, T.J.; Meara, J.R.; Murphy, M.F. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia 2013, 27, 3–9. [Google Scholar] [CrossRef]
- Del Risco Kollerud, R.; Blaasaas, K.G.; Claussen, B. Risk of leukaemia or cancer in the central nervous system among children living in an area with high indoor radon concentrations: Results from a cohort study in Norway. Br. J. Cancer 2014, 111, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Berlivet, J.; Hémon, D.; Cléro, É.; Ielsch, G.; Laurier, D.; Guissou, S.; Lacour, B.; Clavel, J.; Goujon, S. Ecological association between residential natural background radiation exposure and the incidence rate of childhood central nervous system tumors in France, 2000–2012. J. Environ. Radioact. 2020, 211, 106071. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Dufey, F.; Tschense, A.; Schnelzer, M.; Grosche, B.; Kreuzer, M. Radon and the risk of cancer mortality—Internal Poisson models for the German uranium miners cohort. Health Phys. 2010, 99, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.T.; Weiffenbach, C.V.; Norton, S.A. Environmental radon and cancer correlations in Maine. Health Phys. 1983, 45, 339–348. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Dacosta-Urbieta, A.; Barros-Dios, J.M.; Kelsey, K.T. Radon exposure and tumors of the central nervous system. Gac. Sanit. 2018, 32, 567–575. [Google Scholar] [CrossRef]
- Tomásek, L.; Darby, S.C.; Swerdlow, A.J.; Placek, V.; Kunz, E. Radon exposure and cancers other than lung cancer among uranium miners in West Bohemia. Lancet 1993, 341, 919–923. [Google Scholar] [CrossRef]
- Rericha, V.; Kulich, M.; Rericha, R.; Shore, D.L.; Sandler, D.P. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: A case-cohort study. Environ. Health Perspect. 2006, 114, 818–822. [Google Scholar] [CrossRef]
- Zablotska, L.B.; Lane, R.S.; Frost, S.E.; Thompson, P.A. Leukemia, lymphoma and multiple myeloma mortality (1950–1999) and incidence (1969–1999) in the Eldorado uranium workers cohort. Environ. Res. 2014, 130, 43–50. [Google Scholar] [CrossRef]
- Kelly-Reif, K.; Sandler, D.P.; Shore, D.; Schubauer-Berigan, M.; Troester, M.A.; Nylander-French, L.; Richardson, D.B. Mortality and cancer incidence among underground uranium miners in the Czech Republic 1977–1992. Occup. Environ. Med. 2019, 76, 511–518. [Google Scholar] [CrossRef]
- Eatough, J.P.; Henshaw, D.L. Radon and monocytic leukaemia in England. J. Epidemiol. Community Health 1993, 47, 506–507. [Google Scholar] [CrossRef][Green Version]
- Thorne, R.; Foreman, N.K.; Mott, M.G. Radon in Devon and Cornwall and paediatric malignancies. Eur. J. Cancer. 1996, 32, 282–285. [Google Scholar] [CrossRef]
- Steinbuch, M.; Weinberg, C.R.; Buckley, J.D.; Robison, L.L.; Sandler, D.P. Indoor residential radon exposure and risk of childhood acute myeloid leukaemia. Br. J. Cancer 1999, 81, 900–906. [Google Scholar] [CrossRef]
- Lubin, J.H.; Linet, M.S.; Boice, J.D., Jr.; Buckley, J.; Conrath, S.M.; Hatch, E.E.; Kleinerman, R.A.; Tarone, R.E.; Wacholder, S.; Robison, L.L. Case-control study of childhood acute lymphoblastic leukemia and residential radon exposure. J. Natl. Cancer Inst. 1998, 90, 294–300. [Google Scholar] [CrossRef]
- UK Childhood Cancer Study Investigators. The United Kingdom Childhood Cancer Study of exposure to domestic sources of ionising radiation: 1: Radon gas. Br. J. Cancer 2002, 86, 1721–1726. [Google Scholar] [CrossRef]
- Henshaw, D.L.; Eatough, J.P.; Richardson, R.B. Radon as a causative factor in induction of myeloid leukaemia and other cancers. Lancet 1990, 335, 1008–1012. [Google Scholar] [CrossRef]
- Gilman, E.A.; Knox, E.G. Geographical distribution of birth places of children with cancer in the UK. Br. J. Cancer 1998, 77, 842–849. [Google Scholar] [CrossRef]
- Kohli, S.; Noorlind Brage, H.; Löfman, O. Childhood leukaemia in areas with different radon levels: A spatial and temporal analysis using GIS. J. Epidemiol. Community Health 2000, 54, 822–826. [Google Scholar] [CrossRef]
- Lucie, N.P. Radon exposure and leukaemia. Lancet 1989, 2, 99–100. [Google Scholar] [CrossRef]
- Alexander, F.E. Radon and leukaemia. Lancet 1990, 335, 1336–1340. [Google Scholar]
- Muirhead, C.R.; Butland, B.K.; Green, B.M.; Draper, G.J. Childhood leukaemia and natural radiation. Lancet 1991, 337, 503–504. [Google Scholar] [CrossRef]
- Tong, J.; Qin, L.; Cao, Y.; Li, J.; Zhang, J.; Nie, J.; An, Y. Environmental radon exposure and childhood leukemia. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Schüz, J.; Erdmann, F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch. Med. Res. 2016, 47, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Etherington, D.J.; Pheby, D.F.; Bray, F.I. An ecological study of cancer incidence and radon levels in South West England. Eur. J. Cancer 1996, 32, 1189–1197. [Google Scholar] [CrossRef]
- Wheeler, B.W.; Allen, J.; Depledge, M.H.; Curnow, A. Radon and skin cancer in southwest England: An ecologic study. Epidemiology 2012, 23, 44–52. [Google Scholar] [CrossRef]
- Wheeler, B.W.; Kothencz, G.; Pollard, A.S. Geography of non-melanoma skin cancer and ecological associations with environmental risk factors in England. Br. J. Cancer 2013, 109, 235–241. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Loft, S.; Sørensen, M.; Jensen, A.; Andersen, C.E.; Ulbak, K.; Hertel, O.; Pedersen, C.; Tjønneland, A.; Krüger Kjær, S.; et al. Residential Radon Exposure and Skin Cancer Incidence in a Prospective Danish Cohort. PLoS ONE 2015, 10, e0135642. [Google Scholar] [CrossRef]
- Barbosa-Lorenzo, R.; Barros-Dios, J.M.; Raíces Aldrey, M.; Cerdeira Caramés, S.; Ruano-Ravina, A. Residential radon and cancers other than lung cancer: A cohort study in Galicia, a Spanish radon-prone area. Eur. J. Epidemiol. 2016, 31, 437–441. [Google Scholar] [CrossRef]
- Vienneau, D.; de Hoogh, K.; Hauri, D.; Vicedo-Cabrera, A.M.; Schindler, C.; Huss, A.; Röösli, M.; SNC Study Group. Effects of Radon and UV Exposure on Skin Cancer Mortality in Switzerland. Environ. Health Perspect. 2017, 125, 67009. [Google Scholar] [CrossRef]
- Charles, M.W. Radon exposure of the skin: II. Estimation of the attributable risk for skin cancer incidence. J. Radiol. Prot. 2007, 27, 253–274. [Google Scholar] [CrossRef]
- Denman, A.R.; Eatough, J.P.; Gillmore, G.; Phillips, P.S. Assessment of health risks to skin and lung of elevated radon levels in abandoned mines. Health Phys. 2003, 85, 733–739. [Google Scholar] [CrossRef]
- Navaranjan, G.; Berriault, C.; Do, M.; Villeneuve, P.J.; Demers, P.A. Cancer incidence and mortality from exposure to radon progeny among Ontario uranium miners. Occup. Environ. Med. 2016, 73, 838–845. [Google Scholar] [CrossRef]
- Messier, K.P.; Serre, M.L. Lung and stomach cancer associations with groundwater radon in North Carolina, USA. Int. J. Epidemiol. 2017, 46, 676–685. [Google Scholar] [CrossRef][Green Version]
- Kjellberg, S.; Wiseman, J.S. The relationship of radon to gastrointestinal malignancies. Am. Surg. 1995, 61, 822–825. [Google Scholar]
- Auvinen, A.; Salonen, L.; Pekkanen, J.; Pukkala, E.; Ilus, T.; Kurttio, P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: A case-cohort study in Finland. Int. J. Cancer 2005, 114, 109–113. [Google Scholar] [CrossRef]
- Drubay, D.; Ancelet, S.; Acker, A.; Kreuzer, M.; Laurier, D.; Rage, E. Kidney cancer mortality and ionizing radiation among French and German uranium miners. Radiat. Environ. Biophys. 2014, 53, 505–513. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, T.W.; Wang, A.Q.; Zhang, H.; Fang, L.J.; Wu, Q.Q.; Zhang, H.B.; Tao, S.S.; Tian, H.L. Exposure to Radon and Kidney Cancer: A Systematic Review and Meta-analysis of Observational Epidemiological Studies. Biomed. Environ. Sci. 2018, 31, 805–815. [Google Scholar] [CrossRef]
- Neuberger, J.S.; Pierce, J.T.; Lai, S.M. Cancer cluster investigation in a school district. J. Sch. Health 1997, 67, 380–383. [Google Scholar] [CrossRef]
- Kristbjornsdottir, A.; Rafnsson, V. Incidence of cancer among residents of high temperature geothermal areas in Iceland: A census based study 1981 to 2010. Environ. Health 2012, 11, 73. [Google Scholar] [CrossRef]
- VoPham, T.; DuPré, N.; Tamimi, R.M.; James, P.; Bertrand, K.A.; Vieira, V.; Laden, F.; Hart, J.E. Environmental radon exposure and breast cancer risk in the Nurses’ Health Study II. Environ. Health 2017, 16, 97. [Google Scholar] [CrossRef]
- Cohen, B.L. Relationship between exposure to radon and various types of cancer. Health Phys. 1993, 65, 529–531. [Google Scholar] [CrossRef]
- ANPA, Agenzia Nazionale per la Protezione dell’Ambiente, ANPA-RT--00-009. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/32/007/32007841.pdf (accessed on 28 September 2021).
- McNeill, K.A. Epidemiology of Brain Tumors. Neurol. Clin. 2016, 34, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Braganza, M.Z.; Kitahara, C.M.; Berrington de González, A.; Inskip, P.D.; Johnson, K.J.; Rajaraman, P. Ionizing radiation and the risk of brain and central nervous system tumors: A systematic review. Neuro. Oncol. 2012, 14, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Kendall, G.M.; Smith, T.J. Doses to organs and tissues from radon and its decay products. J. Radiol. Prot. 2002, 22, 389–406. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the effects of atomic radiation. Available online: https://www.unscear.org/docs/publications/2013/UNSCEAR_2013_Report_Vol.II.pdf (accessed on 28 September 2021).
- Harley, N.H.; Robbins, E.S. Radon and leukemia in the Danish study: Another source of dose. Health Phys. 2009, 97, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Laurier, D.; Valenty, M.; Tirmarche, M. Radon exposure and the risk of leukemia: A review of epidemiological studies. Health Phys. 2001, 81, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Eatough, J.P.; Henshaw, D.L. Radon and thoron associated dose to the basal layer of the skin. Phys. Med. Biol. 1992, 37, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Eatough, J.P. Alpha-particle dosimetry for the basal layer of the skin and the radon progeny 218-Po and 214-Po. Phys. Med. Biol. 1997, 42, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Lorenzo, R.; Barros-Dios, J.M.; Ruano-Ravina, A. Radon and stomach cancer. Int. J. Epidemiol. 2017, 46, 767–768. [Google Scholar] [CrossRef]
- National Research Council. Risk Assessment of Radon in Drinking Water; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Farkas, A.; Hofmann, W.; Balásházy, I.; Szoke, I.; Madas, B.G.; Moustafa, M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat. Environ. Biophys. 2011, 50, 281–297. [Google Scholar] [CrossRef]
- Bartsch, H.; Hollstein, M.; Mustonen, R.; Schmidt, J.; Spiethoff, A.; Wesch, H.; Wiethege, T.; Müller, K.M. Screening for putative radon-specific p53 mutation hotspot in German uranium miners. Lancet 1995, 346, 121. [Google Scholar] [CrossRef]
- Kennedy, C.H.; Mitchell, C.E.; Fukushima, N.H.; Neft, R.E.; Lechner, J.F. Induction of genomic instability in normal human bronchial epithelial cells by 238Pu alpha-particles. Carcinogenesis 1996, 17, 1671–1676. [Google Scholar] [CrossRef]
- Brooks, A.L.; Bao, S.; Harwood, P.W.; Wood, B.H.; Chrisler, W.B.; Khan, M.A.; Gies, R.A.; Cross, F.T. Induction of micronuclei in respiratory tract following radon inhalation. Int. J. Radiat. Biol. 1997, 72, 485–495. [Google Scholar] [CrossRef]
- Deshpande, A.; Goodwin, E.H.; Bailey, S.M.; Marrone, B.L.; Lehnert, B.E. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: Evidence for an extranuclear target. Radiat. Res. 1996, 145, 260–267. [Google Scholar] [CrossRef]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar]
- Wu, L.J.; Randers-Pehrson, G.; Xu, A.; Waldren, C.A.; Geard, C.R.; Yu, Z.; Hei, T.K. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc. Natl. Acad. Sci. USA 1999, 96, 4959–4964. [Google Scholar] [CrossRef]
- Lorenzo-González, M.; Ruano-Ravina, A.; Torres-Durán, M.; Kelsey, K.T.; Provencio, M.; Parente-Lamelas, I.; Leiro-Fernández, V.; Vidal-García, I.; Castro-Añón, O.; Martínez, C.; et al. Residential radon, genetic polymorphisms in DNA damage and repair-related. Lung Cancer 2019, 135, 10–15. [Google Scholar] [CrossRef]
- Lim, S.M.; Choi, J.W.; Hong, M.H.; Jung, D.; Lee, C.Y.; Park, S.Y.; Shim, H.S.; Sheen, S.; Kwak, K.I.; Kang, D.R.; et al. Indoor radon exposure increases tumor mutation burden in never-smoker patients with lung adenocarcinoma. Lung Cancer 2019, 131, 139–146. [Google Scholar] [CrossRef]
- Alavanja, M.C. Biologic damage resulting from exposure to tobacco smoke and from radon: Implication for preventive interventions. Oncogene 2002, 21, 7365–7375. [Google Scholar] [CrossRef]
- Brenner, D.J.; Sachs, R.K. Do low dose-rate bystander effects influence domestic radon risks? Int. J. Radiat. Biol. 2002, 78, 593–604. [Google Scholar] [CrossRef]
- Azzam, E.I.; De Toledo, S.M.; Spitz, D.R.; Little, J.B. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002, 62, 5436–5442. [Google Scholar]
- Iyer, R.; Lehnert, B.E.; Svensson, R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000, 60, 1290–1298. [Google Scholar] [PubMed]
- Azzam, E.I.; Little, J.B. The radiation-induced bystander effect: Evidence and significance. Hum. Exp. Toxicol. 2004, 23, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sethi, T.K.; El-Ghamry, M.N.; Kloecker, G.H. Radon and lung cancer. Clin. Adv. Hematol. Oncol. 2012, 10, 157–164. [Google Scholar] [PubMed]
- Sanders, C.L.; Scott, B.R. Smoking and hormesis as confounding factors in radiation pulmonary carcinogenesis. Dose-Response 2006, 6, 53–79. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef]
- Luckey, T.D. Radiation hormesis: The good, the bad, and the ugly. Dose-Response 2006, 4, 169–190. [Google Scholar] [CrossRef] [PubMed]
| a: Workers | ||||
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
| Cohort mortality study | 1785 | 70.4 WLM 1 | SMR: 1.89; 95% CI: 0.78–3.89 Expected number of deaths 3: p-value = 0.03 | Tirmarche et al. (1993) [39] |
| Cohort mortality study | 64,209 | 155 WLM 1 | O/E deaths 4: 1.01; 95% CI: 0.95–1.07 No significant association | Darby et al. (1995) [40] |
| Cohort mortality study | 1294 | 89 WLM 1 | O/E deaths 4:1.21; 95% CI 1.03–1.41 No significant association | Darby et al. (1995) [41] |
| Cohort study | 5086 (4140 exposed to radon) | 36.6 WLM 1 | No significant association | Vacquier et al. (2008) [42] |
| Cohort study | 49,268 Ex-E 7931 NE | 279.4 WLM 2 | O/E 4: 1.02; 95% CI: 0.98–1.05 | Kreuzer et al. (2008) [43] |
| Cohort study | 4137 | 794–808 WLM | No significant association | Schubauer-Berigan et al. (2009) [44] |
| Cohort study | 3377 | 17.8 WLM 1 | SMR: 2.00; 95% CI: 1.09–3.35 | Vacquier et al. (2011) [45] |
| b: General Population | ||||
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
| Prospective study | 811,961 | mean ± s.d: 53.5 ± 38.0 Bq/m3 range: 6.3–265.7 | HR: 0.98 per 100 Bq/m3; 95% CI: 0.83–1.15 No clear associations | Turner et al. (2012) [46] |
| Cohort study | 57,053 | 40.5 Bq/m3 | IRR: 1.96; 95% CI: 1.07–3.58 | Bräuner et al. (2013) [47] |
| Ecological study | 251 | GM: 100–200 Bq/m3 | Spearman’s Rho: 0.286 (males; p-value: <0.001;) 0.509 (females; p-value: <0.001) | Ruano-Ravina et al. (2017) [48] |
| Ecological study | 13 | 153.9 Bq/m3 | RR: 1.28 Statistical association | López-Abente et al. (2018) [49] |
| Ecological study | New Jersey: 14,662; Iowa: 8429; Wisconsin: 8023; Pennsylvania: 22,940; Minnesota: 5338 | 4.6–8.6 pCi/L | Negative association: p-value <0.0001 | Monastero et al. (2020) [50] |
| c: Pediatric Population | ||||
| Study design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
| Ecological study | Total death: 2706 Brain and CNS disease: 454 | 0–10,692 pCi/l 1 | Medium exposure RR: 1.28; 95% CI: 1.00–1.62 High exposure RR: 1.18; 95% CI: 0.90–1.54 | Collman et al. (1991) [51] |
| Case-control study | 82 L; 82 ST; 209 Controls | mean: 27 Bq/m3 range: 10–584 Bq/m3 |
Solid Tumor OR: 2.61; 95% CI: 0.96–7.13 | Kaletsch et al. (1999) [52] |
| Case-control study | Cases: 2400 Controls: 6697 | mean: 48 Bq/m3 range: 4–254 Bq/m3 | No significant association | Raaschou-Nielsen et al. (2008) [53] |
| Cohort study | Childhood cancer cases: 997 | median: 77.7 Bq/m3; 90th: 139.9 Bq/m3 | All cancers HR: 0.93; 95% CI: 0.74–1.16 CNS tumors HR: 1.05; 95% CI: 0.68–1.61 | Hauri D et al. (2013) [54] |
| Case-control study | Cases: 27,447 Controls: 36,793 | mean: 22 Bq/m3 | ERR: 3%; 95% CI: 4–11; p-value: 0.35 | Kendall et al. (2013) [55] |
| Cohorts | Total: 712,674 Cancer cases: 864 | mean: 91 Bq/m3 median: 74 Bq/m3 | <50 Bq/m3 HR: 1.00 (Ref.) 50–100 Bq/m3 HR: 0.88; CI: (0.68–1.14) >100 Bq/m3 HR: 1.15; CI: (0.87–1.50) No significant association | Del Risco Kollerud et al. (2014) [56] |
| Ecological study | 5471 cases of CNST | 1 41.0 Bq/m3 | IRR: 1.07; CI: 0,95–1.20 per 100 Bq/m3 No significant association | Berlivet J et al. (2020) [57] |
| a: Workers | ||||
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
| Cohort mortality study | 4320 | 196.8 WLM 1 | O/E deaths: 1.11; 95% CI: 0.98–1.24 No significant association | Tomàsek et al. (1993) [61] |
| Cohort study | 64,209 | 155 WLM 1 | O/E: 1.93; 95% CI: 1.19–2.95 No significant association | Darby et al. (1995) [40] |
| Retrospective case–cohort study | 23,043 | mean ± sd: 64.1 ± 98 WLM | All leukemia RR: 1.75; 95% CI: 1.10–2.78; p-value = 0.014 CLL RR: n.s. | Rericha et al. (2006) [62] |
| Cohort study | 58,987 | 279.4 WLM | No significant association O/E: 0.89; 95% CI: 0.74–1.06 | Kreuzer et al. (2008) [43] |
| Cohort study | 17,660 | 100.2 WLM | All leukemia SMR: 0.69; 95% CI: 0.48–0.97; p-value = 0.031 SIR: 0.79; 95% CI: 0.59–1.03; p-value = 0.088 | Zablotska et al. (2014) [63] |
| Cohort study | 16,434 | 53 WLM | All leukemias: SIR: 1.51; 95% CI: 1.08–2.07 Lymphatic and hematopoietic cancers combined SIR: 1.31; 95% CI: 1.05–1.61 | Kelly-Reif et al. (2019) [64] |
| b: General and Pediatric Population | ||||
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
| Ecological study | 45 | <120 Bq/m3 | Lymphocytic leukemia r = 0.40; p-value < 0.005, ρ = 0.24; p-value < 0.1 Myeloid leukemia r = 0.43; p-value < 0.005, ρ = 0.22 p-value < 0 1 | Eatough et al. (1993) [65] |
| Ecological study | Area ≥ 100 Bq/m3 Cases: 35 Area < 100 Bq/m3 Cases: 73 | Area ≥ 100 Bq/m3 (Mean: 183 Bq/m3) Area < 100 Bq/m3 (Mean: 57 Bq/m3) | Area ≥ 100 Bq/m3: Incidence = 106.7 per million child years Area < 100 Bq/m3: Incidence = 121.7 per million child years No significant difference between Area ≥ 100 Bq/m3 and Area < 100 Bq/m3 (p-value = 0.29). | Thorne et al. (1996) [66] |
| Case-control study |
Cases: 173 Controls: 254 | 1 Cases: 56.0 Bq/m3 1 Controls: 49.8 Bq/m3 | 37–100 Bq/m3 adjusted OR: 1.2; 95% CI: 0.7–1.8 >100 Bq/m3 adjusted OR: 1.1; 95% CI 0.6–2.0 No significant difference | Steinbuch et al. (1999) [67] |
| Case-control study | Cases: 505 Controls: 443 | 1 Cases 65.4 Bq/m3 1 Controls 79.1 Bq/m3 | Rn concentration < 37 Bq/m3 RR: 1; (Reference) Rn concentration 37–73 Bq/m3 RR: 1.22; 95% CI: 0.8–1.9 Rn concentration 74–147 Bq/m3 RR: 0.82; 95% CI: 0.8–1.9 Rn concentration ≥ 148 Bq/m3 RR: 1.02; 95% CI: 0.5–2.0 | Lubin et al. (1998) [68] |
| Case-control study | Cases: 82 Controls: 209 | Median: 27 Bq/m3 Range: 10–584 Bq/m3 | OR: 1.30; 95% CI: 0.32–5.33 | Kaletsch et al. (1999) [52] |
| Case-control study | Cases: 3838 cases (1461 ALL) Controls 7629 | 1 24.0 Bq/m3 | OR: 0.80; 95% CI: 0.64–0.99 | UKCCS [69] (2002) |
| Ecological study | Data not provided | UK: 20 Bq/m3; Cornwall: 110 Bq/m3; World: 50 Bq/m3 | Country data alone r = 0.65; p-value < 0.02; Regional data r = 0.62; p-value <0.02 | Henshaw et al. (1990) [70] |
| Ecological study | Leukemias and lymphomas: 4851 | median: 21 Bq/m3, | RR: 1.06; 95% CI: 0.99–1.12 | Gilman et al. (1998) [71] |
| Ecological correlation study | 53,146 | High risk area: 50,000 Bq/m3; Normal risk area: 10,000–50,000 Bq/m3; Low risk area: <10,000 Bq/m3; | ALL2: RR (normal risk area): 4.64 95% CI: 1.29–28.26 RR (high risk area): 5.67 95% CI: 1.06–42.27 | Kohli et al. (2000) [72] |
| Cohort study | Childhood cancer cases: 997 | 77.7 1 Bq/m3, 90th: 139.9 Bq/m3 | All leukemias AHR: 0.90; 95% CI: 0.56–1.43 Acute lymphoblastic leukemia AHR: 0.90; 95% CI: 0.56- 1.43 | Hauri D et al. (2013) [54] |
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
|---|---|---|---|---|
| Ecological study | 28,989 | 1 40 Bq/m3 1 ≥230 Bq/m3 | Non-melanoma skin cancers, showed a significant increase in incidence in the high-radon postcode sectors (≥100 Bq/m3) compared with the low-radon sectors (<60 Bq/m3) and this effect was observed for both sexes. | Etherington et al. (1996) [78] |
| Prospective study | 811,961 | mean ± sd: 53.5 ± 38.0 Bq/m3 range: 6.3–265.7 Bq/m3 | HR = 0.98; 95% CI: 0.97–1.00, per each 100 Bq/m3 no significant association | Turner et al. (2012) [46] |
| Ecological study | 18,350 | mean ± sd: 98.1 ± 73.1 Bq/m3 | Malignant melanoma Rn concentration ≥ 230 Bq/m3 RR: 0.85; 95% CI: 0.65–1.11 Basal cell carcinoma Rn concentration ≥ 230 Bq/m3 RR: 0.81; 95% CI: 0.66–1.00 Squamous cell carcinoma Rn concentration ≥ 230 Bq/m3 RR: 1.76; 95% CI: 1.46–2.11 | Wheeler et al. (2012) [79] |
| Ecological study | 206,454 | range: 0–≥100 Bq/m3 | 0.182 registrations per 100,000 population per year 95% CI: 0.04–0.32 p-value = 0.011 | Wheeler et al. (2013) [80] |
| Prospectic cohort | 57,053 | median: 38.3 Bq/m3 | BCC Adjusted IRR: 1.14; 95% CI: 1.03–1.27 SCC Adjusted IRR: 0.90; 95% CI: 0.70- 1.37 MM Adjusted IRR: 1.08; 95% CI: 0.77–1.50 | Bräuner et al. (2015) [81] |
| Ecological study | 2294 | Cutpoint set: 50 Bq/m3 | Risk of non-pulmonary cancer HR: 1.2; 95% CI: 0.9–1.6 Connective tissue and others of the skin HR: 1.5; 95% CI: 0.6–3.8 (except melanoma) | Barbosa-Lorenzo et al. (2016) [82] |
| Mortality cohorts | Tot: 5,249,462 skin cancer deaths: 2989 | mean ± sd: 91.8 ± 47.8 Bq/m3 | MM HR3: 1.41; 95% CI: 1.09–1.80 at 30 years HR3: 1.05; 0.94–1.18 at 75 years Adjusted HR: 1.16; 95% CI: 1.04–1.29 at 60 years | Vienneau et al. (2017) [83] |
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
|---|---|---|---|---|
| Cohort study | 64,209 | 155 WLM | O/E: 1.33; 95% CI: 1.169–1.52 Mortality: no significant association | Darby et al. (1995) [40] |
| Cohort study | 1415 | 89 WLM | O/E: 1.45; 95% CI: 1.04–1.98 Mortality: no significant association | Darby et al. (1995) [41] |
| Cohort study | 28,546 | 21 WLM | Incidence: Overall excess 1 RR: −0.12; 95% CI: −0.59–0.35 Mortality: overall excess 1 RR: −0.082; 95% CI: −0.61–0.45 No significant association | Navaranjan et al. (2016) [86] |
| Ecological study | 5218 | Groundwater: 100 Bq/L Indoor air: 100 Bq/m3 | Groundwater Rn exposure and stomach cancer OR: 1.24; 95% CI 1.03–1.49 | Messier et al. (2017) [87] |
| Ecological study | 56,385 | IQ range: 53–184 Bq/m3 | Statistical association in women RR: 1.17; 95% CI 1.02–1.32 | López-Abente et al. (2018) [49] |
| Cohort study | 16,434 | 53 WLM | SMR: 1.27; 95% CI: 1.02–1.51 SIR: 1.37; 95% CI: 1.11–1.63 | Kelly-Reif et al. (2019) [64] |
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
|---|---|---|---|---|
| Cohort study | 5086 | 36.6 WLM | A significant excess of kidney cancer deaths was observed (n = 20; SMR = 2.0; 95% CI: 1.22–3.09), which was not associated with cumulative Rn exposure | Vacquier et al. (2008) [42] |
| Cohort study | 28,546 | 21 WLM | No association for kidney cancer with increasing cumulative Rn exposure | Navaranjan et al. (2016) [86] |
| Cohort study | French cohort: 3377; German cohort: 58,986 |
median: 4.7 WLM range: 0–128.4 WLM median: 18.4 WLM range: 0–3224.5 WLM | French cohort SMR: 1.49; 95% CI: 0.73–2.67 German cohort SMR = 0.91; 95% CI: 0.77–1.06 No significant association | Drubay et al. (2014) [90] |
| Study Design | Sample Size (n) | Radon Conc. | Results | Reference (Year) |
|---|---|---|---|---|
| Cohort study | School employees: 520 | Classrooms: <2 pCi/L Utility tunnels: 29–33 pCi/L | SMR: 5; 95% CI: 1.03–14.6 | Neuberger et al. (1997) [92] |
| Observational cohort study | 74,806 | 1 water: 1.3–9 Bq/L | HR: 1.59; 95%CI: 1.10–2.31 | Kristbjornsdottir et al. (2012) [93] |
| Prospective cohort study | 811,961 | mean ± sd: 53.5 ± 38.0 Bq/m3 range: 6.3–265.7 Bq/m3 | HR: 0.91; 95% CI: 0.82–1.01 | Turner et al. (2012) [46] |
| Prospective cohort study | 112,639 female | 2 P20: <27.0 Bq/m32 P40: ≥27.0–37.7 Bq/m3 2 P60: ≥37.7–50.1 Bq/m3 2 P80: ≥50.1–74.9 Bq/m3 2 P100: ≥74.9 Bq/m3 | For women in the highest quintile of exposure (≥74.9 Bq/m3) HR = 1.38; 95% CI: 0.97–1.96 (ER-/PR-) No association was observed for ER+/PR+ breast cancer | VoPham et al. (2017) [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozzoni, P.; Pinelli, S.; Corradi, M.; Ranzieri, S.; Cavallo, D.; Poli, D. Environmental/Occupational Exposure to Radon and Non-Pulmonary Neoplasm Risk: A Review of Epidemiologic Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10466. https://doi.org/10.3390/ijerph181910466
Mozzoni P, Pinelli S, Corradi M, Ranzieri S, Cavallo D, Poli D. Environmental/Occupational Exposure to Radon and Non-Pulmonary Neoplasm Risk: A Review of Epidemiologic Evidence. International Journal of Environmental Research and Public Health. 2021; 18(19):10466. https://doi.org/10.3390/ijerph181910466
Chicago/Turabian StyleMozzoni, Paola, Silvana Pinelli, Massimo Corradi, Silvia Ranzieri, Delia Cavallo, and Diana Poli. 2021. "Environmental/Occupational Exposure to Radon and Non-Pulmonary Neoplasm Risk: A Review of Epidemiologic Evidence" International Journal of Environmental Research and Public Health 18, no. 19: 10466. https://doi.org/10.3390/ijerph181910466
APA StyleMozzoni, P., Pinelli, S., Corradi, M., Ranzieri, S., Cavallo, D., & Poli, D. (2021). Environmental/Occupational Exposure to Radon and Non-Pulmonary Neoplasm Risk: A Review of Epidemiologic Evidence. International Journal of Environmental Research and Public Health, 18(19), 10466. https://doi.org/10.3390/ijerph181910466








