Women’s Lives Matter—The Critical Need for Women to Prioritize Optimal Physical Activity to Reduce COVID-19 Illness Risk and Severity
Abstract
:1. Introduction
2. Mental Health Benefits of PA
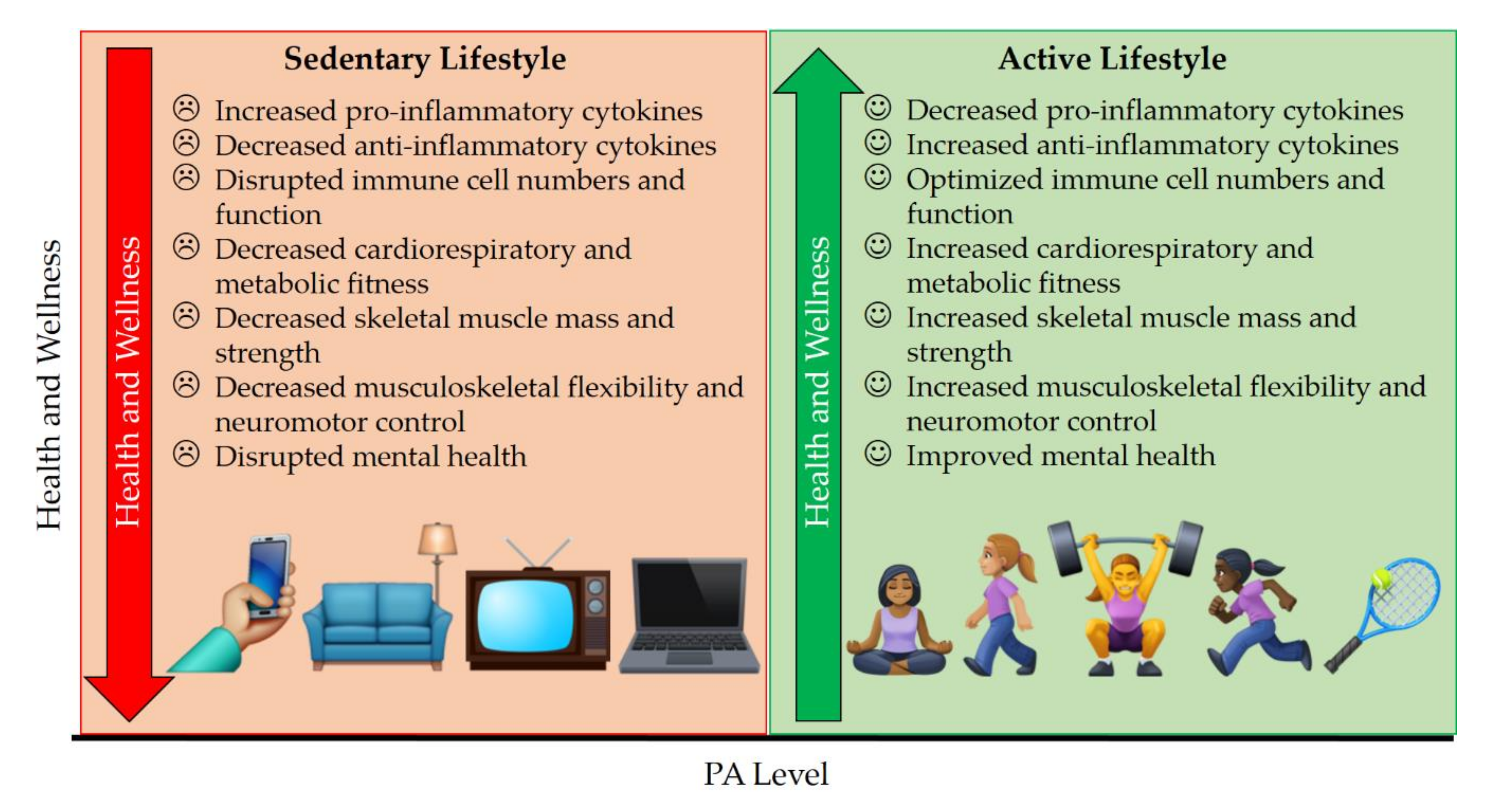
3. Physical Health Benefits of PA
4. The Specific Role of Mucosal Immunity and Immunoglobulin A (IgA) in Protection against Respiratory Infections and Symptoms
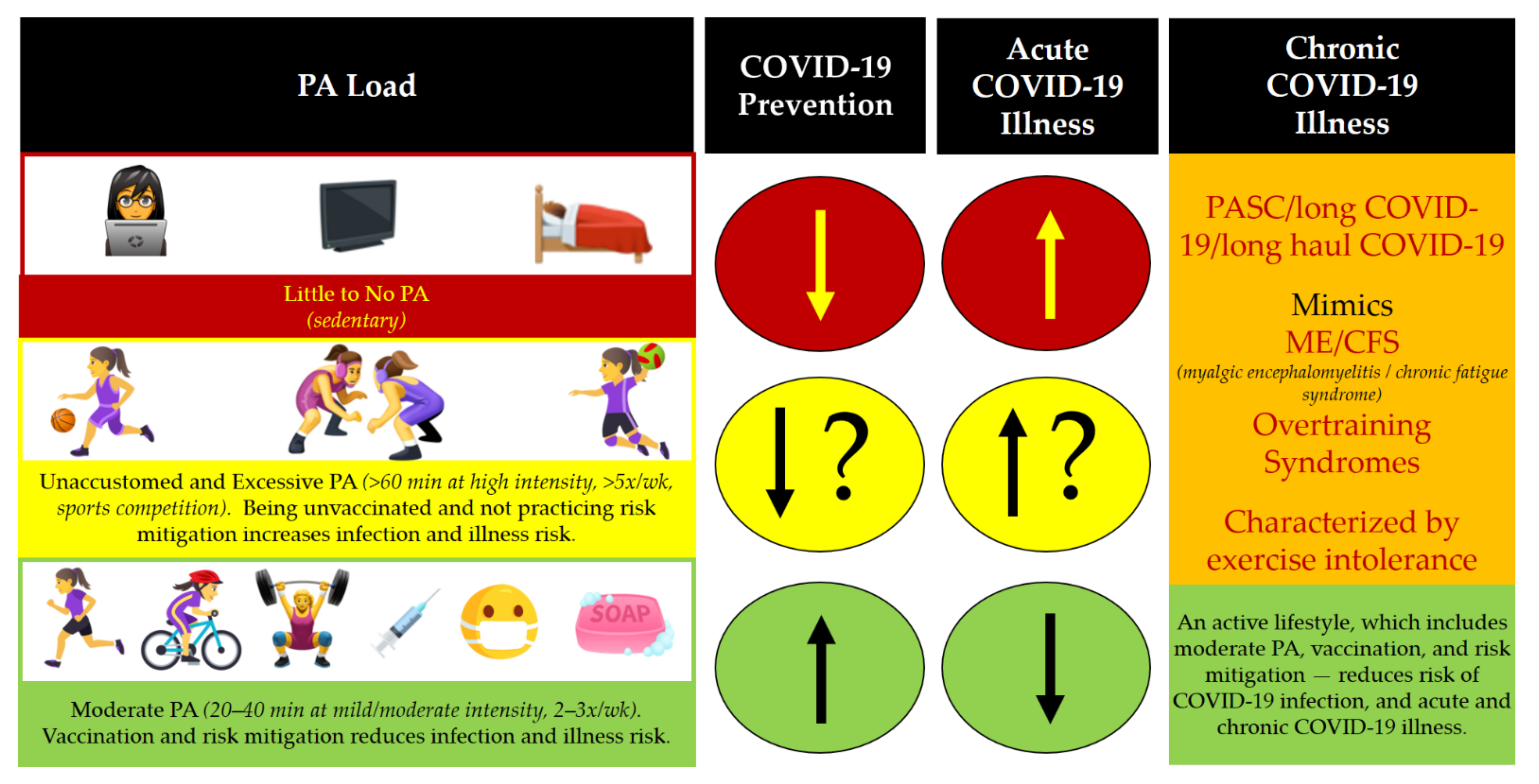
5. Women, PA, and Post-Acute Sequelae of SARS-CoV-2 Infection (PASC)
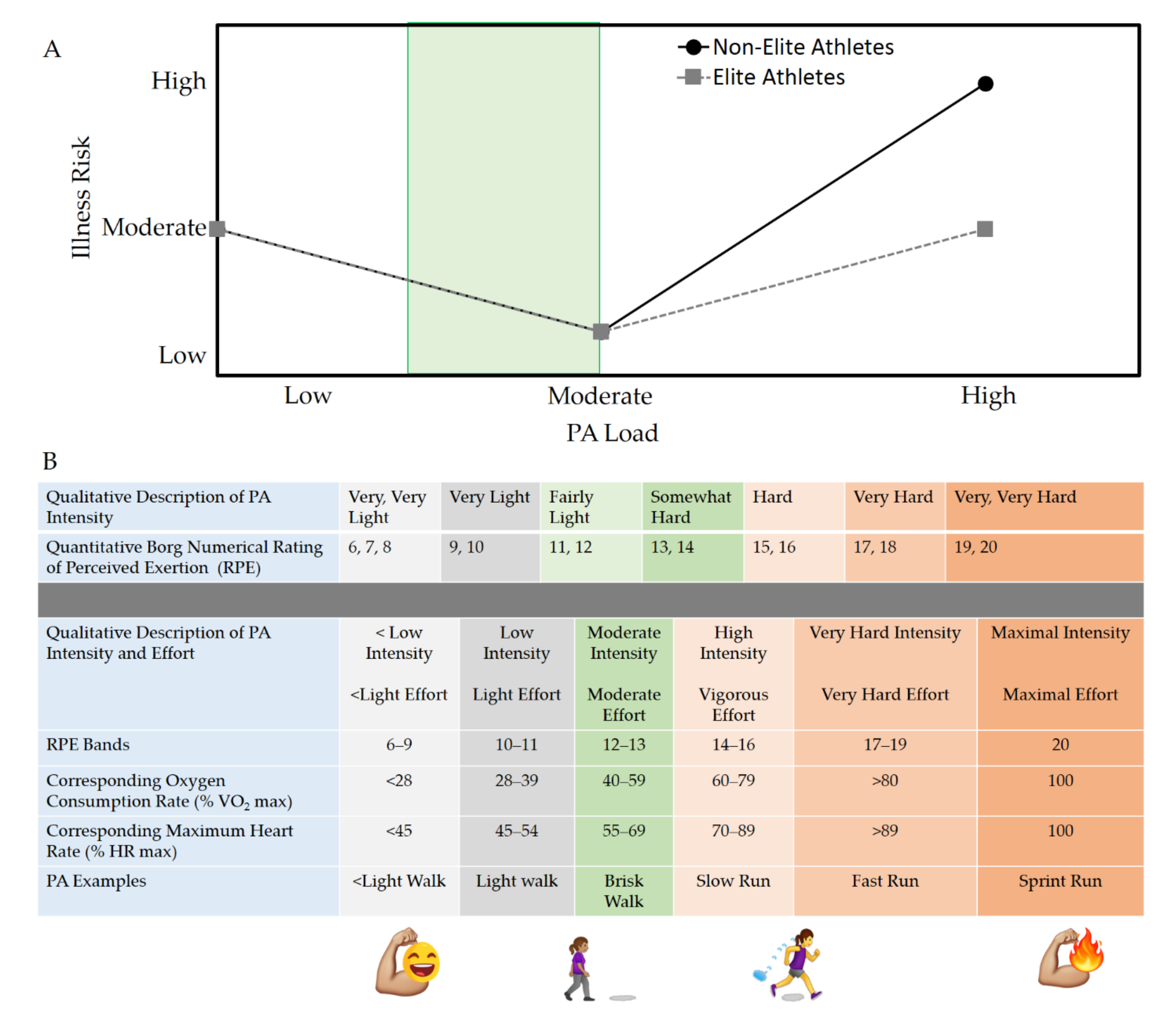
6. Recommendations for Staying Active during a the COVID-19 Pandemic
7. COVID-19 Vaccination and Its Relevance to Women’s Health and Maintaining an Active Lifestyle
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Timeline of ECDC’s Response to COVID-19. Available online: https://www.ecdc.europa.eu/en/covid-19/timeline-ecdc-response (accessed on 20 July 2021).
- World Health Organization (WHO). Archived: WHO Timeline—COVID-19. Available online: https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 (accessed on 10 September 2021).
- Jones, N.R.; Qureshi, Z.U.; Temple, R.J.; Larwood, J.P.J.; Greenhalgh, T.; Bourouiba, L. Two metres or one: What is the evidence for physical distancing in COVID-19? BMJ 2020, 370, m3223. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.R.; Cook, A.R.; Park, M.; Sun, Y.; Sun, H.; Lim, J.T.; Tam, C.; Dickens, B.L. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: A modelling study. Lancet Infect. Dis. 2020, 20, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Lewnard, J.A.; Lo, N.C. Scientific and ethical basis for social-distancing interventions against COVID-19. Lancet Infect. Dis. 2020, 20, 631–633. [Google Scholar] [CrossRef] [Green Version]
- Gokmen, Y.; Baskici, C.; Ercil, Y. Effects of non-pharmaceutical interventions against COVID-19: A cross-country analysis. Int. J. Health Plann Manag. 2021, 36, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.J.; Dunstan, D.W.; Owen, N.; Bonfa, E.; Gualano, B. Combating physical inactivity during the COVID-19 pandemic. Nat. Rev. Rheumatol. 2020, 16, 347–348. [Google Scholar] [CrossRef]
- Hall, G.; Laddu, D.R.; Phillips, S.A.; Lavie, C.J.; Arena, R. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog. Cardiovasc. Dis. 2021, 64, 108–110. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Coping with Stress. Available online: https://www.cdc.gov/mentalhealth/stress-coping/cope-with-stress/index.html (accessed on 10 September 2021).
- Mattioli, A.V.; Sciomer, S.; Cocchi, C.; Maffei, S.; Gallina, S. Quarantine during COVID-19 outbreak: Changes in diet and physical activity increase the risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1409–1417. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M.; Sanchis-Gomar, F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19). Eur. J. Prev. Cardiol. 2020, 27, 906–908. [Google Scholar] [CrossRef]
- Lind, A.; Gonzalez Laya, A. What the COVID-19 Pandemic Tells Us about Gender Gquality. Available online: https://www.weforum.org/agenda/2020/05/what-the-covid-19-pandemic-tells-us-about-gender-equality/ (accessed on 10 September 2021).
- Cunningham, C.; O’Sullivan, R. Why physical activity matters for older adults in a time of pandemic. Eur. Rev. Aging Phys. Act. 2020, 17, 16. [Google Scholar] [CrossRef]
- Rezende, L.F.M.; Lee, D.H.; Ferrari, G.; Eluf-Neto, J.; Giovannucci, E.L. Physical activity for cancer patients during COVID-19 pandemic: A call to action. Cancer Causes Control 2021, 32, 1–3. [Google Scholar] [CrossRef]
- Hemphill, N.M.; Kuan, M.T.Y.; Harris, K.C. Reduced Physical Activity During COVID-19 Pandemic in Children With Congenital Heart Disease. Can. J. Cardiol. 2020, 36, 1130–1134. [Google Scholar] [CrossRef]
- Marcal, I.R.; Fernandes, B.; Viana, A.A.; Ciolac, E.G. The Urgent Need for Recommending Physical Activity for the Management of Diabetes During and Beyond COVID-19 Outbreak. Front. Endocrinol. 2020, 11, 584642. [Google Scholar] [CrossRef]
- Nienhuis, C.P.; Lesser, I.A. The Impact of COVID-19 on Women’s Physical Activity Behavior and Mental Well-Being. Int. J. Environ. Res. Public Health 2020, 17, 9036. [Google Scholar] [CrossRef]
- United Nations (UN) Department of Global Communications (DGC). Gender Equality in the Time of COVID-19. Available online: https://www.un.org/en/un-coronavirus-communications-team/gender-equality-time-covid-19 (accessed on 10 September 2021).
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. Available online: https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines (accessed on 9 September 2021).
- World Health Organization (WHO). Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 10 September 2021).
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocr. 2017, 46, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports, M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Yang, J.; Marziano, V.; Deng, X.; Guzzetta, G.; Zhang, J.; Trentini, F.; Cai, J.; Poletti, P.; Zheng, W.; Wang, W.; et al. Despite vaccination, China needs non-pharmaceutical interventions to prevent widespread outbreaks of COVID-19 in 2021. Nat. Hum. Behav. 2021, 5, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Hill, E.M.; Tildesley, M.J.; Dyson, L.; Keeling, M.J. Vaccination and non-pharmaceutical interventions for COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2021, 21, 793–802. [Google Scholar] [CrossRef]
- Contreras, S.; Priesemann, V. Risking further COVID-19 waves despite vaccination. Lancet Infect. Dis. 2021, 21, 745–746. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Li, G.; Xu, X.; Sun, Y.; Wang, F.; Shi, X.; Li, X.; Xie, G.; Zhang, L. Differential impact of non-pharmaceutical public health interventions on COVID-19 epidemics in the United States. BMC Public Health 2021, 21, 965. [Google Scholar] [CrossRef]
- Bhuyan, A. Experts criticise India’s complacency over COVID-19. Lancet 2021, 397, 1611–1612. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Social Media Postcards: Be Active and Stay Healthy at Home (COVID-19). Available online: https://www.paho.org/en/covid-19-communication-materials/social-media-postcards-be-active-and-stay-healthy-home-covid-19 (accessed on 26 July 2021).
- Collins, F. NIH Launches New Initiative to Study “Long COVID”. Available online: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid (accessed on 22 July 2021).
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012, 13, 596–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- De Loof, A. Only two sex forms but multiple gender variants: How to explain? Commun. Integr. Biol. 2018, 11, e1427399. [Google Scholar] [CrossRef] [PubMed]
- Guss, C.; Shumer, D.; Katz-Wise, S.L. Transgender and gender nonconforming adolescent care: Psychosocial and medical considerations. Curr. Opin. Pediatr. 2015, 27, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Dasso, N.A. How is exercise different from physical activity? A concept analysis. Nurs. Forum 2019, 54, 45–52. [Google Scholar] [CrossRef] [Green Version]
- United Nations Development Programme (UNDP). What Does Coronavirus Mean for Women. Available online: https://www.undp.org/blogs/what-does-coronavirus-mean-women (accessed on 10 September 2021).
- Global Health 5050. The Sex, Gender and COVID-19 Project. Available online: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/ (accessed on 10 September 2021).
- Li, S.H.; Graham, B.M. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 2017, 4, 73–82. [Google Scholar] [CrossRef]
- Bromberger, J.T.; Schott, L.L.; Avis, N.E.; Crawford, S.L.; Harlow, S.D.; Joffe, H.; Kravitz, H.M.; Matthews, K.A. Psychosocial and health-related risk factors for depressive symptom trajectories among midlife women over 15 years: Study of Women’s Health Across the Nation (SWAN). Psychol. Med. 2019, 49, 250–259. [Google Scholar] [CrossRef]
- Li, G.; Miao, J.; Wang, H.; Xu, S.; Sun, W.; Fan, Y.; Zhang, C.; Zhu, S.; Zhu, Z.; Wang, W. Psychological impact on women health workers involved in COVID-19 outbreak in Wuhan: A cross-sectional study. J. Neurol. Neurosurg. Psychiatry 2020, 91, 895–897. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Leow, S.; Jackson, B.; Alderson, J.A.; Guelfi, K.J.; Dimmock, J.A. A Role for Exercise in Attenuating Unhealthy Food Consumption in Response to Stress. Nutrients 2018, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Youngstedt, S.D. Sleep quality improved following a single session of moderate-intensity aerobic exercise in older women: Results from a pilot study. J. Sport Health Sci. 2014, 3, 338–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBouthillier, D.M.; Asmundson, G.J. A Single Bout of Aerobic Exercise Reduces Anxiety Sensitivity But Not Intolerance of Uncertainty or Distress Tolerance: A Randomized Controlled Trial. Cogn. Behav. Ther. 2015, 44, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Colledge, F.; Ludyga, S.; Mucke, M.; Puhse, U.; Gerber, M. The effects of an acute bout of exercise on neural activity in alcohol and cocaine craving: Study protocol for a randomised controlled trial. Trials 2018, 19, 713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Jiang, W.T.; Wang, X.; Cai, Z.D.; Liu, Z.H.; Liu, G.R. Exercise, brain plasticity, and depression. CNS Neurosci. 2020, 26, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.; Bourbeau, K.; Bellovary, B.; Zuhl, M.N. Exercise Intensity Influences Prefrontal Cortex Oxygenation during Cognitive Testing. Behav. Sci. 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, D.B.; Bullen, B.A.; Skrinar, G.S.; Arnold, M.A.; Rosenblatt, M.; Beitins, I.Z.; Martin, J.B.; McArthur, J.W. Physical conditioning facilitates the exercise-induced secretion of beta-endorphin and beta-lipotropin in women. N. Engl. J. Med. 1981, 305, 560–563. [Google Scholar] [CrossRef]
- Monaghesh, E.; Hajizadeh, A. The role of telehealth during COVID-19 outbreak: A systematic review based on current evidence. BMC Public Health 2020, 20, 1193. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Teleconsultations during a Pandemic. Available online: https://iris.paho.org/handle/10665.2/52006 (accessed on 10 September 2021).
- Zhou, X.; Snoswell, C.L.; Harding, L.E.; Bambling, M.; Edirippulige, S.; Bai, X.; Smith, A.C. The Role of Telehealth in Reducing the Mental Health Burden from COVID-19. Telemed. J. E Health 2020, 26, 377–379. [Google Scholar] [CrossRef] [Green Version]
- Diamond, R.; Waite, F. Physical activity in a pandemic: A new treatment target for psychological therapy. Psychol. Psychother. 2021, 94, 357–364. [Google Scholar] [CrossRef]
- Tian, D.; Meng, J. Exercise for Prevention and Relief of Cardiovascular Disease: Prognoses, Mechanisms, and Approaches. Oxidative Med. Cell. Longev. 2019, 2019, 3756750. [Google Scholar] [CrossRef] [Green Version]
- Zeiher, J.; Ombrellaro, K.J.; Perumal, N.; Keil, T.; Mensink, G.B.M.; Finger, J.D. Correlates and Determinants of Cardiorespiratory Fitness in Adults: A Systematic Review. Sports Med. Open 2019, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 September 2021).
- Schwellnus, M.; Soligard, T.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hagglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Vanhees, L.; De Sutter, J.; Gelada, S.N.; Doyle, F.; Prescott, E.; Cornelissen, V.; Kouidi, E.; Dugmore, D.; Vanuzzo, D.; Borjesson, M.; et al. Importance of characteristics and modalities of physical activity and exercise in defining the benefits to cardiovascular health within the general population: Recommendations from the EACPR (Part I). Eur. J. Prev. Cardiol. 2012, 19, 670–686. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Al-Mallah, M.H.; Juraschek, S.P.; Whelton, S.; Dardari, Z.A.; Ehrman, J.K.; Michos, E.D.; Blumenthal, R.S.; Nasir, K.; Qureshi, W.T.; Brawner, C.A.; et al. Sex Differences in Cardiorespiratory Fitness and All-Cause Mortality: The Henry Ford ExercIse Testing (FIT) Project. Mayo Clin. Proc. 2016, 91, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, H.; Parfitt, G.; Davison, K.; Eston, R. Validity of Submaximal Step Tests to Estimate Maximal Oxygen Uptake in Healthy Adults. Sports Med. 2016, 46, 737–750. [Google Scholar] [CrossRef]
- Smith, A.E.; Evans, H.; Parfitt, G.; Eston, R.; Ferrar, K. Submaximal Exercise-Based Equations to Predict Maximal Oxygen Uptake in Older Adults: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 1003–1012. [Google Scholar] [CrossRef]
- Kang, J.; Chaloupka, E.C.; Mastrangelo, M.A.; Biren, G.B.; Robertson, R.J. Physiological comparisons among three maximal treadmill exercise protocols in trained and untrained individuals. Eur. J. Appl. Physiol. 2001, 84, 291–295. [Google Scholar] [CrossRef]
- Chen, M.J.; Fan, X.; Moe, S.T. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: A meta-analysis. J. Sports Sci. 2002, 20, 873–899. [Google Scholar] [CrossRef]
- Hughes, D.C.; Cox, M.G.; Serice, S.; Baum, G.; Harrison, C.; Basen-Engquist, K. Using rating of perceived exertion in assessing cardiorespiratory fitness in endometrial cancer survivors. Physiother. Theory Pract. 2017, 33, 758–765. [Google Scholar] [CrossRef]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef] [Green Version]
- Eston, R.; Connolly, D. The use of ratings of perceived exertion for exercise prescription in patients receiving beta-blocker therapy. Sports Med. 1996, 21, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Mytinger, M.; Nelson, R.K.; Zuhl, M. Exercise Prescription Guidelines for Cardiovascular Disease Patients in the Absence of a Baseline Stress Test. J. Cardiovasc. Dev. Dis. 2020, 7, 15. [Google Scholar] [CrossRef]
- Zeiher, J.; Duch, M.; Kroll, L.E.; Mensink, G.B.M.; Finger, J.D.; Keil, T. Domain-specific physical activity patterns and cardiorespiratory fitness among the working population: Findings from the cross-sectional German Health Interview and Examination Survey. BMJ Open 2020, 10, e034610. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Harber, M.P.; Whaley, M.H.; Finch, W.H.; Bishop, D.L.; Kaminsky, L.A. Cardiorespiratory Fitness and Mortality in Healthy Men and Women. J. Am. Coll. Cardiol. 2018, 72, 2283–2292. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Klein, S.L.; Levin, E.R. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology 2020, 161, bqaa127. [Google Scholar] [CrossRef]
- Moolman, J.A. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc. Res. 2006, 69, 777–780. [Google Scholar] [CrossRef]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Goodman, K.E.; Magder, L.S.; Baghdadi, J.D.; Pineles, L.; Levine, A.R.; Perencevich, E.N.; Harris, A.D. Impact of Sex and Metabolic Comorbidities on COVID-19 Mortality Risk Across Age Groups: 66,646 Inpatients Across 613 U.S. Hospitals. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020. [Google Scholar] [CrossRef]
- da Silveira, M.P.; da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, E.; Rossi, R.C.; de Resende, E.S.D.T. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 2021, 21, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Dantas, W.S.; Neves, W.D.; Gil, S.; Barcellos, C.R.G.; Rocha, M.P.; de Sa-Pinto, A.L.; Roschel, H.; Gualano, B. Exercise-induced anti-inflammatory effects in overweight/obese women with polycystic ovary syndrome. Cytokine 2019, 120, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Thompson, P.D.; Al-Zaiti, S.S.; Albert, C.M.; Hivert, M.F.; Levine, B.D.; Lobelo, F.; Madan, K.; Sharrief, A.Z.; Eijsvogels, T.M.H.; et al. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks Into Perspective-An Update: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e705–e736. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Davison, G. Exercise, Immunity, and Illness. Muscle Exerc. Physiol. 2019, 317–344. [Google Scholar] [CrossRef]
- Rawson, E.S.; Clarkson, P.M.; Tarnopolsky, M.A. Perspectives on Exertional Rhabdomyolysis. Sports Med. 2017, 47, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, E.; Marinho, D.A.; Neiva, H.P.; Lourenco, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2019, 10, 1550. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Jae, S.Y. Exercise as medicine for COVID-19: An ACE in the hole? Med. Hypotheses 2020, 142, 109835. [Google Scholar] [CrossRef]
- Magalhaes, D.M.; Nunes-Silva, A.; Rocha, G.C.; Vaz, L.N.; de Faria, M.H.S.; Vieira, E.L.M.; Rocha, N.P.; Simoes, E.S.A.C. Two protocols of aerobic exercise modulate the counter-regulatory axis of the renin-angiotensin system. Heliyon 2020, 6, e03208. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Dulson, D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine 2018, 104, 136–142. [Google Scholar] [CrossRef]
- Cavalcante, P.A.M.; Gregnani, M.F.; Henrique, J.S.; Ornellas, F.H.; Araujo, R.C. Aerobic but not Resistance Exercise Can Induce Inflammatory Pathways via Toll-Like 2 and 4: A Systematic Review. Sports Med. Open 2017, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, A.; Rasmuson, J.; Vu, J.; Mulvagh, S.L.; Yip, C.Y.Y.; Norris, C.M.; Oudit, G.Y. Sex differences in COVID-19: Candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H296–H304. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher mortality of COVID-19 in males: Sex differences in immune response and cardiovascular comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, A.; Koberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldan, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019, 144, S4–S18. [Google Scholar] [CrossRef] [Green Version]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef] [PubMed]
- Witlox, L.; Schagen, S.B.; de Ruiter, M.B.; Geerlings, M.I.; Peeters, P.H.M.; Koevoets, E.W.; van der Wall, E.; Stuiver, M.; Sonke, G.; Velthuis, M.J.; et al. Effect of physical exercise on cognitive function and brain measures after chemotherapy in patients with breast cancer (PAM study): Protocol of a randomised controlled trial. BMJ Open 2019, 9, e028117. [Google Scholar] [CrossRef]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; Cohen, D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, J.; Moon, S.Y.; Jin, H.Y.; Yang, J.M.; Ogino, S.; Song, M.; Hong, S.H.; Abou Ghayda, R.; Kronbichler, A.; et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: A nationwide cohort study. Br. J. Sports Med. 2021. [Google Scholar] [CrossRef]
- Tahaghoghi-Hajghorbani, S.; Zafari, P.; Masoumi, E.; Rajabinejad, M.; Jafari-Shakib, R.; Hasani, B.; Rafiei, A. The role of dysregulated immune responses in COVID-19 pathogenesis. Virus Res. 2020, 290, 198197. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Gronesova, P.; Cholujova, D.; Kozic, K.; Korbuly, M.; Vlcek, M.; Penesova, A.; Imrich, R.; Sedlak, J.; Hunakova, L. Effects of short-term Pilates exercise on selected blood parameters. Gen. Physiol. Biophys. 2018, 37, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.A.M.; Dantas, P.M.S.; Dos Santos, I.K.; Dantas, M.; da Silva, D.C.P.; Cabral, B.; Guerra, R.O.; Junior, G.B.C. Effect of Acute and Chronic Aerobic Exercise on Immunological Markers: A Systematic Review. Front. Physiol. 2019, 10, 1602. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Impact of aerobic versus resisted exercise training on systemic inflammation biomarkers and quality of Life among obese post-menopausal women. Afr. Health Sci. 2019, 19, 2881–2891. [Google Scholar] [CrossRef]
- Barros, E.S.; Nascimento, D.C.; Prestes, J.; Nobrega, O.T.; Cordova, C.; Sousa, F.; Boullosa, D.A. Acute and Chronic Effects of Endurance Running on Inflammatory Markers: A Systematic Review. Front. Physiol. 2017, 8, 779. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Roche, J.A.; Roche, R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 2020, 34, 7265–7269. [Google Scholar] [CrossRef]
- Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Brandtzaeg, P.; Kiyono, H.; Pabst, R.; Russell, M.W. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008, 1, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Pilette, C.; Ouadrhiri, Y.; Godding, V.; Vaerman, J.P.; Sibille, Y. Lung mucosal immunity: Immunoglobulin-A revisited. Eur. Respir. J. 2001, 18, 571–588. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Bishop, N.C.; Gleeson, M. Acute and chronic effects of exercise on markers of mucosal immunity. Front. Biosci. 2009, 14, 4444–4456. [Google Scholar] [CrossRef] [Green Version]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.A.; Tree, J.A.; Wallace, A.; Amcheslavsky, A.; Kurt Yilmaz, N.; Buttigieg, K.R.; Elmore, M.J.; et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat. Commun. 2020, 11, 4198. [Google Scholar] [CrossRef]
- Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Viant, C.; Gaebler, C.; Cipolla, M.; Hoffmann, H.H.; Oliveira, T.Y.; Oren, D.A.; et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claer, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Wisnewski, A.V.; Campillo Luna, J.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef] [PubMed]
- See, R.H.; Zakhartchouk, A.N.; Petric, M.; Lawrence, D.J.; Mok, C.P.Y.; Hogan, R.J.; Rowe, T.; Zitzow, L.A.; Karunakaran, K.P.; Hitt, M.M.; et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J. Gen. Virol. 2006, 87, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Senchina, D.S.; Kohut, M.L. Immunological outcomes of exercise in older adults. Clin. Interv. Aging 2007, 2, 3–16. [Google Scholar] [CrossRef]
- Rouw, A.; Wexler, A.; Kates, J.; Michaud, J. Global COVID-19 Vaccine Access: A Snapshot of Inequality. Available online: https://www.kff.org/policy-watch/global-covid-19-vaccine-access-snapshot-of-inequality/ (accessed on 16 July 2021).
- Burki, T. Global COVID-19 vaccine inequity. Lancet. Infect. Dis. 2021, 21, 922–923. [Google Scholar] [CrossRef]
- Akimoto, T.; Kumai, Y.; Akama, T.; Hayashi, E.; Murakami, H.; Soma, R.; Kuno, S.; Kono, I. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br. J. Sports Med. 2003, 37, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, M.M.; Morgan, A.L.; McNevin, N.; Boardley, D.J.; Topp, R. Salivary s-IgA response to training in functionally limited elders. J. Aging Phys. Act. 2003, 11, 502–515. [Google Scholar] [CrossRef]
- Wilson, E.; Donovan, C.V.; Campbell, M.; Chai, T.; Pittman, K.; Sena, A.C.; Pettifor, A.; Weber, D.J.; Mallick, A.; Cope, A.; et al. Multiple COVID-19 Clusters on a University Campus—North Carolina, August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Walsh-Messinger, J.; Manis, H.; Vrabec, A.; Sizemore Bs, J.; Bishof, K.; Debidda, M.; Malaspina, D.; Greenspan, N. The kids are not alright: A preliminary report of Post-COVID syndrome in university students. J. Am. Coll. Health 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. Eclinicalmedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.H.; Wing, Y.K.; Yu, M.W.; Leung, C.M.; Ma, R.C.; Kong, A.P.; So, W.Y.; Fong, S.Y.; Lam, S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Lian, X.; Su, X.; Wu, W.; Marraro, G.A.; Zeng, Y. From SARS and MERS to COVID-19: A brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Ghosh, S.; Klein, R.S. Sex Drives Dimorphic Immune Responses to Viral Infections. J. Immunol. 2017, 198, 1782–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.F.; Cotler, J.; Jason, L.A. Post-viral fatigue and COVID-19: Lessons from past epidemics. Fatigue 2020, 8, 61–69. [Google Scholar] [CrossRef]
- Wise, J. Long Covid: Doctors call for research and surveillance to capture disease. BMJ 2020, 370, m3586. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Galecki, P.; Maes, M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol. Neurobiol. 2014, 49, 741–756. [Google Scholar] [CrossRef]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N. Why myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may kill you: Disorders in the inflammatory and oxidative and nitrosative stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuro Endocrinol. Lett. 2009, 30, 677–693. [Google Scholar]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- Wilshire, C.E.; Kindlon, T.; Courtney, R.; Matthees, A.; Tuller, D.; Geraghty, K.; Levin, B. Rethinking the treatment of chronic fatigue syndrome-a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychol. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, diagnosis and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science (ECSS) and the American College of Sports Medicine (ACSM). Eur. J. Sport Sci. 2013, 13, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and post-infective fatigue syndrome—A review. Open Forum Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Treatment of ME/CFS. Available online: https://www.cdc.gov/me-cfs/treatment/index.html (accessed on 26 July 2021).
- Hasson, R.; Sallis, J.F.; Coleman, N.; Kaushal, N.; Nocera, V.G.; Keith, N. COVID-19: Implications for Physical Activity, Health Disparities, and Health Equity. Am. J. Lifestyle Med. 2021. [Google Scholar] [CrossRef]
- American College of Sports Medicine (ACSM). Staying Physically Active during the COVID-19 Pandemic. Available online: https://www.acsm.org/read-research/newsroom/news-releases/news-detail/2020/03/16/staying-physically-active-during-covid-19-pandemic (accessed on 10 September 2021).
- Bourouiba, L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef]
- Chang, C.; Khurana, S.; Strodel, R.; Camp, A.; Magenheimer, E.; Hawley, N. Perceived Barriers to Physical Activity Among Low-Income Latina Women at Risk for Type 2 Diabetes. Diabetes Educ. 2018, 44, 444–453. [Google Scholar] [CrossRef]
- Craft, B.B.; Carroll, H.A.; Lustyk, M.K. Gender Differences in Exercise Habits and Quality of Life Reports: Assessing the Moderating Effects of Reasons for Exercise. Int. J. Lib. Arts Soc. Sci. 2014, 2, 65–76. [Google Scholar] [PubMed]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 26 July 2021).
- Kalil, A.C. Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA 2020, 323, 1897–1898. [Google Scholar] [CrossRef] [PubMed]
- Rome, B.N.; Avorn, J. Drug Evaluation during the Covid-19 Pandemic. N. Engl. J. Med. 2020, 382, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- BBC News (USA). Covid-19: First Vaccine Given in US as Roll-Out Begins. Available online: https://www.bbc.com/news/world-us-canada-55305720 (accessed on 26 July 2021).
- BBC News (UK). Covid: Brian Pinker 82 First to get Oxford-AstraZeneca Vaccine. Available online: https://www.bbc.com/news/uk-55525542 (accessed on 26 July 2021).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Stowe, J.; Andrews, N.; Gower, C.; Gallagher, E.; Utsi, L.; Simmons, R.; Thelwall, S.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against Hospital Admission with the Delta (B.1.617.2) Variant. Available online: https://khub.net/web/phe-national/public-library/-/document_library/v2WsRK3ZlEig/view_file/479607329 (accessed on 4 August 2021).
- World Health Organization (WHO). Regulation and Prequalification (COVID-19 Vaccines). Available online: https://www.who.int/teams/regulation-prequalification/eul/covid-19 (accessed on 30 July 2021).
- Islam, M.S.; Kamal, A.M.; Kabir, A.; Southern, D.L.; Khan, S.H.; Hasan, S.M.M.; Sarkar, T.; Sharmin, S.; Das, S.; Roy, T.; et al. COVID-19 vaccine rumors and conspiracy theories: The need for cognitive inoculation against misinformation to improve vaccine adherence. PLoS ONE 2021, 16, e0251605. [Google Scholar] [CrossRef]
- Hotez, P.; Batista, C.; Ergonul, O.; Figueroa, J.P.; Gilbert, S.; Gursel, M.; Hassanain, M.; Kang, G.; Kim, J.H.; Lall, B.; et al. Correcting COVID-19 vaccine misinformation: Lancet Commission on COVID-19 Vaccines and Therapeutics Task Force Members. EClinicalMedicine 2021, 33, 100780. [Google Scholar] [CrossRef]
- Pathak, E.B.; Menard, J.; Garcia, R. Population Age-Ineligible for COVID-19 Vaccine in the United States: Implications for State, County, and Race/Ethnicity Vaccination Targets. medRxiv 2021. [Google Scholar] [CrossRef]
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First Month of COVID-19 Vaccine Safety Monitoring—United States, December 14, 2020-January 13, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef]
- Chang, W.H. A review of vaccine effects on women in light of the COVID-19 pandemic. Taiwan J. Obs. Gynecol. 2020, 59, 812–820. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in the Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, P.Y.; Garrett, D.A.; Galal, O. Women and family health: The role of mothers in promoting family and child health. Int. J. Glob. Health Health Disparities 2005, 4, 30–42. [Google Scholar]
- Bustreo, F. She Decides on Her Health, Her Future. Available online: https://www.who.int/news-room/commentaries/detail/she-decides-on-her-health-her-future (accessed on 2 August 2021).
- Choi, H. Can You Exercise before or After You’re Vaccinated for COVID-19? Available online: https://health.clevelandclinic.org/can-you-exercise-before-or-after-youre-vaccinated-for-covid-19/ (accessed on 2 August 2021).
- Business.Govt.Nz. COVID-19 Vaccinations: Q+A for Employers. Available online: https://www.business.govt.nz/news/covid-19-vaccinations-q-a-for-employers (accessed on 2 August 2021).
- World Health Organization (WHO). The Effects of Virus Variants on COVID-19 Vaccines. Available online: https://www.who.int/news-room/feature-stories/detail/the-effects-of-virus-variants-on-covid-19-vaccines (accessed on 26 July 2021).
- Kustin, T.; Harel, N.; Finkel, U.; Perchik, S.; Harari, S.; Tahor, M.; Caspi, I.; Levy, R.; Leshchinsky, M.; Ken Dror, S.; et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat. Med. 2021, 27, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Victor, P.J.; Mathews, K.P.; Paul, H.; Mammen, J.J.; Murugesan, M. Protective Effect of COVID-19 Vaccine Among Health Care Workers During the Second Wave of the Pandemic in India. Mayo Clin. Proc. 2021, 96, 2493–2494. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef] [Green Version]
- United Nations (UN). Vaccine Inequity Triggers ‘Huge Disconnect’ between Countries. Available online: https://news.un.org/en/story/2021/05/1092092 (accessed on 26 July 2021).
- World Health Organization (WHO). COVAX—Working for Global Equitable Access to COVID-19 Vaccines. Available online: https://www.who.int/initiatives/act-accelerator/covax (accessed on 26 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Pelagio, K.P.; Hew-Butler, T.; Fahlman, M.M.; Roche, J.A. Women’s Lives Matter—The Critical Need for Women to Prioritize Optimal Physical Activity to Reduce COVID-19 Illness Risk and Severity. Int. J. Environ. Res. Public Health 2021, 18, 10271. https://doi.org/10.3390/ijerph181910271
Garcia-Pelagio KP, Hew-Butler T, Fahlman MM, Roche JA. Women’s Lives Matter—The Critical Need for Women to Prioritize Optimal Physical Activity to Reduce COVID-19 Illness Risk and Severity. International Journal of Environmental Research and Public Health. 2021; 18(19):10271. https://doi.org/10.3390/ijerph181910271
Chicago/Turabian StyleGarcia-Pelagio, Karla P., Tamara Hew-Butler, Mariane M. Fahlman, and Joseph A. Roche. 2021. "Women’s Lives Matter—The Critical Need for Women to Prioritize Optimal Physical Activity to Reduce COVID-19 Illness Risk and Severity" International Journal of Environmental Research and Public Health 18, no. 19: 10271. https://doi.org/10.3390/ijerph181910271
APA StyleGarcia-Pelagio, K. P., Hew-Butler, T., Fahlman, M. M., & Roche, J. A. (2021). Women’s Lives Matter—The Critical Need for Women to Prioritize Optimal Physical Activity to Reduce COVID-19 Illness Risk and Severity. International Journal of Environmental Research and Public Health, 18(19), 10271. https://doi.org/10.3390/ijerph181910271






