Menstrual Cycle Hormonal Changes and Energy Substrate Metabolism in Exercising Women: A Perspective
Abstract
1. Introduction
- Those who believe menstrual cycle hormonal changes do affect a woman’s metabolism during exercise, and
- Those who believe the menstrual cycle hormonal changes do not affect a woman’s metabolism during exercise.
2. Research Background
2.1. Muscle Glycogen—Glucose
2.2. Substrate Oxidation
2.3. Lactate
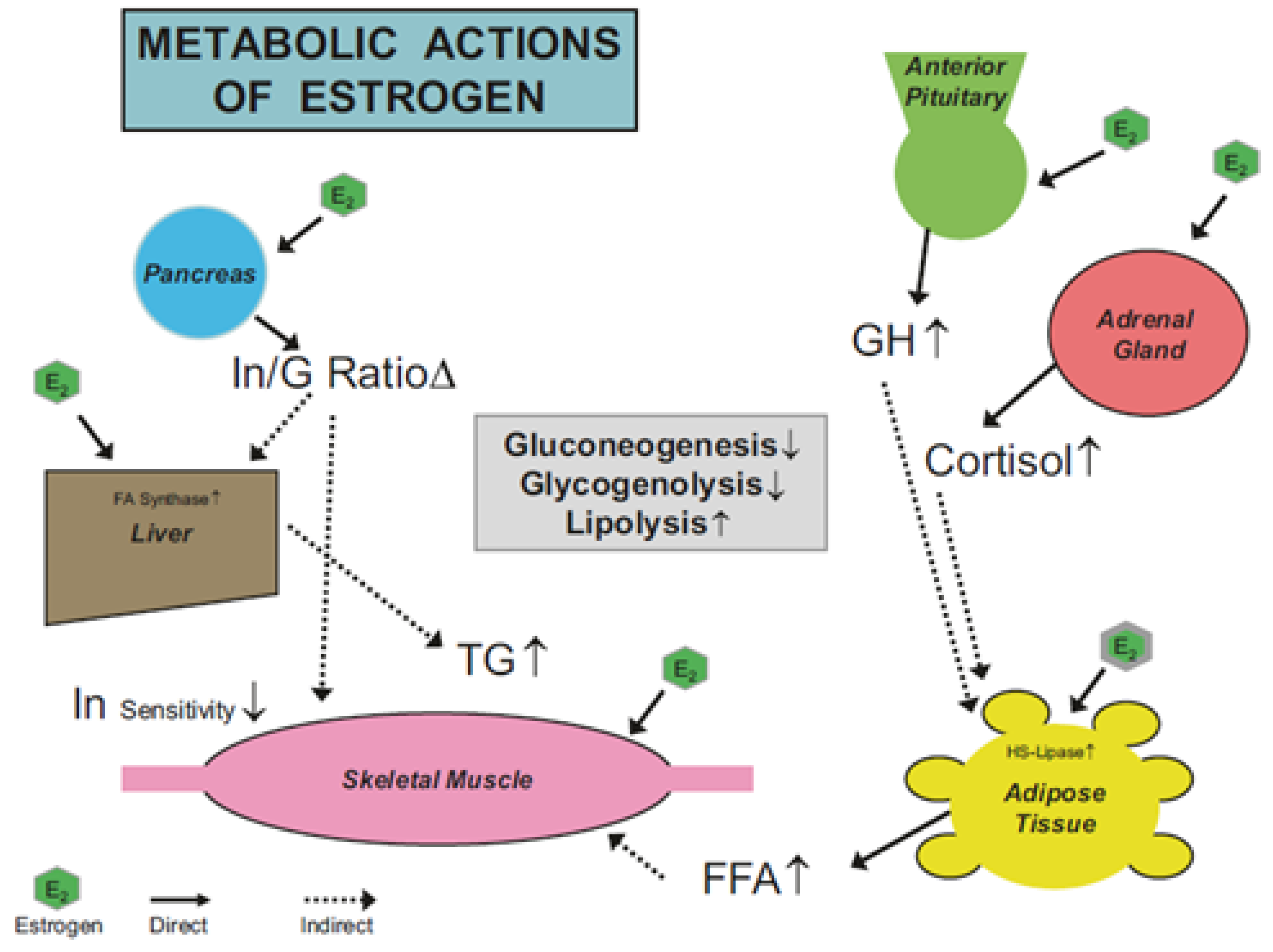
2.4. Mechanism of Estrogens Action
3. Factors Affecting Research Studies
3.1. All Women Are Not Alike and as Such, Menstrual Cycles Are Not Alike
- Cycle length varies between women (cycles can be 21 to 35 days, with 2 to 7 days of menses).
- Cycle length can vary within a woman by up to 8 days.
- Cycle phase length can also vary within women (FP = ±6 days, LP = ±4 days).
3.2. What’s in A Name?—Menstrual Cycle Terminology Confusion
3.3. Inadequate Screening for Medical Conditions
3.4. A Hormone Is a Hormone, but Biological Specimens Differ
- Saliva is good for assessing free levels of FSSH, while in blood you can examine free, bound, and total levels of such hormones.
- Saliva and blood hormone levels are not always in perfect equilibrium and therefore do not always reflect each other (i.e., efflux from the blood to the saliva can be impeded).
- Not all hormones can be measured in saliva (i.e., molecular weight prevents transport into the saliva).
- Contamination of saliva specimens can be a problem (e.g., blood from brushing teeth, food particles).
- Reference standards for saliva hormone levels are not well established yet.
- Historically early salivary hormonal assays used in prior research studies were problematic, lacking good sensitivity and accuracy.
3.5. Statistical Miss-Steps
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mujika, I.; Taipale, R. Sport Science on Women, Women in Sport Science. Int. J. Sports Physiol. Perform. 2019, 14, 1013–1014. [Google Scholar] [CrossRef] [PubMed]
- Stachenfeld, N.S. Including women in research. It’s necessary, and really not so hard to do. Exp. Physiol. 2018, 103, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Heather, A.K. Myths and Methodologies: Reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp. Physiol. 2018, 103, 1309–1317. [Google Scholar] [CrossRef]
- Bunt, J.C. Metabolic actions of estradiol: Significance for acute and chronic exercise responses. Med. Sci. Sports Exerc. 1990, 22, 286–290. [Google Scholar] [CrossRef]
- Davis, H.C.; Hackney, A.C. The hypothalamic-pituitary-ovarian axis and oral contraceptives: Regulation and function. In Sex Hormones, Exercise and Women; Hackney, A.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–18. [Google Scholar]
- Newell-Fugate, A.E. The role of sex steroids in white adipose tissue adipocyte function. Reproduction 2017, 153, R133–R149. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; McCracken-Compton, M.A.; Ainsworth, B. Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle. Int. J. Sport Nutr. 1994, 4, 299–308. [Google Scholar] [CrossRef]
- Costill, D.L.; Hargreaves, M. Carbohydrate Nutrition and Fatigue1. Sports Med. 1992, 13, 86–92. [Google Scholar] [CrossRef]
- Wolfe, R.R. Fat metabolism in exercise. Adv. Exp. Med. Biol. 1998, 441, 147–156. [Google Scholar] [PubMed]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.A.K.J.; Ackerman, K.E.; Sipilä, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological considerations for studies in sport and exercise science with women as participants: A working guide for standards of practice for research on women. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Koenig, R.; Rosen, C.; Auchus, R.; Goldfine, A. William’s Textbook of Endocrinology, 14th ed.; Elsevier Publishing: New York, NY, USA, 2019. [Google Scholar]
- Isacco, L.; Boisseau, N. Sex hormones and substrate metabolism during endurance exercise. In Sex Hormones, Exercise and Women: Scientific and Clinical Aspects; Hackney, A.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 35–58. [Google Scholar]
- Hackney, A.C. Effects of the menstrual cycle on resting muscle glycogen content. Horm. Metab. Res. 1990, 22, 647. [Google Scholar] [CrossRef]
- Chappell, S.; Hackney, A.C. Association between menstrual cycle phase, physical activity level and dietary macro-nutrient intake. Biol. Sport 1997, 14, 251–258. [Google Scholar]
- Beckett, T.; Tchernof, A.; Toth, M.J. Effect of ovariectomy and estradiol replacement on skeletal muscle enzyme activity in female rats. Metabolism 2002, 51, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Hackney, A.C.; Sharp, R.L. The Menstrual Cycle and Exercise: Performance, Muscle Glycogen, and Substrate Responses. Int. J. Sports Med. 1989, 10, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. Influence of oestrogen on muscle glycogen utilization during exercise. Acta Physiol. Scand. 1999, 167, 273–274. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1120–R1128. [Google Scholar] [CrossRef] [PubMed]
- Zderic, T.W.; Coggan, A.R.; Ruby, B.C. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J. Appl. Physiol. 2001, 90, 447–453. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrhoeic women. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef]
- Horton, T.J.; Miller, E.K.; Glueck, D.; Tench, K. No effect of menstrual cycle phase on glucose kinetics and fuel oxidation during moderate-intensity exercise. Am. J. Physiol. Metab. 2002, 282, E752–E762. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; Muoio, D.; Meyer, W.R. The effect of sex steroid hormones on substrate oxidation during prolonged submaximal exercise in women. Jpn. J. Physiol. 2000, 50, 489–494. [Google Scholar] [CrossRef][Green Version]
- Hackney, A.C.; Curley, C.S.; Nicklas, B.J. Physiological responses to submaximal exercise at the mid-follicular, ovulatory and mid-luteal phases of the menstrual cycle. Scand. J. Med. Sci. Sports 1991, 1, 94–98. [Google Scholar] [CrossRef]
- McCracken, M.; Ainsworth, B.; Hackney, A.C. Effects of the menstrual cycle phase on the blood lactate responses to exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 1994, 69, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Berend, J.Z.; Brammeier, M.R.; Jones, N.A.; Holliman, S.C.; Hackney, A.C. Effect of the menstrual cycle phase and diet on blood lactate responses to exercise. Biol. Sport 1994, 11, 241–248. [Google Scholar] [PubMed]
- Wenz, M.; Berend, J.Z.; Lynch, N.A.; Chappell, S.; Hackney, A.C. Substrate oxidation at rest and during exercise: Effects of menstrual cycle phase and diet composition. J. Physiol. Pharmacol. 1997, 48, 851–860. [Google Scholar] [PubMed]
- Lariviere, F.; Moussalli, R.; Garrel, D.R. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am. J. Physiol. Metab. 1994, 267, E422–E428. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, J.E.; Jones, N.L.; Toews, C.J.; Sutton, J.R. Effects of menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J. Appl. Physiol. 1981, 51, 1493–1499. [Google Scholar] [CrossRef]
- Mesaki, N.; Sasaki, J.; Shoji, M.; Iwasaki, H.; Asano, K.; Eda, M. Effect of menstrual cycle on cardiorespiratory system during incremental exercise. Nihon Sanka Fujinka Gakkai Zasshi 1986, 38, 1–9. [Google Scholar]
- Dombovy, M.L.; Bonekat, H.W.; Williams, T.J.; Staats, B.A. Exercise performance and ventilatory response in the menstrual cycle. Med. Sci. Sports Exerc. 1987, 19, 111–117. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. Oestrogen’s regulation of fat metabolism during exercise and gender specific effects. Curr. Opin. Pharmacol. 2012, 12, 363–371. [Google Scholar] [CrossRef]
- Bessinger, R.C.; McMurray, R.G.; Hackney, A.C. Substrate utilization and hormonal responses to moderate intensity exercise during pregnancy and after delivery. Am. J. Obstet. Gynecol. 2002, 186, 757–764. [Google Scholar] [CrossRef]
- Campbell, S.E.; Febbraio, M.A. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am. J. Physiol. 2002, 282, E1139–E4640. [Google Scholar] [CrossRef]
- Campbell, S.E.; Febbraio, M.A. Effect of ovarian hormones on mitochondrial enzyme activity in fat oxidation pathway of skeletal muscle. Am. J. Physiol. 2001, 281, E803–E841. [Google Scholar] [CrossRef]
- Hatta, H.; Atomi, Y.; Shinohara, S.; Yamamoto, Y.; Yamada, S. The Effects of Ovarian Hormones on Glucose and Fatty Acid Oxidation during Exercise in Female Ovariectomized Rats. Horm. Metab. Res. 1988, 20, 609–611. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Sharoff, C.; Chipkin, S.R.; Grow, D.; Ruby, B.C.; Braun, B. Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1046–E1055. [Google Scholar] [CrossRef]
- Hacker, N.F.; Gambone, J.C.; Hobel, C.J. (Eds.) Hacker & Moore’s Essentials of Obstetrics and Gynecology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Cole, L.A.; Ladner, D.G.; Byrn, F.W. The normal variabilities of the menstrual cycle. Fertil. Steril. 2009, 91, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, U.M. Importance of daily sex hormone measurements within the menstrual cycle for fertility estimates in cyclical shifts studies. Evol. Psychol. 2020, 18, 1474704919897913. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Deswal, R.; Narwal, V.; Dang, A. The prevalence of polycystic ovary syndrome: A brief systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Liepa, G.U.; Sengupta, A.; Karsies, D. Polycystic ovary syndrome (PCOS) and other androgen excess–related conditions: Can changes in dietary intake make a difference? Nutr. Clin. Pract. 2008, 23, 63–71. [Google Scholar] [CrossRef]
- Hackney, A.C.; Viru, A. Research methodology: Endocrinologic measurements in exercise science and sports Medicine. J. Athl. Train. 2008, 43, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Streckfus, C.F. Advances in Salivary Diagnostics; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wilson, D.W.; Walker, R.F.; Griffiths, K. Saliva as a medium for chronobiological studies: Its particular potential in steroid endocrinology. Ann.-Ist. Super. Sanita 1993, 29, 607–611. [Google Scholar] [PubMed]
- Viru, A.; Hackney, A.C.; Valja, E.; Karelson, K.; Janson, T.; Viru, M. Influence of prolonged continuous exercise on hormonal responses to subsequent intensive exercise. Eur. J. Appl. Physiol. 2001, 85, 578–585. [Google Scholar] [CrossRef]
- Nathalie, B.; Laurie, I. Substrate metabolism during exercise: Sexual dimorphism and women’s specificities. Eur. J. Sport Sci. 2021, 17, 1–21. [Google Scholar]
- Romijn, J.A.; Coyle, E.F.; Hibbert, J.; Wolfe, R.R. Comparison of indirect calorimetry and a new breath 13C/12C ratio method during strenuous exercise. Am. J. Physiol. 1992, 263 Pt 1, E64–E71. [Google Scholar] [CrossRef]
- Solli, G.S.; Sandbakk, S.B.; Noordhof, D.A.; Ihalainen, J.K.; Sandbakk, Ø. Changes in self-reported physical fitness, performance, and side effects across the phases of the menstrual cycle among competitive endurance athletes. Int. J. Sports Physiol. Perform. 2020, 15, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Sygo, J.; Morton, J.P. Fuelling the female athlete: Carbohydrate and protein recommendations. Eur. J. Sport Sci. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackney, A.C. Menstrual Cycle Hormonal Changes and Energy Substrate Metabolism in Exercising Women: A Perspective. Int. J. Environ. Res. Public Health 2021, 18, 10024. https://doi.org/10.3390/ijerph181910024
Hackney AC. Menstrual Cycle Hormonal Changes and Energy Substrate Metabolism in Exercising Women: A Perspective. International Journal of Environmental Research and Public Health. 2021; 18(19):10024. https://doi.org/10.3390/ijerph181910024
Chicago/Turabian StyleHackney, Anthony C. 2021. "Menstrual Cycle Hormonal Changes and Energy Substrate Metabolism in Exercising Women: A Perspective" International Journal of Environmental Research and Public Health 18, no. 19: 10024. https://doi.org/10.3390/ijerph181910024
APA StyleHackney, A. C. (2021). Menstrual Cycle Hormonal Changes and Energy Substrate Metabolism in Exercising Women: A Perspective. International Journal of Environmental Research and Public Health, 18(19), 10024. https://doi.org/10.3390/ijerph181910024




