Associations between Oral Hypofunction Tests, Age, and Sex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
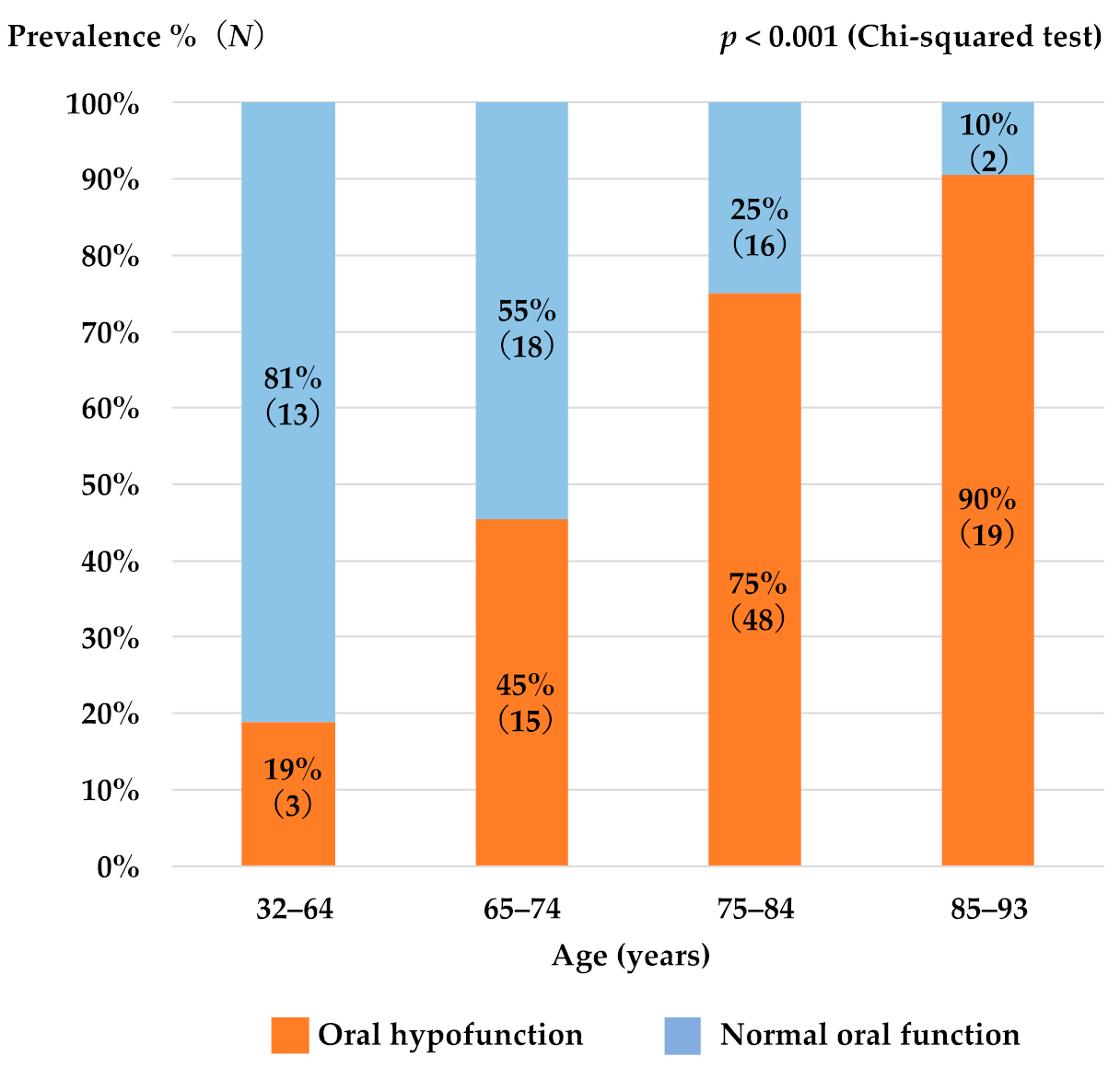
3. Results
3.1. Patient Characteristics
3.2. Factors Associated with Susceptibility to Oral Hypofunction
3.3. Correlation between Each of the Oral Hypofunction Test Item and Age
3.4. Relationship between Each Oral Hypofunction Test Item and Age, Sex, and Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research Agenda for Frailty in Older Adults: Toward a Better Understanding of Physiology and Etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef]
- Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R.; Zhou, J.; Fried, L.P. Initial Manifestations of Frailty Criteria and the Development of Frailty Phenotype in the Women’s Health and Aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 984–990. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Hirano, H.; Arai, H.; Morishita, S.; Ohara, Y.; Edahiro, A.; Murakami, M.; Shimada, H.; Kikutani, T.; Suzuki, T. Relationship between Frailty and Oral Function in Community-Dwelling Elderly Adults. J. Am. Geriatr. Soc. 2017, 65, 66–76. [Google Scholar] [CrossRef]
- Watanabe, Y.; Okada, K.; Kondo, M.; Matsushita, T.; Nakazawa, S.; Yamazaki, Y. Oral Health for Achieving Longevity. Geriatr. Gerontol. Int. 2020, 20, 526–538. [Google Scholar] [CrossRef]
- Ohara, Y.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Inagaki, H.; Motohashi, Y.; Edahiro, A.; Hirano, H.; Kitamura, A.; Awata, S.; et al. Association of Eating Alone with Oral Frailty among Community-Dwelling Older Adults in Japan. Arch. Gerontol. Geriatr. 2020, 87, 104014. [Google Scholar] [CrossRef]
- Hironaka, S.; Kugimiya, Y.; Watanabe, Y.; Motokawa, K.; Hirano, H.; Kawai, H.; Kera, T.; Kojima, M.; Fujiwara, Y.; Ihara, K.; et al. Association between Oral, Social, and Physical Frailty in Community-Dwelling Older Adults. Arch. Gerontol. Geriatr. 2020, 89, 104105. [Google Scholar] [CrossRef]
- Minakuchi, S.; Tsuga, K.; Ikebe, K.; Ueda, T.; Tamura, F.; Nagao, K.; Furuya, J.; Matsuo, K.; Yamamoto, K.; Kanazawa, M.; et al. Oral Hypofunction in the Older Population: Position Paper of the Japanese Society of Gerodontology in 2016. Gerodontology 2018, 35, 317–324. [Google Scholar] [CrossRef]
- Motokawa, K.; Mikami, Y.; Shirobe, M.; Edahiro, A.; Ohara, Y.; Iwasaki, M.; Watanabe, Y.; Kawai, H.; Kera, T.; Obuchi, S.; et al. Relationship between Chewing Ability and Nutritional Status in Japanese Older Adults: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 1216. [Google Scholar] [CrossRef] [PubMed]
- Takehara, S.; Hirani, V.; Wright, F.A.C.; Naganathan, V.; Blyth, F.M.; Le Couteur, D.G.; Waite, L.M.; Seibel, M.J.; Handelsman, D.J.; Cumming, R.G. Appetite, Oral Health and Weight Loss in Community-Dwelling Older Men: An Observational Study from the Concord Health and Ageing in Men Project (C.H.A.M.P.). BMC Geriatr. 2021, 21, 255. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, R.; Furuya, J.; Nishiyama, A.; Suzuki, H.; Aoyagi, M.; Matsubara, C.; Yoshizumi, Y.; Yoshimi, K.; Nakane, A.; Tohara, H.; et al. Structural Equation Modeling of Tongue Function and Tongue Hygiene in Acute Stroke Patients. Int. J. Environ. Res. Public Health 2021, 18, 4567. [Google Scholar] [CrossRef]
- Choo, A.; Delac, D.M.; Messer, L.B. Oral hygiene measures and promotion: Review and considerations. Aust. Dent. J. 2001, 46, 166–173. [Google Scholar] [CrossRef]
- Tan, E.C.K.; Lexomboon, D.; Sandborgh-Englund, G.; Haasum, Y.; Johnell, K. Medications That Cause Dry Mouth As an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 76–84. [Google Scholar] [CrossRef]
- JATOS Study Group. Principal Results of the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (J.A.T.O.S.). Hypertens. Res. 2008, 31, 2115–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugimiya, Y.; Iwasaki, M.; Ohara, Y.; Motokawa, K.; Edahiro, A.; Shirobe, M.; Watanabe, Y.; Obuchi, S.; Kawai, H.; Fujiwara, Y.; et al. Relationship between Oral Hypofunction and Sarcopenia in Community-Dwelling Older Adults: The Otassha Study. Int. J. Environ. Res. Public Health 2021, 18, 6666. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Ohara, Y.; Edahiro, A.; Kawai, H.; Fujiwara, Y.; Kim, H.; Ihara, K.; et al. Oral hypofunction and malnutrition among community-dwelling older adults: Evidence from the Otassha study. Gerodontology 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Nonoyama, T.; Tsushita, K.; Arai, H.; Matsushita, K.; Uchibori, N. Oral Hypofunction and Its Association with Frailty in Community-Dwelling Older People. Geriatr. Gerontol. Int. 2020, 20, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hamada, T.; Tanaka, A.; Nishi, K.; Kume, K.; Goto, Y.; Beppu, M.; Hijioka, H.; Higashi, Y.; Tabata, H.; et al. Association of Oral Hypofunction with Frailty, Sarcopenia, and Mild Cognitive Impairment: A Cross-Sectional Study of Community-Dwelling Japanese Older Adults. J. Clin. Med. 2021, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Onuki, W.; Magara, J.; Tsujimura, T.; Ito, K.; Sakai, H.; Kulvanich, S.; Nakajima, Y.; Saka, N.; Inoue, M. Survey of Oral Hypofunction in Older Outpatients at a Dental Hospital. J. Oral Rehabil. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Shimizu, T.; Ueda, T.; Sakurai, K. New method for evaluation of tongue-coating status. J. Oral. Rehabil. 2007, 34, 442–447. [Google Scholar] [CrossRef]
- Yamada, H.; Nakagawa, Y.; Nomura, Y.; Yamamoto, K.; Suzuki, M.; Watanabe, N.Y.; Saito, I.; Seto, K. Preliminary results of moisture checker for Mucus in diagnosing dry mouth. Oral Dis. 2005, 11, 405–407. [Google Scholar] [CrossRef]
- Ikebe, K.; Matsuda, K.; Morii, K.; Nokubi, T.; Ettinger, R.L. The relationship between oral function and body mass index among independently living older Japanese people. Int. J. Prosthodont. 2006, 19, 539–546. [Google Scholar] [PubMed]
- Horibe, Y.; Matsuo, K.; Ikebe, K.; Minakuchi, S.; Sato, Y.; Sakurai, K.; Ueda, T. Relationship between two pressure-sensitive films for testing reduced occlusal force in diagnostic criteria for oral hypofunction. Gerodontology 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Ergun, A.; Oder, W. Oral diadochokinesis and velocity of narrative speech: A prognostic parameter for the outcome of diffuse axonal injury in severe head trauma. Brain Inj. 2008, 22, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Mihara, Y.; Matsuda, K.I.; Hatta, K.; Gondo, Y.; Masui, Y.; Nakagawa, T.; Kamide, K.; Ishizaki, T.; Arai, Y.; Maeda, Y.; et al. Relationship between gerotranscendence and oral health-related quality of life. J. Oral. Rehabil. 2018, 45, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Utanohara, Y.; Hayashi, R.; Yoshikawa, M.; Yoshida, M.; Tsuga, K.; Akagawa, Y. Standard Values of Maximum Tongue Pressure Taken Using Newly Developed Disposable Tongue Pressure Measurement Device. Dysphagia 2008, 23, 286–290. [Google Scholar] [CrossRef]
- Shirobe, M.; Watanabe, Y.; Tanaka, T.; Hirano, H.; Kikutani, T.; Nakajo, K.; Sato, T.; Furuya, J.; Minakuchi, S.; Iijima, K. Effect of an Oral Frailty Measures Program on Community-Dwelling Elderly People: A Cluster-Randomized Controlled Trial. Gerontology 2021, 1–10, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]

| Measurement Items | Mean ± SD | |||
|---|---|---|---|---|
| Number (%) | ||||
| Overall (n = 134) | Male (n = 53) | Female (n = 81) | p-Value | |
| Age * | 75.2 ± 11.2 | 77.6 ± 9.2 | 73.6 ± 12.2 | 0.034 |
| CCI | 0.5 ± 0.8 | 0.7 ± 1.0 | 0.4 ± 0.7 | 0.077 |
| Hypertension | 50 (37.3) | 25 (18.7) | 25 (18.7) | 0.056 |
| Mental disorder | 7 (5.2) | 3 (2.2) | 4 (3.0) | 0.854 |
| Neurodegenerative disease | 1 (0.02) | 1 (0.02) | 0 (0.0) | 0.215 |
| Oral cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a |
| Oral hypofunction | 85 (63.4) | 35 (66.0) | 50 (61.7) | 0.613 |
| Oral hygiene | 28.3 ± 21.7 | 26.1 ± 22.2 | 29.6 ± 21.0 | 0.304 |
| Oral dryness | 28.0 ± 3.2 | 28.6 ± 3.4 | 27.7 ± 3.0 | 0.091 |
| Occlusal force | 563.4 ± 391.5 | 645.0 ± 398.4 | 510.0 ± 379.9 | 0.039 |
| Number of remaining teeth | 15.3 ± 9.2 | 15.1 ± 9.0 | 15.4 ± 9.4 | 0.748 |
| ODK /pa/ * | 5.9 ± 1.1 | 5.7 ± 1.0 | 6.0 ± 1.1 | 0.028 * |
| ODK /ta/ | 6.0 ± 1.0 | 5.9 ± 0.9 | 6.1 ± 1.1 | 0.132 |
| ODK /ka/ * | 5.6 ± 1.0 | 5.4 ± 0.9 | 5.7 ± 1.0 | 0.046 * |
| Tongue pressure | 26.9 ± 8.6 | 27.9 ± 8.5 | 26.2 ± 8.7 | 0.290 |
| Masticatory function | 132.6 ± 58.9 | 143.6 ± 62.1 | 125.4 ± 55.9 | 0.087 |
| Swallowing function | 1.6 ± 4.0 | 1.3 ± 3.7 | 1.8 ± 4.2 | 0.207 |
| Explanatory Variables | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age * | 1.076 | 1.006–1.151 | 0.033 * |
| Sex (0 = male, 1 = female) | 0.310 | 0.075–1.285 | 0.107 |
| CCI | 0.682 | 0.280–1.661 | 0.400 |
| Hypertension (1 = positive) | 1.548 | 0.422–5.677 | 0.510 |
| Oral hygiene | 1.154 | 0.960–1.386 | 0.127 |
| Oral dryness * | 0.636 | 0.475–0.852 | 0.002 * |
| Occlusal force * | 0.995 | 0.993–0.998 | <0.001 * |
| ODK lowest * | 0.221 | 0.085–0.577 | 0.002 * |
| Tongue pressure * | 0.890 | 0.796–0.994 | 0.038 * |
| Masticatory function * | 0.982 | 0.969–0.995 | 0.008 * |
| Swallowing function | 1.298 | 0.886–1.903 | 0.180 |
| Oral Hypofunction Tests | All (n = 134) | Male (n = 53) | Female (n = 81) | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Oral hygiene | −0.074 | 0.395 | −0.049 | 0.729 | −0.061 | 0.590 |
| Oral dryness | 0.104 | 0.232 | 0.117 | 0.403 | 0.031 | 0.786 |
| Occlusal force | −0.239 | 0.005 * | −0.199 | 0.154 | −0.305 | 0.006 * |
| Number of remaining teeth | −0.407 | <0.001 * | −0.301 | 0.029 * | −0.446 | <0.001 * |
| ODK /pa/ | −0.389 | <0.001 * | −0.318 | 0.020 * | −0.392 | <0.001 * |
| ODK /ta/ | −0.324 | <0.001 * | −0.232 | 0.094 | −0.345 | 0.002 * |
| ODK /ka/ | −0.426 | <0.001 * | −0.357 | 0.009 * | −0.454 | <0.001 * |
| Tongue pressure | −0.274 | 0.001 * | −0.413 | 0.002 * | −0.226 | 0.042 * |
| Masticatory function | −0.238 | 0.006 * | −0.184 | 0.186 | −0.316 | 0.004 * |
| Swallowing function | 0.289 | 0.001 * | 0.24 | 0.083 | 0.346 | 0.002 * |
| Oral Hypofunction Tests | Explanatory Variables | B | SE | β | p-Value | 95% CI | Variance Inflation Factor |
|---|---|---|---|---|---|---|---|
| Oral hygiene | Age | −0.006 | 0.032 | −0.018 | 0.850 | −0.069 to 0.057 | 1.113 |
| Sex | 0.595 | 0.709 | 0.076 | 0.403 | −0.807 to 1.998 | 1.070 | |
| CCI | −0.299 | 0.432 | −0.062 | 0.491 | −1.154 to 0.557 | 1.062 | |
| HT | 0.662 | 0.722 | 0.084 | 0.360 | −0.765 to 2.090 | 1.085 | |
| Oral moisture | Age | 0.005 | 0.026 | 0.019 | 0.837 | −0.046 to 0.056 | 1.113 |
| Sex | −0.885 | 0.579 | −0.138 | 0.129 | −2.031 to 0.260 | 1.070 | |
| CCI | −0.014 | 0.353 | −0.003 | 0.969 | −0.712 to 0.685 | 1.062 | |
| HT | 0.198 | 0.589 | 0.030 | 0.738 | −0.968 to 1.364 | 1.085 | |
| Occlusal force | Age * | −9.031 | 3.044 | −0.259 | 0.004 * | −15.055 to −3.008 | 1.113 |
| Sex * | −175.828 | 68.258 | −0.220 | 0.011 * | −310.879 to −40.777 | 1.070 | |
| CCI | −63.037 | 41.636 | −0.129 | 0.132 | −145.415 to 19.342 | 1.062 | |
| HT | 79.469 | 69.478 | 0.099 | 0.255 | −57.994 to 216.932 | 1.085 | |
| Number of remaining teeth | Age * | −0.345 | 0.069 | −0.420 | <0.001 * | −0.482 to −0.208 | 1.113 |
| Sex | −1.025 | 1.553 | −0.055 | 0.510 | −4.097 to 2.047 | 1.070 | |
| CCI | −0.727 | 0.947 | −0.063 | 0.444 | −2.601 to 1.147 | 1.062 | |
| HT | 1.029 | 1.580 | 0.054 | 0.516 | −2.098 to 4.155 | 1.085 | |
| ODK/pa/ | Age * | −0.033 | 0.008 | −0.346 | <0.001 * | −0.049 to −0.017 | 1.113 |
| Sex | 0.151 | 0.181 | 0.070 | 0.405 | −0.207 to 0.510 | 1.070 | |
| CCI | −0.126 | 0.111 | −0.095 | 0.257 | −0.345 to 0.093 | 1.062 | |
| HT | 0.011 | 0.184 | 0.005 | 0.954 | −0.354 to 0.376 | 1.085 | |
| ODK/ta/ | Age * | −0.028 | 0.008 | −0.302 | 0.001 * | −0.043 to −0.012 | 1.113 |
| Sex | 0.010 | 0.175 | 0.005 | 0.957 | −0.337 to 0.356 | 1.070 | |
| CCI | −0.175 | 0.107 | −0.136 | 0.104 | −0.386 to 0.037 | 1.062 | |
| HT | −0.227 | 0.178 | −0.107 | 0.204 | −0.580 to 0.125 | 1.085 | |
| ODK/ka/ | Age * | −0.030 | 0.007 | −0.345 | <0.001 * | −0.045 to −0.015 | 1.113 |
| Sex | 0.122 | 0.167 | 0.061 | 0.466 | −0.208 to 0.451 | 1.070 | |
| CCI | −0.090 | 0.102 | −0.074 | 0.377 | −0.291 to 0.111 | 1.062 | |
| HT | −0.067 | 0.170 | −0.034 | 0.692 | −0.403 to 0.268 | 1.085 | |
| Tongue pressure | Age * | −0.171 | 0.069 | −0.223 | 0.014 * | −0.307 to −0.036 | 1.113 |
| Sex | −2.745 | 1.538 | −0.156 | 0.077 | −5.787 to 0.297 | 1.070 | |
| CCI | −0.684 | 0.938 | −0.064 | 0.467 | −2.539 to 1.172 | 1.062 | |
| HT | −0.868 | 1.565 | −0.049 | 0.580 | −3.965 to 2.228 | 1.085 | |
| Masticatory function | Age * | −1.172 | 0.461 | −0.223 | 0.012 * | −2.084 to −0.260 | 1.113 |
| Sex * | −25.522 | 10.335 | −0.213 | 0.015 * | −45.969 to −5.075 | 1.070 | |
| CCI | −12.194 | 6.304 | −0.166 | 0.055 | −24.666 to 0.279 | 1.062 | |
| HT | 4.578 | 10.519 | 0.038 | 0.664 | −16.235 to 25.390 | 1.085 | |
| Swallowing function | Age * | 0.100 | 0.032 | 0.280 | 0.002* | 0.037 to 0.164 | 1.113 |
| Sex | 0.738 | 0.718 | 0.090 | 0.306 | −0.683 to 2.159 | 1.070 | |
| CCI | −0.034 | 0.438 | −0.007 | 0.939 | −0.900 to 0.833 | 1.062 | |
| HT | −0.944 | 0.731 | −0.114 | 0.199 | −2.391 to 0.502 | 1.085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatanaka, Y.; Furuya, J.; Sato, Y.; Uchida, Y.; Shichita, T.; Kitagawa, N.; Osawa, T. Associations between Oral Hypofunction Tests, Age, and Sex. Int. J. Environ. Res. Public Health 2021, 18, 10256. https://doi.org/10.3390/ijerph181910256
Hatanaka Y, Furuya J, Sato Y, Uchida Y, Shichita T, Kitagawa N, Osawa T. Associations between Oral Hypofunction Tests, Age, and Sex. International Journal of Environmental Research and Public Health. 2021; 18(19):10256. https://doi.org/10.3390/ijerph181910256
Chicago/Turabian StyleHatanaka, Yukiko, Junichi Furuya, Yuji Sato, Yoshiki Uchida, Toshiharu Shichita, Noboru Kitagawa, and Tokiko Osawa. 2021. "Associations between Oral Hypofunction Tests, Age, and Sex" International Journal of Environmental Research and Public Health 18, no. 19: 10256. https://doi.org/10.3390/ijerph181910256
APA StyleHatanaka, Y., Furuya, J., Sato, Y., Uchida, Y., Shichita, T., Kitagawa, N., & Osawa, T. (2021). Associations between Oral Hypofunction Tests, Age, and Sex. International Journal of Environmental Research and Public Health, 18(19), 10256. https://doi.org/10.3390/ijerph181910256






