Estrogen-Receptor-Positive Breast Cancer in Postmenopausal Women: The Role of Body Composition and Physical Exercise
Abstract
:1. Introduction
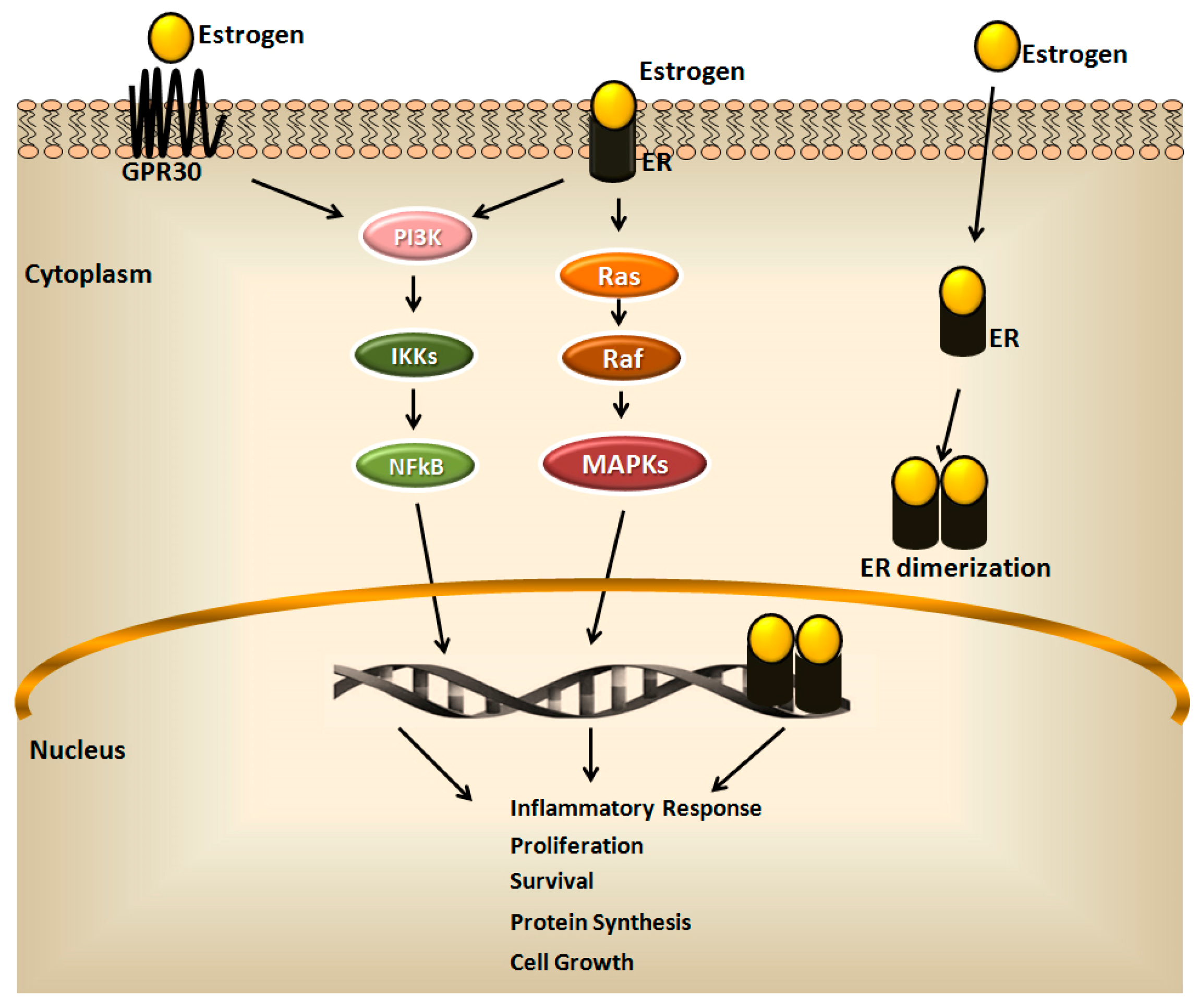
2. Estrogen Receptors in Breast Cancer and Their Clinical Implications
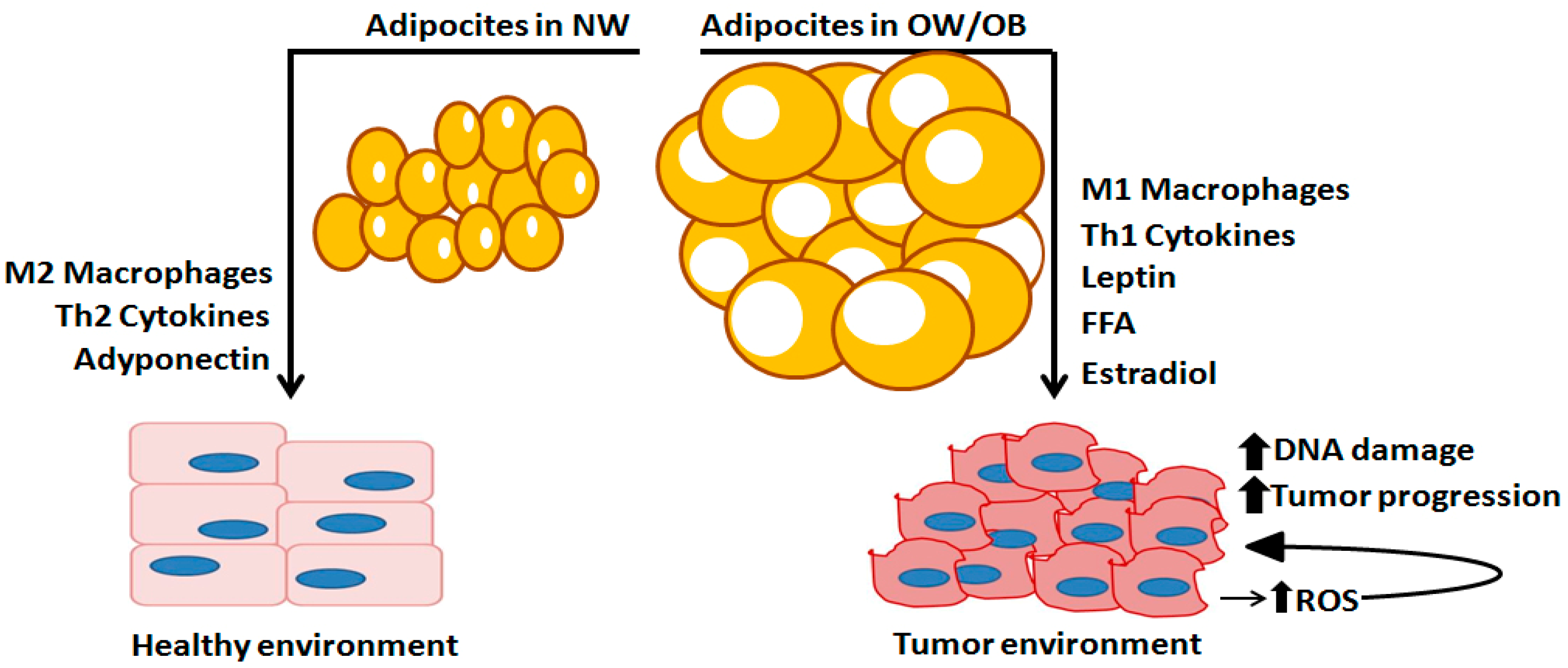
3. The Role of Adipose Tissue
4. Physical Activity and Physical Exercise in Breast Cancer Prevention
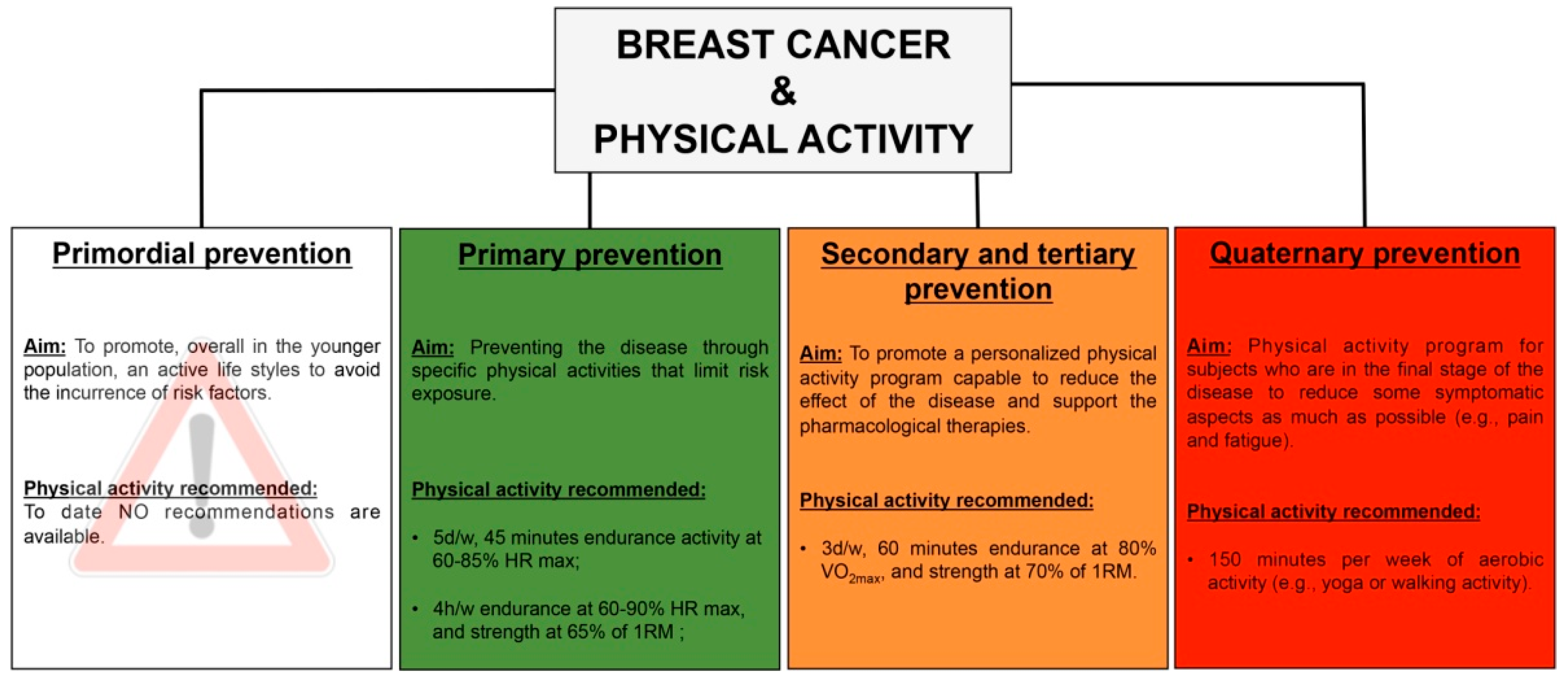
- Primordial prevention—It consists in programs and campaigns, usually addressed to the younger population, aimed at promoting a healthy lifestyle and avoiding the incurrence of risk factors;
- Primary prevention—It consists in measures, addressed to a susceptible but healthy population, aimed at preventing a disease through specific activities that limit risk exposure or increase the immunity;
- Secondary prevention—It consists in procedures that increase the early disease detection, and its target is healthy-appearing individuals with subclinical forms of the disease, and often occurs in the form of screenings. The objective is the early identification of sick or high-risk subjects to achieve healing or prevent the onset and progression of the disease;
- Tertiary prevention—It targets both clinical and outcome stages of a disease, with the aim to reduce the severity of the disease as well as of any associated consequences, and to reduce the effects of the disease once established in an individual, through a tailored rehabilitation program;
- Quaternary prevention—It consist in practice able to protect patients from medical interventions that cause more harm than benefits, due to the over-treatment condition or final-stage of the disease.
5. Physical Activity and Exercise as Fundamental Approaches within ER-Positive Breast Cancer at Each Disease Prevention Level
5.1. Primary Prevention
5.2. Secondary and Tertiary Prevention
5.3. Quaternary Prevention
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Harbeck, N. Advances in targeting HER2-positive breast cancer. Curr. Opin. Obs. Gynecol. 2018, 30, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, M.R.; Agahozo, M.C.; Koppert, L.B.; van Deurzen, C.H.M. A retrospective alternative for active surveillance trials for ductal carcinoma in situ of the breast. Int. J. Cancer 2020, 146, 1189–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheang, M.C.; Martin, M.; Nielsen, T.O.; Prat, A.; Voduc, D.; Rodriguez-Lescure, A.; Ruiz, A.; Chia, S.; Shepherd, L.; Ruiz-Borrego, M.; et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 2015, 20, 474–482. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Breast Cancer Facts & Figures 2019–2020; American Cancer Society, Inc.: Atlanta, GA, USA, 2019. [Google Scholar]
- Carreira, H.; Williams, R.; Funston, G.; Stanway, S.; Bhaskaran, K. Associations between breast cancer survivorship and adverse mental health outcomes: A matched population-based cohort study in the United Kingdom. PLoS Med. 2021, 18, e1003504. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Moses, M.A. ADAM12 induces estrogen-independence in breast cancer cells. Breast Cancer Res. Treat. 2012, 131, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Siersbæk, R.; Kumar, S.; Carroll, J.S. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018, 32, 1141–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Warner, M.; Gustafsson, J.Å. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol. Cell Endocrinol. 2015, 15, 240–244. [Google Scholar] [CrossRef]
- Zekas, E.; Prossnitz, E.R. Estrogen-mediated inactivation of FOXO3a by the G protein-coupled estrogen receptor GPER. BMC Cancer 2015, 15, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. Methods Mol. Biol. 2016, 1366, 1–10. [Google Scholar]
- Girgert, R.; Emons, G.; Gründker, C. Estrogen Signaling in ERα-Negative Breast Cancer: ERβ and GPER. Front. Endocrinol. 2019, 9, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gérard, C.; Brown, K.A. Obesity and breast cancer—Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell Endocrinol. 2018, 466, 15–30. [Google Scholar] [CrossRef] [PubMed]
- NIH Common Fund. Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2013; pp. 2004–2013. [Google Scholar]
- Saczko, J.; Michel, O.; Chwiłkowska, A.; Sawicka, E.; Mączyńska, J.; Kulbacka, J. Estrogen Receptors in Cell Membranes: Regulation and Signaling. Adv. Anat. Embryol. Cell Biol. 2017, 227, 93–105. [Google Scholar] [PubMed]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, C.; Cheng, G.J.; Saji, S.; Zelada-Hedman, M.; Wärri, A.; Weihua, Z.; Van Noorden, S.; Wahlstrom, T.; Coombes, R.C.; Warner, M.; et al. Estrogen receptor beta in breast cancer. Endocr. Relat. Cancer 2002, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Speirs, V.; Skliris, G.P.; Burdall, S.E.; Carder, P.J. Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J. Clin. Pathol. 2002, 55, 371–374. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X. The role of estrogen receptor beta in breast cancer. Biomark Res. 2020, 8, 39. [Google Scholar] [CrossRef]
- Deng, Y.; Miki, Y.; Nakanishi, A. Estradiol/GPER affects the integrity of mammary duct-like structures in vitro. Sci. Rep. 2020, 10, 1386. [Google Scholar] [CrossRef] [Green Version]
- Fox, E.M.; Davis, R.J.; Shupnik, M.A. ERbeta in breast cancer--onlooker, passive player, or active protector? Steroids 2008, 11, 1039–1051. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Li, Y.; Dong, Y.; Liang, Y.; Qu, H.; Qi, D.; Lu, Y.; Jin, X.; Guo, Y.; Jia, Y.; et al. Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J. Exp. Clin. Cancer Res. 2019, 38, 354. [Google Scholar] [CrossRef] [Green Version]
- Pupo, M.; Maggiolini, M.; Musti, A.M. GPER Mediates Non-Genomic Effects of Estrogen. Methods Mol. Biol. 2016, 1366, 471–488. [Google Scholar]
- Anbalagan, M.; Rowan, B.G. Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer. Mol. Cell Endocrinol. 2015, 418, 264–272. [Google Scholar] [CrossRef]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; Masushige, S.; Gotoh, Y.; Nishida, E.; Kawashima, H.; et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270, 1491–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunone, G.; Briand, P.A.; Miksicek, R.J.; Picard, D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996, 15, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Stavro, P.M.; Thompson, L.U. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr. Cancer 2002, 43, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lupien, M.; Meyer, C.A.; Bailey, S.T.; Eeckhoute, J.; Cook, J.; Westerling, T.; Zhang, X.; Carroll, J.S.; Rhodes, D.R.; Liu, X.S.; et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 2010, 24, 2219–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnani, L.; Patten, D.K.; Nguyen, V.T.; Hong, S.P.; Steel, J.H.; Patel, N.; Lombardo, Y.; Faronato, M.; Gomes, A.R.; Woodley, L.; et al. The pioneer factor PBX1 is a novel driver of metastatic progression in ERα-positive breast cancer. Oncotarget 2015, 6, 21878–21891. [Google Scholar] [CrossRef]
- Harrod, A.; Fulton, J.; Nguyen, V.T.M.; Periyasamy, M.; Ramos-Garcia, L.; Lai, C.F.; Metodieva, G.; de Giorgio, A.; Williams, R.L.; Santos, D.B.; et al. Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene 2017, 36, 2286–2296. [Google Scholar] [CrossRef] [Green Version]
- Jeselsohn, R.; Bergholz, J.S.; Pun, M.; Cornwell, M.; Liu, W.; Nardone, A.; Xiao, T.; Li, W.; Qiu, X.; Buchwalter, G.; et al. Allele-Specific Chromatin Recruitment and Therapeutic Vulnerabilities of ESR1 Activating Mutations. Cancer Cell 2018, 33, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalides, R.; Griekspoor, A.; Balkenende, A.; Verwoerd, D.; Janssen, L.; Jalink, K.; Floore, A.; Velds, A.; van’t Veer, L.; Neefjes, J. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell 2004, 5, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.A.; Mazumdar, A.; Vadlamudi, R.K.; Kumar, R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 2002, 21, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, R.; Flach, K.; Bentin Toaldo, C.; Alexi, X.; Canisius, S.; Neefjes, J.; Michalides, R.; Zwart, W. PKA phosphorylation redirects ERα to promoters of a unique gene set to induce tamoxifen resistance. Oncogene 2013, 32, 3543–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stender, J.D.; Nwachukwu, J.C.; Kastrati, I.; Kim, Y.; Strid, T.; Yakir, M.; Srinivasan, S.; Nowak, J.; Izard, T.; Rangarajan, E.S.; et al. Structural and Molecular Mechanisms of Cytokine-Mediated Endocrine Resistance in Human Breast Cancer Cells. Mol. Cell 2017, 65, 1122–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado, A.; Holmes, K.A.; Ross-Innes, C.S.; Schmidt, D.; Carroll, J.S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat. Genet. 2011, 43, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.K.; Lin, Z.H.; Chang, C.W.; Varang, V.; Chng, K.R.; Pan, Y.F.; Yong, E.L.; Sung, W.K.; Cheung, E. AP-2γ regulates oestrogen receptor-mediated long-range chromatin interaction and gene transcription. EMBO J. 2011, 30, 2569–2581. [Google Scholar] [CrossRef]
- Theodorou, V.; Stark, R.; Menon, S.; Carroll, J.S. GATA3 acts upstream of FOXA1 in mediating ESR1 binding by shaping enhancer accessibility. Genome Res. 2013, 23, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siewierska, K.; Malicka, I.; Kobierzycki, C.; Grzegrzolka, J.; Piotrowska, A.; Paslawska, U.; Cegielski, M.; Podhorska-Okolow, M.; Dziegiel, P.; Wozniewski, M. Effect of Physical Training on the Levels of Sex Hormones and the Expression of Their Receptors in Rats With Induced Mammary Cancer in Secondary Prevention Model—Preliminary Study. In Vivo 2020, 2, 495–501. [Google Scholar] [CrossRef]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 1, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulya, A.; Kirwan, J.P. Brown and Beige Adipose Tissue: Therapy for Obesity and Its Comorbidities? Endocrinol. Metab. Clin. N. Am. 2016, 3, 605–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, S.; Ader, I.; Creff, J.; Leménager, H.; Achard, P.; Casteilla, L.; Sensebé, L.; Carrière, A.; Deschaseaux, F. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci. Rep. 2019, 1, 7250. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Kulenkampff, E.; Wolfrum, C. Adipose Tissue Stem Cells. Handb. Exp. Pharm. 2016, 233, 251–263. [Google Scholar]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells 2014, 5, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, A.; Li, Y.; Liang, R.; Li, D.; Li, B. Adipose Tissue-Resident Regulatory T Cells. Adv. Exp. Med. Biol. 2017, 1011, 153–162. [Google Scholar]
- Murawska-Ciałowicz, E. Adipose tissue—Morphological and biochemical characteristic of different depots. Postepy Hig. Med. Dosw. 2017, 71, 466–484. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 1, 1–58. [Google Scholar]
- Cleary, M.P.; Grossmann, M.E. Obesity and Breast Cancer: The Estrogen Connection. Endocrinology 2009, 150, 2537–2542. [Google Scholar] [CrossRef] [PubMed]
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5760. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Dimauro, I.; Grazioli, E.; Palombo, R.; Guidotti, F.; Fantini, C.; Sgrò, P.; De Francesco, D.; Di Luigi, L.; Capranica, L.; et al. Exercise-mediated downregulation of MALAT1 expression and implications in primary and secondary cancer prevention. Free Radic. Biol. Med. 2020, 160, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Paronetto, M.P.; Caporossi, D. Exercise, redox homeostasis and the epigenetic landscape. Redox Biol. 2020, 35, 101477. [Google Scholar] [CrossRef]
- Grazioli, E.; Dimauro, I.; Mercatelli, N.; Wang, G.; Pitsiladis, Y.; Di Luigi, L.; Caporossi, D. Physical activity in the prevention of human diseases: Role of epigenetic modifications. BMC Genom. 2017, 18, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalafi, M.; Malandish, A.; Rosenkranz, S.K. The impact of exercise training on inflammatory markers in postmenopausal women: A systemic review and meta-analysis. Exp. Gerontol. 2021, 150, 111398. [Google Scholar] [CrossRef]
- Sgrò, P.; Emerenziani, G.P.; Antinozzi, C.; Sacchetti, M.; Di Luigi, L. Exercise as a drug for glucose management and prevention in type 2 diabetes mellitus. Curr. Opin. Pharm. 2021, 59, 95–102. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Enikolopov, G.N.; Mintz, A.; Delbono, O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013, 16, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 5, 275–297. [Google Scholar] [CrossRef]
- Jaworski, K.; Sarkadi-Nagy, E.; Duncan, R.E.; Ahmadian, M.; Sul, H.S. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 1, G1–G4. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Potter, V.J.; Zidon, T.M.; Padilla, J. Exercise and Estrogen Make Fat Cells “Fit”. Exerc. Sport Sci. Rev. 2015, 43, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Dasso, N.A. How is exercise different from physical activity? A concept analysis. Nurs. Forum 2019, 54, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- van Waart, H.; Stuiver, M.M.; van Harten, W.H.; Geleijn, E.; Kieffer, J.M.; Buffart, L.M.; de Maaker-Berkhof, M.; Boven, E.; Schrama, J.; Geenen, M.M.; et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J. Clin. Oncol. 2015, 33, 1918–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travier, N.; Velthuis, M.J.; Steins Bisschop, C.N.; van den Buijs, B.; Monninkhof, E.M.; Backx, F.; Los, M.; Erdkamp, F.; Bloemendal, H.J.; Rodenhuis, C.; et al. Effects of an 18-week exercise programme started early during breast cancer treatment: A randomised controlled trial. BMC Med. 2015, 13, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijwel, S.; Cardinale, D.A.; Norrbom, J.; Chapman, M.; Ivarsson, N.; Wengström, Y.; Sundberg, C.J.; Rundqvist, H. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J. 2018, 32, 5495–5505. [Google Scholar] [CrossRef] [Green Version]
- Thorsen, L.; Skovlund, E.; Strømme, S.B.; Hornslien, K.; Dahl, A.A.; Fosså, S.D. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J. Clin. Oncol. 2005, 23, 2378–2388. [Google Scholar] [CrossRef]
- Rezende, L.F.M.; Sá, T.H.; Markozannes, G.; Rey-López, J.P.; Lee, I.M.; Tsilidis, K.K.; Ioannidis, J.P.A.; Eluf-Neto, J. Physical activity and cancer: An umbrella review of the literature including 22 major anatomical sites and 770,000 cancer cases. Br. J. Sports Med. 2018, 52, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, Q.; Zhang, Y.; Xie, Q.; Tan, X. Physical Activity and Risk of Breast Cancer: A Meta-Analysis of 38 Cohort Studies in 45 Study Reports. Value Health 2019, 22, 104–128. [Google Scholar] [CrossRef] [Green Version]
- Grazioli, E.; Cerulli, C.; Dimauro, I.; Moretti, E.; Murri, A.; Parisi, A. New Strategy of Home-Based Exercise during Pandemic COVID-19 in Breast Cancer Patients: A Case Study. Sustainability 2020, 12, 6940. [Google Scholar] [CrossRef]
- Natalucci, V.; Marini, C.F.; Flori, M.; Pietropaolo, F.; Lucertini, F.; Annibalini, G.; Vallorani, L.; Sisti, D.; Saltarelli, R.; Villarini, A.; et al. Effects of a Home-Based Lifestyle Intervention Program on Cardiometabolic Health in Breast Cancer Survivors during the COVID-19 Lockdown. J. Clin. Med. 2021, 10, 2678. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Di Filippo, E.S.; Bondi, D.; Verratti, V.; Doria, C.; Caporossi, D.; Sabatini, S.; Dimauro, I.; Pietrangelo, T. Corrigendum to “Endurance training improves plasma superoxide dismutase activity in healthy elderly”. Mech. Ageing Dev. 2020, 186, 111214. [Google Scholar] [CrossRef]
- Dimauro, I.; Sgura, A.; Pittaluga, M.; Magi, F.; Fantini, C.; Mancinelli, R.; Sgadari, A.; Fulle, S.; Caporossi, D. Regular exercise participation improves genomic stability in diabetic patients: An exploratory study to analyse telomere length and DNA damage. Sci. Rep. 2017, 23, 4137. [Google Scholar] [CrossRef] [Green Version]
- Pittaluga, M.; Sgadari, A.; Dimauro, I.; Tavazzi, B.; Parisi, P.; Caporossi, D. Physical exercise and redox balance in type 2 diabetics: Effects of moderate training on biomarkers of oxidative stress and DNA damage evaluated through comet assay. Oxidative Med. Cell Longev. 2015, 2015, 981242. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, E.M. Attività fisica, strumento di prevenzione e gestione delle malattie croniche [Physical activity, tool for the prevention and management of chronic diseases]. G Ital. Med. Lav. Erg. 2018, 40, 106–119. [Google Scholar]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Boshnjaku, A.; Dimauro, I.; Krasniqi, E.; Grazioli, E.; Tschan, H.; Migliaccio, S.; Di Luigi, L.; Caporossi, D. Effect of sport training on forearm bone sites in female handball and soccer players. J. Sports Med. Phys. Fit. 2016, 56, 1503–1510. [Google Scholar]
- Kisling, L.A.; Das, J.M. Prevention Strategies. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. Adv. Exp. Med. Biol. 2019, 1152, 9–29. [Google Scholar] [PubMed]
- Nomura, S.J.; Inoue-Choi, M.; Lazovich, D.; Robien, K. WCRF/AICR recommendation adherence and breast cancer incidence among postmenopausal women with and without non-modifiable risk factors. Int. J. Cancer 2016, 138, 2602–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef]
- Filippini, S.E.; Vega, A. Breast cancer genes: Beyond BRCA1 and BRCA2. Front. Biosci. 2013, 18, 1358–1372. [Google Scholar]
- Walter, K.R.; Ford, M.E.; Gregoski, M.J.; Kramer, R.M.; Knight, K.D.; Spruill, L.; Nogueira, L.M.; Krisanits, B.A.; Phan, V.; La Rue, A.C.; et al. Advanced glycation end products are elevated in estrogen receptor-positive breast cancer patients, alter response to therapy, and can be targeted by lifestyle intervention. Breast Cancer Res. Treat. 2019, 173, 559–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, M.; Honma, N. Estrogen receptor β. Arch. Pathol. Lab. Med. 2011, 135, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Linowiecka, K.; Urbanowska-Domańska, O.; Guz, J.; Foksiński, M. The potential influence of breast cancer estrogen receptors’ distribution on active DNA demethylation. Contemp. Oncol. 2019, 23, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Blaha, M.J.; Nasir, K.; Rivera, J.J.; Blumenthal, R.S. Effects of physical activity on cardiovascular disease. Am. J. Cardiol. 2012, 109, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major noncommunicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Ceci, R.; Beltran Valls, M.R.; Duranti, G.; Dimauro, I.; Quaranta, F.; Pittaluga, M.; Sabatini, S.; Caserotti, P.; Parisi, P.; Parisi, A.; et al. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014, 2, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Dimauro, I.; Scalabrin, M.; Fantini, C.; Grazioli, E.; Beltran Valls, M.R.; Mercatelli, N.; Parisi, A.; Sabatini, S.; Di Luigi, L.; Caporossi, D. Resistance training and redox homeostasis: Correlation with age-associated genomic changes. Redox Biol. 2016, 10, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Magi, F.; Dimauro, I.; Margheritini, F.; Duranti, G.; Mercatelli, N.; Fantini, C.; Ripani, F.R.; Sabatini, S.; Caporossi, D. Telomere length is independently associated with age, oxidative biomarkers, and sport training in skeletal muscle of healthy adult males. Free Radic. Res. 2018, 52, 639–647. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 10, 2920–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matelot, D.; Schnell, F.; Kervio, G.; Ridard, C.; Thillaye du Boullay, N.; Wilson, M.; Carre, F. Cardiovascular benefits of endurance training in seniors: 40 is not too late to start. Int. J. Sports Med. 2016, 37, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sigal, R.J.; Kenny, G.P.; Boulé, N.G.; Wells, G.A.; Prud’homme, D.; Fortier, M.; Reid, R.D.; Tulloch, H.; Coyle, D.; Phillips, P.; et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Jervaeus, A.; Bolam, K.A.; Norrbom, J.; Bergh, J.; Rundqvist, H.; Wengström, Y. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J. Cancer Surviv. Res. Pract. 2019, 13, 244–256. [Google Scholar] [CrossRef] [Green Version]
- De Luca, V.; Minganti, C.; Borrione, P.; Grazioli, E.; Cerulli, C.; Guerra, E.; Bonifacino, A.; Parisi, A. Effects of concurrent aerobic and strength training on breast cancer survivors: A pilot study. Public Health 2016, 136, 126–132. [Google Scholar] [CrossRef]
- Milne, H.M.; Wallman, K.E.; Gordon, S.; Courneya, K.S. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2008, 108, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.; Lane, K.; et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef]
- Coyle, Y.M.; Xie, X.J.; Lewis, C.M.; Bu, D.; Milchgrub, S.; Euhus, D.M. Role of physical activity in modulating breast cancer risk as defined by APC and RASSF1A promoter hypermethylation in nonmalignant breast tissue. Cancer Epidemiol. Biomark. Prev. 2007, 16, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.A.; Davis, J.M.; Barrilleaux, T.L.; McClellan, J.L.; Steiner, J.L.; Carmichael, M.D.; Pena, M.M.; Hebert, J.R.; Green, J.E. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine 2011, 55, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Cruz, V.; Peng, Y.; Harker-Murray, A.; Haley, B.B.; Zhao, H.; Xie, X.J.; Euhus, D. Bootcamp during neoadjuvant chemotherapy for breast cancer: A randomized pilot trial. Breast Cancer 2012, 6, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Buss, L.A.; Dachs, G.U. Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE-/- mice. J. Appl. Physiol. 2018, 124, 938–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, A.; Sligar, A.D.; Chavarria, D.; Lee, J.; Choksi, D.; Patil, N.P.; Lee, H.; Veith, A.P.; Riley, W.J.; Desai, S.; et al. Biomechanical regulation of breast cancer metastasis and progression. Sci. Rep. 2021, 11, 9838. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Sheikholeslami-Vatani, D.; Khosrobakhsh, F.; Khaledi, N. Synergistic Effects of Exercise Training and Vitamin D Supplementation on Mitochondrial Function of Cardiac Tissue, Antioxidant Capacity, and Tumor Growth in Breast Cancer in Bearing-4T1 Mice. Front. Physiol. 2021, 12, 640237. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol. Res. 2021, 10. [Google Scholar] [CrossRef]
- Eschke, R.K.; Lampit, A.; Schenk, A.; Javelle, F.; Steindorf, K.; Diel, P.; Bloch, W.; Zimmer, P. Impact of Physical Exercise on Growth and Progression of Cancer in Rodents-A Systematic Review and Meta-Analysis. Front Oncol. 2019, 5, 35. [Google Scholar] [CrossRef]
- Khori, V.; Amani Shalamzari, S.; Isanejad, A.; Alizadeh, A.M.; Alizadeh, S.; Khodayari, S.; Khodayari, H.; Shahbazi, S.; Zahedi, A.; Sohanaki, H.; et al. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: Possible underlying pathway of miR-21. Eur. J. Pharm. 2015, 765, 179–187. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; McCullough, M.; McTiernan, A.; Gansler, T.; Andrews, K.S.; et al. American cancer society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project External Report; World Cancer Research Fund: London, UK, 2018. [Google Scholar]
- Friedenreich, C.M.; Woolcott, C.G.; McTiernan, A.; Ballard-Barbash, R.; Brant, R.F.; Stanczyk, F.Z.; Terry, T.; Boyd, N.F.; Yaffe, M.J.; Irwin, M.L.; et al. Alberta physical activity and breast cancer prevention trial: Sex hormone changes in a year-long exercise intervention among postmenopausal women. J. Clin. Oncol. 2010, 28, 1458–1466. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Neilson, H.K.; O’Reilly, R.; Duha, A.; Yasui, Y.; Morielli, A.R.; Adams, S.C.; Courneya, K.S. Effects of a high vs moderate volume of aerobic exercise on adiposity outcomes in postmenopausal women: A randomized clinical trial. JAMA Oncol. 2015, 1, 766–776. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Wang, Q.; Yasui, Y.; Stanczyk, F.Z.; Duha, A.; Brenner, D.R.; Courneya, K.S. Long-term Effects of Moderate versus High Durations of Aerobic Exercise on Biomarkers of Breast Cancer Risk: Follow-up to a Randomized Controlled Trial. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.L.; Foster-Schubert, K.E.; Alfano, C.M.; Wang, C.C.; Wang, C.Y.; Duggan, C.R.; Mason, C.; Imayama, I.; Kong, A.; Xiao, L.; et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: Randomized controlled trial. J. Clin. Oncol. 2012, 30, 2314–2326. [Google Scholar] [CrossRef]
- van Gemert, W.A.; May, A.M.; Schuit, A.J.; Oosterhof, B.Y.; Peeters, P.H.; Monninkhof, E.M. Effect of Weight Loss with or without Exercise on Inflammatory Markers and Adipokines in Postmenopausal Women: The SHAPE-2 Trial, A Randomized Controlled Trial. Cancer Epidemiol. Biomark. Prev. 2016, 25, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, N.; Eskandari, Z.; Farajivafa, V.; Hanson, E.D.; Agha-Alinejad, H.; Abdollah-Pour, A.; Haghighat, S. Effect of 6 months of aerobic training on adipokines as breast cancer risk factors in postmenopausal women: A randomized controlled trial. J. Cancer Res. 2018, 14, 1336–1340. [Google Scholar]
- Matthews, C.E.; Sampson, J.N.; Brenner, D.R.; Moore, S.C.; Courneya, K.S.; Ziegler, R.G.; Friedenreich, C.M. Effects of Exercise and Cardiorespiratory Fitness on Estrogen Metabolism in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1480–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, C.; Tapsoba, J.D.; Stanczyk, F.; Wang, C.Y.; Schubert, K.F.; McTiernan, A. Long-term weight loss maintenance, sex steroid hormones, and sex hormone-binding globulin. Menopause 2019, 26, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Encabo, P.; Valadés, D.; García-Honduvilla, N.; de Cos Blanco, A.; Friedenreich, C.M.; Pérez-López, A. Exercise type and fat mass loss regulate breast cancer-related sex hormones in obese and overweight postmenopausal women. Eur. J. Appl. Physiol. 2020, 120, 1277–1287. [Google Scholar] [CrossRef]
- de Roon, M.; van Gemert, W.A.; Peeters, P.H.; Schuit, A.J.; Monninkhof, E.M. Long-term effects of a weight loss intervention with or without exercise component in postmenopausal women: A randomized trial. Prev. Med. Rep. 2016, 5, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.Y.; Blackburn, G.L.; McTiernan, A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012, 20, 1628–1638. [Google Scholar] [CrossRef] [Green Version]
- Monninkhof, E.M.; Velthuis, M.J.; Peeters, P.H.; Twisk, J.W.; Schuit, A.J. Effect of exercise on postmenopausal sex hormone levels and role of body fat: A randomized controlled trial. J. Clin. Oncol. 2009, 27, 4492–4499. [Google Scholar] [CrossRef]
- McTiernan, A.; Tworoger, S.S.; Rajan, K.B.; Yasui, Y.; Sorenson, B.; Ulrich, C.M.; Chubak, J.; Stanczyk, F.Z.; Bowen, D.; Irwin, M.L.; et al. Effect of exercise on serum androgens in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1099–1105. [Google Scholar]
- McTiernan, A.; Tworoger, S.S.; Ulrich, C.M.; Yasui, Y.; Irwin, M.L.; Rajan, K.B.; Sorenson, B.; Rudolph, R.E.; Bowen, D.; Stanczyk, F.Z.; et al. Effect of exercise on serum estrogens in postmenopausal women: A 12-month randomized clinical trial. Cancer Res. 2004, 64, 2923–2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 3, 543–568. [Google Scholar]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 6, 1281–1298. [Google Scholar] [CrossRef] [Green Version]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 6, 1320S–1329S. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basen-Engquist, K.; Carmack, C.; Brown, J.; Jhingran, A.; Baum, G.; Song, J.; Scruggs, S.; Swartz, M.C.; Cox, M.G.; Lu, K.H. Response to an exercise intervention after endometrial cancer: Differences between obese and non-obese survivors. Gynecol. Oncol. 2014, 133, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoh, K.; Nishikawa, H.; Enomoto, H.; Ishii, N.; Iwata, Y.; Ishii, A.; Yuri, Y.; Miyamoto, Y.; Hasegawa, K.; Nakano, C.; et al. Effect of exercise therapy on sarcopenia in pancreatic cancer: A study protocol for a randomised controlled trial. BMJ Open Gastroenterol. 2018, 5, e000194. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, A.F.; Foidart-Dessalle, M.; Tomasella, M.; Coucke, P.; Devos, M.; Bruyère, O.; Bury, T.; Deflandre, D.; Jerusalem, G.; Lifrange, E.; et al. Multidisciplinary rehabilitation program after breast cancer: Benefits on physical function, anthropometry and quality of life. Eur. J. Phys. Rehabil. Med. 2017, 53, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Fallowfield, L.J. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res. Treat. 2008, 107, 167–180. [Google Scholar] [CrossRef]
- Saghatchian, M.; Lesur, A. Gestion des effets secondaires de l’hormonothérapie du cancer du sein chez la femme jeune [Management of side effects related to adjuvant hormone therapy in young women with breast cancer]. Bull Cancer 2019, 106, S37–S42. [Google Scholar] [CrossRef]
- Bundred, N.J. The effects of aromatase inhibitors on lipids and thrombosis. Br. J. Cancer 2005, 93, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Fredslund, S.O.; Gravholt, C.H.; Laursen, B.E.; Jensen, A.B. Key metabolic parameters change significantly in early breast cancer survivors: An explorative PILOT study. J. Transl. Med. 2019, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Gibb, F.W.; Dixon, J.M.; Clarke, C.; Homer, N.Z.; Faqehi, A.M.M.; Andrew, R.; Walker, B.R. Higher Insulin Resistance and Adiposity in Postmenopausal Women With Breast Cancer Treated With Aromatase Inhibitors. J. Clin. Endocrinol. Metab. 2019, 104, 3670–3678. [Google Scholar] [CrossRef]
- Foglietta, J.; Inno, A.; de Iuliis, F.; Sini, V.; Duranti, S.; Turazza, M.; Tarantini, L.; Gori, S. Cardiotoxicity of Aromatase Inhibitors in Breast Cancer Patients. Clin. Breast Cancer 2017, 17, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ren, D.; Shen, G.; Ahmad, R.; Dong, L.; Du, F.; Zhao, J. Toxicity of extended adjuvant endocrine with aromatase inhibitors in patients with postmenopausal breast cancer: A Systemtic review and Meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 156, 103114. [Google Scholar] [CrossRef]
- Jacobse, J.N.; Schaapveld, M.; Boekel, N.B.; Hooning, M.J.; Jager, A.; Baaijens, M.H.A.; Hauptmann, M.; Russell, N.S.; Rutgers, E.J.T.; Aleman, B.M.P.; et al. Risk of heart failure after systemic treatment for early breast cancer: Results of a cohort study. Breast Cancer Res. Treat. 2021, 185, 205–214. [Google Scholar] [CrossRef]
- Cheung, A.M.; Heisey, R.; Srighanthan, J. Breast cancer and osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y. Secondary osteoporosis. Cancer treatment-induced bone loss. Clin. Calcium 2018, 28, 1665–1670. [Google Scholar] [PubMed]
- Leslie, W.D.; Morin, S.N.; Lix, L.M.; Niraula, S.; McCloskey, E.V.; Johansson, H.; Harvey, N.C.; Kanis, J.A. Fracture Risk in Women with Breast Cancer Initiating Aromatase Inhibitor Therapy: A Registry-Based Cohort Study. Oncologist 2019, 24, 1432–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagykálnai, T.; Landherr, L.; Mészáros, E. Aromatázgátlók és artralgia [Aromatase inhibitors and arthralgia]. Magy. Onkol. 2011, 55, 32–39. [Google Scholar]
- Niravath, P. Aromatase inhibitor-induced arthralgia: A review. Ann. Oncol. 2013, 24, 1443–1449. [Google Scholar] [CrossRef]
- Romero, S.A.D.; Su, H.I.; Satagopan, J.; Li, Q.S.; Seluzicki, C.M.; Dries, A.; DeMichele, A.M.; Mao, J.J. Clinical and genetic risk factors for aromatase inhibitor-associated arthralgia in breast cancer survivors. Breast 2020, 49, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, K.H.; Ahmed, R.L.; Hannan, P.J.; Yee, D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1672–1680. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.L.; Winters-Stone, K. Effects of a 12-month randomized controlled trial of aerobic or resistance exercise during and following cancer treatment in women. Phys. Sportsmed. 2009, 37, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Campbell, N.; Chen, H.; Salinardi, T.; Chen, W.; Partridge, A.; Mantzoros, C.; Winer, E. Impact of physical activity on insulin levels in breast cancer survivors. J. Clin. Oncol. 2008, 26, 907–912. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; George, S.L.; Switzer, B.R.; Snyder, D.C.; Madden, J.F.; Polascik, T.J.; Ruffin, M.T.; Vollmer, R.T. Overcoming challenges in designing and implementing a phase II randomized controlled trial using a presurgical model to test a dietary intervention in prostate cancer. Clin. Trials 2008, 5, 262–272. [Google Scholar] [CrossRef]
- Irwin, M.L.; Alvarez-Reeves, M.; Cadmus, L.; Mierzejewski, E.; Mayne, S.T.; Yu, H.; Chung, G.G.; Jones, B.; Knobf, M.T.; DiPietro, L. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring) 2009, 17, 1534–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinan, E.; Hussey, J.; Broderick, J.M.; Lithander, F.E.; O’Donnell, D.; Kennedy, M.J.; Connolly, E.M. The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors—A pilot study. Support. Care Cancer 2013, 21, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Daley, A.J.; Doll, H.; Woodroofe, N.; Coleman, R.E.; Mutrie, N.; Crank, H.; Powers, H.J.; Saxton, J.M. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: A randomized controlled trial. Cancer Causes Control. 2013, 24, 181–191. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Fogleman, A.; Trammell, R.; Hopkins-Price, P.; Spenner, A.; Vicari, S.; Rao, K.; Courneya, K.S.; Hoelzer, K.; Robbs, R.; et al. Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: Pilot randomized controlled trial. Psychooncology 2015, 24, 302–310. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Vicari, S.; Trammell, R.; Hopkins-Price, P.; Fogleman, A.; Spenner, A.; Rao, K.; Courneya, K.S.; Hoelzer, K.; Robbs, R.; et al. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Med. Sci. Sports Exerc. 2014, 46, 1077–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courneya, K.S.; Segal, R.J.; McKenzie, D.C.; Dong, H.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Crawford, J.J.; Mackey, J.R. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc. 2014, 46, 1744–1751. [Google Scholar] [CrossRef] [Green Version]
- Artene, D.; Blidaru, A. Factors that influence oncology nutrition efficacy in breast cancer patients under antiestrogenic treatment. Ann. Oncol. 2018, 29, 603–640. [Google Scholar] [CrossRef]
- de Paulo, T.R.S.; Winters-Stone, K.M.; Viezel, J.; Rossi, F.E.; Simões, R.R.; Tosello, G.; Freitas, I.F. Junior. Effects of resistance plus aerobic training on body composition and metabolic markers in older breast cancer survivors undergoing aromatase inhibitor therapy. Exp. Gerontol. 2018, 111, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijwel, S.; Backman, M.; Bolam, K.A.; Jervaeus, A.; Sundberg, C.J.; Margolin, S.; Browall, M.; Rundqvist, H.; Wengström, Y. Adding high-intensity interval training to conventional training modalities: Optimizing health-related outcomes during chemotherapy for breast cancer: The OptiTrain randomized controlled trial. Breast Cancer Res. Treat. 2018, 168, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuri, R.; Kordi, M.R.; Moghaddasi, M.; Rahnama, N.; Damirchi, A.; Rahmani-Nia, F.; Emami, H. Effect of combination exercise training on metabolic syndrome parameters in postmenopausal women with breast cancer. J. Cancer Res. Ther. 2012, 8, 238–242. [Google Scholar] [CrossRef]
- Karimi, N.; Roshan, V.D. Change in adiponectin and oxidative stress after modifiable lifestyle interventions in breast cancer cases. Asian Pac. J. Cancer Prev. 2013, 14, 2845–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.W.; Lee, J.; Suh, S.H.; Ligibel, J.; Courneya, K.S.; Jeon, J.Y. Effects of Exercise on Insulin, IGF Axis, Adipocytokines, and Inflammatory Markers in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2017, 26, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittus, K.L.; Gramling, R.E.; Ades, P.A. Exercise interventions for individuals with advanced cancer: A systematic review. Prev. Med. 2017, 104, 124–132. [Google Scholar] [CrossRef]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Safety and feasibility of exercise interventions in patients with advanced cancer: A systematic review. Support. Care Cancer 2017, 25, 3031–3050. [Google Scholar] [CrossRef]
- Carson, J.W.; Carson, K.M.; Olsen, M.; Sanders, L.; Westbrook, K.; Keefe, F.J.; Porter, L.S. Yoga Practice Predicts Improvements in Day-to-Day Pain in Women with Metastatic Breast Cancer. J. Pain Symptom Manag. 2020, 14, 30804–30806. [Google Scholar]
- Ligibel, J.A.; Giobbie-Hurder, A.; Shockro, L.; Campbell, N.; Partridge, A.H.; Tolaney, S.M.; Lin, N.U.; Winer, E.P. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer 2016, 122, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Iyengar, N.M.; Nilsen, T.S.; Michalski, M.; Thomas, S.M.; Herndon, J.; Sasso, J.; Yu, A.; Chandarlapaty, S.; Dang, C.T.; et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: A randomized controlled trial. Cancer 2018, 124, 2552–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, J.; Davis, G.M.; Hackett, D.; Beith, J.M.; Wilcken, N.; Currow, D.; Emery, J.; Phillips, J.; Martin, A.; Hui, R.; et al. Physical Activity for Symptom Management in Women With Metastatic Breast Cancer: A Randomized Feasibility Trial on Physical Activity and Breast Metastases. J. Pain Symptom Manag. 2019, 58, 929–939. [Google Scholar] [CrossRef]
- Carson, J.W.; Carson, K.M.; Porter, L.S.; Keefe, F.J.; Shaw, H.; Miller, J.M. Yoga for women with metastatic breast cancer: Results from a pilot study. J. Pain Symptom Manag. 2007, 33, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, L.; Pialoux, V.; Pérol, O.; Morelle, M.; Martin, A.; Friedenreich, C.; Febvey-Combes, O.; Pérol, D.; Belladame, E.; Clémençon, M.; et al. Feasibility and Health Benefits of an Individualized Physical Activity Intervention in Women With Metastatic Breast Cancer: Intervention Study. JMIR mHealth uHealth 2020, 8, e12306. [Google Scholar] [CrossRef]
- Wang, M.; Yu, B.; Westerlind, K.; Strange, R.; Khan, G.; Patil, D.; Boeneman, K.; Hilakivi-Clarke, L. Prepubertal physical activity up-regulates estrogen receptor beta, BRCA1 and p53 mRNA expression in the rat mammary gland. Breast Cancer Res. Treat. 2009, 1, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Enger, S.M.; Ross, R.K.; Paganini-Hill, A.; Carpenter, C.L.; Bernstein, L. Carpenter and Leslie Bernstein Body Size, Physical Activity, and Breast Cancer Hormone Receptor Status: Results from Two Case-Control Studies Cancer Epidemiol. Biomark. Prev. 2000, 9, 681–687. [Google Scholar]
| Subtypes | Molecular Signatures | % Incidence |
|---|---|---|
| Luminal A | ER+, PR±, HER2−, Low Ki67 | ≈70% |
| Luminal B | ER+, PR±, HER2±, High Ki67 | 10–20% |
| Triple Negative | ER−, PR−, HER2− | 15–20% |
| HER2 | ER−, PR−, HER+ | 5–15% |
| REF. | GROUPS | EXERCISE CHARACTERISTICS | MAIN OUTCOMES |
|---|---|---|---|
| [128] | (n = 173) age 50 to 79 years BMI ≥ 25 kg/m2 (mean 30.4 ± 4.1) Groups: AG (n = 84) CG (n = 86) | 12 months AG: endurance exercise, 5 d/w progressively increase to 45 min at 60–75% HRmax; CG: no interventions | ↓Fat mass ↓ Testosterone and free testosterone = DHEA, DHEA-S =androstenedione |
| [129] | (n = 169) age 50 to 75 years BMI ≥ 25 kg/m2 (mean 30.4 ± 4.1) Groups: AG (n = 84) CG (n = 85) | 12 months AG: endurance exercise, 5 d/w progressively increase to 45 min at 60–75% HRmax; CG: no interventions | ↓Fat mass ↓ Estrone ↓ Estradiol and free estradiol 🡹 SHBG |
| [127] | (n = 189) age 50 to 69 years BMI 22–40 kg/m2 (mean 27.3 ± 3.6) Groups: AG (n = 96) CG (n = 93) | 12 months AG: 3 d/w of combined endurance + strength program (from 60–85% HRmax) (2.5 h/w) CG: no interventions | ↓Fat mass =estrogen levels =androgen levels =SHBG |
| [116] | (n = 320) age 50–74 years BMI 22–40 kg/m2 (mean 29.1 ± 4.5) Groups: AG (n = 160) CG (n = 160) | 12 months AG: 225 min/w (average of 3.6 d/w for 178 min/w) at 70% to 80% HRR CG: no interventions | ↓ Estradiol and free estradiol 🡹 SHBG ↓ Body weight = estrone, androstenedione and testosterone |
| [119] | (n = 439) age 50–75 years BMI ≥ 25 kg/m2 (mean 30.9) Groups: DG (n = 118) AG (n = 117) DAG (n = 117) CG (n = 87) | 12 months; DG: daily energy intake of 1200 to 2000 kcal/d based on baseline weight; AG: ≥45 min MVPA (70% to 85% heart rate max), 5 d/w; DAG: both interventions; CG: no interventions | ↓Fat mass in all groups vs. CG ↓ Insulin in DG and DAG ↓ hs-CRP in DG and DAG ↓ Leptin in all groups vs. CG 🡹 Adiponectin in DG and DAG ↓ Estron, estradiol, free estradiol, and free testosterone in all in all intervention groups vs. CG ↓ Total testosterone in DAG 🡹 SHBG in DG and DAG |
| [126] | (n = 439) aged 50–75 years BMI ≥ 25 kg/m2 (mean 30.9) Groups: DG (n = 118) AG (n = 117) DAG (n = 117) CG (n = 87) | 12 months; DG: daily energy intake of 1200–2000 kcal/d based on baseline weight; AG: ≥45 min of MVPA (70–85% heart rate max), 5 d/w (225 min/w). DAG: both interventions (diet + exercise) CG: no interventions | ↓Fat mass in all intervention groups vs. CG; ↓ Waist circumference in all intervention groups vs. CG; |
| [117] | (n = 382) age 50–74 years BMI 22–40 kg/m2 (mean 29.4 ± 4.4) Groups: COG (n = 193) MVG (n = 189) | 12 months of endurance activity (5 d/w, 3 supervised, 2 unsupervised); COG: 60 min/session, 60–80% HRR MVG: 30 min/session, 60–80% HRR | ↓Fat mass depending on exercise volume (high or moderate); =sex hormone levels between groups |
| [120] | (n = 243) age 50–69 years BMI 25–35 kg/m2 (mean 29.5 ± 2.6) Groups: DG (n = 97) COG (n = 98) CG (n = 48) | 16 weeks; DG: caloric deficit of 3500 kcal/w with habitual physical activity level; COG: 4 h/w of combined endurance (from 60% to 90% HRR) and strength program with an average energy expenditure of 2530 kcal/week; CG: habitual physical activity level + standardized diet | ↓Fat mass in DG and COG ↓ hs-CRP in DG and COG; =IL6 in all groups; ↓ Adiponectin in COG; ↓ Leptin in DG and COG |
| [121] | (n = 41) age 50–74 years BMI 23.8–32.9 kg/m2 (mean 28.2 ± 3.4) Groups: AG (n = 22) CG (n = 19) | 6 months; AG: 3 d/w progressively increase to 50 min at 70–80% HRmax; CG: no interventions | =Leptin =Resistin =Fat mass 🡹 Aerobic Fitness level ↓ BMI |
| [122] | (n = 306) age 50–74 years BMI 22–40 kg/m2 (mean 29.0) Groups: AG (n = 153) CG (n = 154) | 12 months; AG: 45 min/d, 5 d/w (70% to 80% HRR); CG: no interventions | ↓ Total estradiol =estrogen metabolites and metabolic pathways |
| [123] | (n = 439) age 50–75 years BMI ≥ 25 kg/m2 (mean 30.0 ± 3.7) Groups: DG (n = 118) AG (n = 117) DAG (n = 117) CG (n = 87) | 12 months + 18 months follow-up (FU); DG: daily energy intake of 1200–2000 kcal/d based on baseline weight; AG: ≥45 min of MVPA (70–85% heart rate max), 5 d/w (225 min/w); DAG: both interventions (diet + exercise); CG: no interventions | 🡹SHBG in DAG; =SHBG in DG and AG; Participants who reported weight loss had statistically greater decreases in free estradiol, free testosterone, and increases in SHBG |
| [118] | (n = 333) age 50–74 years BMI 22–40 kg/m2 (mean 28.9 ± 4.4) Groups: COG (n = 168) MVG (n = 165) | 12 months of endurance activity (5 d/w, 3 supervised, 2 unsupervised) + 12 months follow-up (FU); COG: 60 min/session, 65% to 75% HRR; MVG: 30 min/session, 65% to 75% HRR | ↓Fat mass depending on exercise volume (high or moderate); ↓ hs-CRP, insulin, glucose, HOMA-IR, estrone, estradiol, free estradiol at 12 months; SHBG at 12 months; ↓ Glucose, insulin, HOMA-IR, estrone at FU; 🡹 hs-CRP, free estradiol, estradiol at FU; ↓ SHBG at FU =biomarker changes over the time between groups. |
| [124] | (n = 35) age 50–65 years BMI ≥ 25 kg/m2 (mean 33.2 ± 1.4) Groups: AG (n = 10) COG (n = 13) CG (n = 12) | 12 weeks, 3 d/w; AG: 60 min/session of endurance exercise, 55–75% of HRR; COG: 40 mn resistance (6 exercises, 3 sets of 8–12 repetition at 65% of 1RM) + 20 min endurance exercise; CG: no interventions | ↓Fat mass in AG and COG; ↓ Lean body mass in the COG; ↓ DHEA-S (−13%), total (−40%) and free testosterone (−41%) in AG; ↓ Total (25%) and free testosterone (21%) in COG; =estrogen levels in both groups. The decrease in fat mass and DHEA-S correlates with an increase in circulating SHBG. |
| REF. | GROUPS | EXERCISE CHARACTERISTICS | MAIN OUTCOMES |
| [152] |
(n = 85) age 52–62 years IRG (n = 42) DRG (n = 42) | 6 months (after treatment) 2 d/w IRG and DRG: 3 set 12 repetition (13 w supervised + 13 w no-supervised) | ↓ Body fat and IGF-II in IRG 🡹 IGFBP-3 in IRG |
| [153] | (n = 66) age 46–58 years HAG (n = 22) HRG (n = 21) CG (n = 23) | 6 months (before and during adjuvant CH) 4 d/w HAG: 15–30 min HRG: 2 sets 10 repetitions CG: no intervention | 🡹 Aerobic capacity (25%) in HAG and (4%) in HRG ↓ BMD (6.2%) in CG, (4.9%) in HRG and (0.7%) in HAG ↓ Aerobic capacity (10%) in CG |
| [154] | (n = 101) Age HARG (n = 51) CG (n = 50) | 16 weeks (after CH, RT, during HT) 2 d/w HARG: 50-min supervised strength + 90 min unsupervised aerobic CG: no intervention | ↓ Fast insulin and hip circumference in HARG = insulin resistance, fasting glucose and BMI |
| [155] | (n = 90) age 41–48 years DG (n = 29) DHARG (n = 29) DHARG + FVLF (32) | 6 months 5 d/w DG: Calcium reach Diet DHARG: Calcium Diet + 150 min of MVPA AT + RT DHARG + FVLF: Calcium Diet + exercise + FVLF | ↓ Waist circumference and % body fat in DHARG + FVLF = insulin, proinsulin, IGF-1, CRP, cholesterol, SHBG, IL-1B, and TNFR2 in all groups 🡹 In QoL in all groups |
| [156] | (n = 75) age 55–64 years AG + HAG (n = 37) CG (n = 38) | 6 months (after CH) 5 d/w AG + HAG: 3 d/w 150 min/week of supervised gym- and 2 d/w home-based moderate-intensity aerobic exercise CG: no intervention | ↓ FAT in AG + HAG 🡹 LM in AG + HAG ↓ FAT, LM and BMD in CG |
| [157] | (n = 26) Age 40–60 years AG (n = 16) CG (n = 10) | 8 weeks AG: moderate intensity CG: no intervention | ↓ Waist circumference in AG 🡹 PA level = blood pressure, HDL, insulin resistance and CRP |
| [158] | (n = 90) age 55–65 years DARG (n = 47) CG (n = 43) | 6 months (duirng and after CH) 3 d/w DARG: 30 min 65–80% predicted HRmax + 10–15 min resistance band exercise + total daily caloric intake 600 kcal below their requirements CG: no intervenation | ↓ Central adiposity, WHR, total cholesterol and leptin in DARG 🡹 Predicted VO2max in DARG |
| [159] | (n = 28) age 56–66 years ARG (n = 15) CG (n = 13) | 12 weeks, supervised 6 wks, unsupervised 6 wks ARG:, 150 min/wk aerobic moderate intensity + resistance exercise, 2 d/wk CG: no intervention | 🡹 IL6 and predicted O2 in ARG ↓ IL-10, adiponectin, fatigue and sleep disturbance in ARG |
| [160] | (n = 46) age 30–70 years ARG (n = 22) CG (n = 24) | 12 weeks 5 d/w ARG: 160 min/wk at 48–52% of heart rate reserve +resistance exercise CG: no intervention | ↓ %BF, IL10, anxiety, sleep dysfunction, exercise social support in ARG 🡹 VO2max in ARG |
| [161] | (n = 242) age ≥ 18 years AG (n = 78) RG (n = 82) CG (n = 82) | 3 d/w during CH AG: 45 min at 80% VO2max RG: two sets of 8–12 at 60–70% of estimated 1-RM GC: no intervention | 🡹DFS and RFI in AG and RG |
| [162] | (n = 165) DG (n = 83) DRG (n = 82) | 12 months (after CH, during antiestrogenic treatment) 7 d/w DG: food naturally high in proteins, calcium, probiotics and prebiotics DRG: diet + 4 reps of 1 isometric exercise | ↓ Weight and fat in DG and DRG ↓ Visceral fat in DRG |
| [163] |
(n = 36) age 63–75 years ARG (n = 18) CG (n = 18) | 9 months (during AI) 3 d/w ARG: 30 min at 75/80% HRmax + 3 sets 8–10 reps at 75% 1RM CG: no intervention | 🡹 Osteocalcin in ARG ↓ Total mass, total fat and HDL in ARG |
| [164] | (n = 240) age 52–64 years ARG-HIIT (n = 79) AG-HIIT (n = 80) CG (n = 81) | 16 weeks 2 d/w + 12 months FU (during and after CH) ARG-HIIT: 3 sets 10 rep at 70–80% 1RM + 3 × 3-min bouts on cycle ergometer, 1 min of recovery AG-HIIT: from 20 min MACT to aerobic part of ARG-HIIT CG: no intervention | 🡹 Role functioning in RG-HIIT and AG-HIIT ↓ Total cancer-related fatigue in RG-HIIT and AG-HIIT 🡹 Pain in CG |
| [69] | (n = 23) age 51–63 years ARG-HIIT (n = 8) AG-HIIT (n = 9) CG (n = 13) | 16 weeks 2 d/w + 12 months FU (during and after CH) ARG-HIIT: 3 sets 10 rep at 70–80% 1RM + 3 × 3-min bouts on cycle ergometer, 1 min of recovery AG-HIIT: from 20 min MACT to aerobic part of ARG-HIIT CG: no intervention | 🡹 Muscle fiber CSA and SC count per fiber in ARG-HIIT ↓ Symptoms and displayed gains in lower limb in ARG-HIIT and AG-HIIT 🡹 Number of capillaries per fiber in AG-HIIT ↓ MHC isoform type I and protein levels of PINK1 in CG 🡹 SOD2 level in CG |
| REF. | GROUPS | EXERCISE CHARACTERISTICS | MAIN OUTCOMES |
|---|---|---|---|
| [170] |
(n = 13) Life expectancy ≥ 6 months age 44–75 years YG (n = 13) | YG: 8 weekly group session (during CH) | ↓ Pain, fatigue distress 🡹 Relaxation, invigoration |
| [171] | (n = 101) Life expectancy ≥ 12 months age 59–59 years AG (n = 48) CG (n = 53) | 16 weeks (during CH) AG: 150 min MVPA per week CG: no intervention | =min/w exercise and physical functioning in AG |
| [172] | (n = 65) age 62–72 years AG (n = 33) CG (n = 32) | 12 weeks 3 d/w (during CH) AG: 55–80 % VO2peak on treadmill CG: no intervention | 🡹VO2peak and functional capacity in AG. Attendance rate 63%, permanent discontinuation 27%, dose modification 49%, acceptable tolerability 42% in AG. |
| [173] | (n = 14) Life expectancy at least 4 months age ≥ 18 years HARG (n = 8) CG (n = 6) | 8 weeks 2 d/w (during CH and HT) HARG: supervised RT 2 sets of 12 repetitions 1 min recovery, intensity 6–7 Adult OMNI Scale + unsupervised 10–15 min walking CG: no intervention | 🡹FACIT-F score, VO2max and six-minute walking test in HARG. Adherence 100% in RT and 25% in walking. |
| [174] | (n = 48) Life expectancy ≥ 9 months age 56–67 years YG (n = 30) CG (n = 18) | 8 week 5–6 d/w (undergoing treatments) YG: meditation, gentle postures, breathing techniques, presentations on yogic principles for optimal coping. 15–30 min/d CG: Discussion about several topic related to the disease concerns | ↓ Pain levels in YG and CG Dose–response relationship between YG, duration and daily pain. |
| [175] | (n = 49) age 55–65 years HPA (n = 49) | 6 months (during CH, RT, HT, TT) HPA: reach 10,000 steps per day. | 🡹HPA increases 6-MWT, quadriceps strength ↓ BMI =muscle CSA, skeletal muscle radiodensity, LM. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimauro, I.; Grazioli, E.; Antinozzi, C.; Duranti, G.; Arminio, A.; Mancini, A.; Greco, E.A.; Caporossi, D.; Parisi, A.; Di Luigi, L. Estrogen-Receptor-Positive Breast Cancer in Postmenopausal Women: The Role of Body Composition and Physical Exercise. Int. J. Environ. Res. Public Health 2021, 18, 9834. https://doi.org/10.3390/ijerph18189834
Dimauro I, Grazioli E, Antinozzi C, Duranti G, Arminio A, Mancini A, Greco EA, Caporossi D, Parisi A, Di Luigi L. Estrogen-Receptor-Positive Breast Cancer in Postmenopausal Women: The Role of Body Composition and Physical Exercise. International Journal of Environmental Research and Public Health. 2021; 18(18):9834. https://doi.org/10.3390/ijerph18189834
Chicago/Turabian StyleDimauro, Ivan, Elisa Grazioli, Cristina Antinozzi, Guglielmo Duranti, Alessia Arminio, Annamaria Mancini, Emanuela A. Greco, Daniela Caporossi, Attilio Parisi, and Luigi Di Luigi. 2021. "Estrogen-Receptor-Positive Breast Cancer in Postmenopausal Women: The Role of Body Composition and Physical Exercise" International Journal of Environmental Research and Public Health 18, no. 18: 9834. https://doi.org/10.3390/ijerph18189834
APA StyleDimauro, I., Grazioli, E., Antinozzi, C., Duranti, G., Arminio, A., Mancini, A., Greco, E. A., Caporossi, D., Parisi, A., & Di Luigi, L. (2021). Estrogen-Receptor-Positive Breast Cancer in Postmenopausal Women: The Role of Body Composition and Physical Exercise. International Journal of Environmental Research and Public Health, 18(18), 9834. https://doi.org/10.3390/ijerph18189834











