Effects of Montmorency Tart Cherry and Blueberry Juice on Cardiometabolic Outcomes in Healthy Individuals: Protocol for a 3-Arm Placebo Randomized Controlled Trial
Abstract
:Strengths and Limitations of this Study
- -
- This study will be the first randomized placebo-controlled trial to examine the effectiveness of both Montmorency tart cherry and blueberry juice on cardiometabolic outcomes.
- -
- Primary and secondary outcomes measures are central to the treatment of cardiometabolic disease and its comorbidities.
1. Introduction
1.1. Aims and Objectives
1.2. Hypotheses
2. Materials and Methods
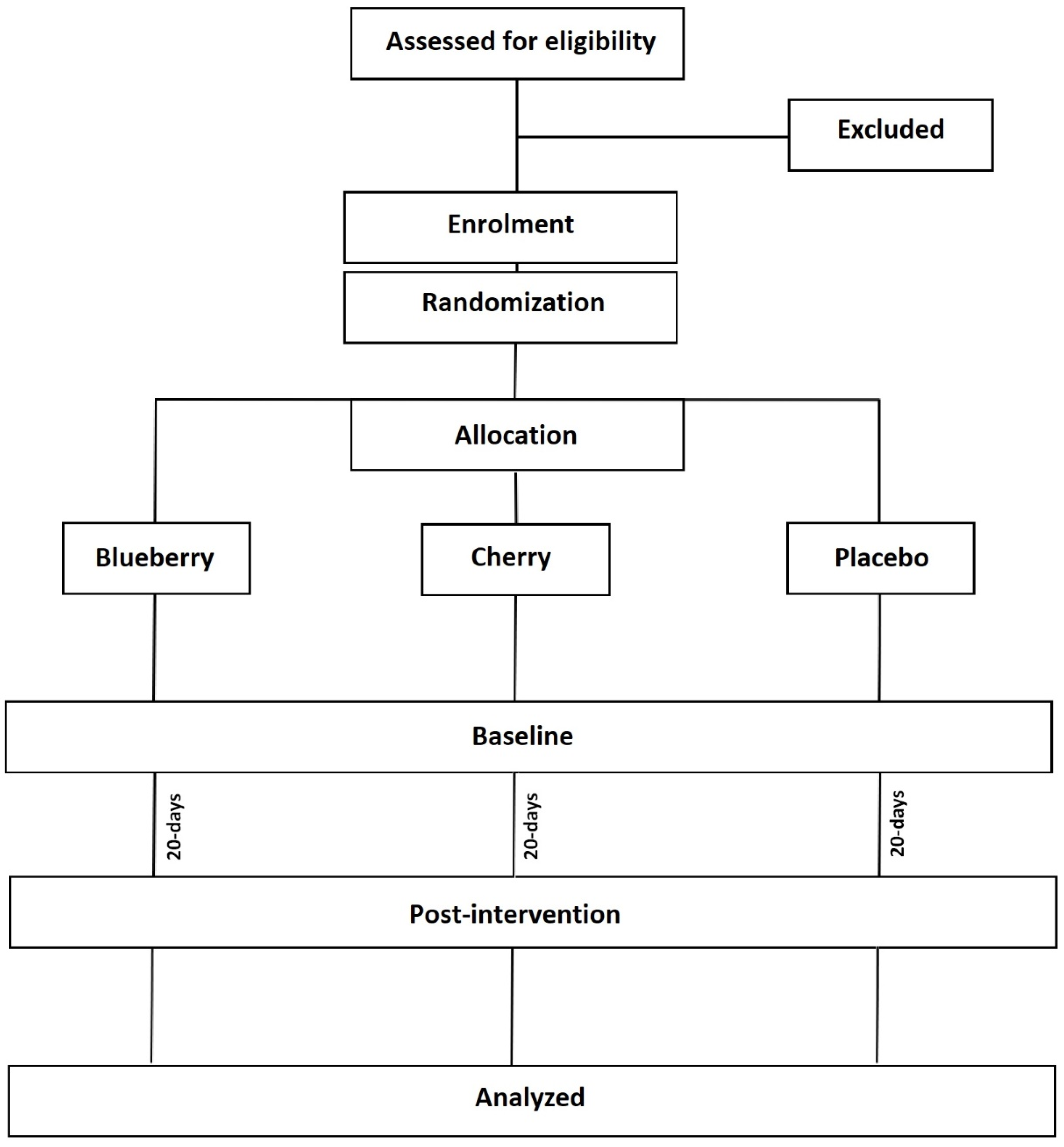
2.1. Study Design and Setting
2.2. Participants
2.2.1. Inclusion Criteria
- -
- Eighteen years of age and above
- -
- Non-smoker
- -
- BMI < 30
- -
- Able to give informed consent
2.2.2. Exclusion Criteria
- -
- Pregnancy
- -
- Sixty-five years of age and above
- -
- Diabetes or any other metabolic/uncontrolled hypertensive conditions
- -
- Food allergies to cherries or blueberries
- -
- Habitual consumption of blueberries/cherries and/or blueberry/cherry products
- -
- Not regularly taking medication or antioxidant supplements
2.2.3. Sample Size
2.3. Recruitment
2.4. Dietary Intervention
2.5. Data Collection
2.5.1. Laboratory Visit Data
2.5.2. Anthropometric Measurements
2.5.3. Energy Expenditure and Substrate Oxidation
2.5.4. Haematological Testing
2.5.5. Blood Pressure and Resting Heart Rate
2.5.6. Questionnaires
2.6. Data Management
2.7. Statistical Analysis
3. Ethics and Dissemination
4. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Danaei, G.; Lu, Y.; Singh, G.M.; Carnahan, E.; Stevens, G.A.; Cowan, M.J.; Kobayashi, J. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet Diabetes Endocrinol. 2014, 2, 634–647. [Google Scholar]
- Ahmed, C.M.S.; Jiang, H.; Chen, J.Y.; Lin, Y.-H. Traffic-Related Particulate Matter and Cardiometabolic Syndrome: A Review. Atmosphere 2018, 9, 336. [Google Scholar] [CrossRef] [Green Version]
- Farah, C.; Michel, L.Y.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chai, S.C.; Davis, K.; Wright, R.S.; Kuczmarski, M.F.; Zhang, Z. Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: A randomized controlled trial. Food Funct. 2018, 9, 3185–3194. [Google Scholar] [CrossRef] [Green Version]
- Qato, D.M.; Alexander, G.C.; Conti, R.M.; Johnson, M.; Schumm, P.; Lindau, S.T. Use of Prescription and Over-the-counter Medications and Dietary Supplements Among Older Adults in the United States. JAMA 2008, 300, 2867–2878. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.; Dominguez, J.; Nguyen, L.; Gullapalli, N. Hypertension Management: An Update. Am. Health Drug Benefits 2010, 3, 47–56. [Google Scholar]
- Oyebode, O.; Gordon-Dseagu, V.; Walker, A.; Mindell, J.S. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: Analysis of Health Survey for England data. J. Epidemiol. Community Health 2014, 68, 856–862. [Google Scholar] [CrossRef]
- Anand, S.S.; Hawkes, C.; De Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Popkin, B.M. Food consumption and its impact on cardiovascular disease: Importance of solutions focused on the globalized food system: A report from the workshop convened by the World Heart Federation. J. Am. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Desai, T.; Bottoms, L.; Roberts, M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 118, 2523–2539. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.S.; Gomes, R.D.S.; Carmo, M.M.D.; Da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef] [Green Version]
- Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity of small dark fruits: Influence of cultivars and harvest time. J. Berry Res. 2014, 4, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Nemzer, B.; Vargas, L.; Xia, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef]
- Traustadóttir, T.; Davies, S.; Stock, A.A.; Su, Y.; Heward, C.B.; Roberts, L.J.; Harman, S.M. Tart Cherry Juice Decreases Oxidative Stress in Healthy Older Men and Women. J. Nutr. 2009, 139, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency Cherries Reduce the Oxidative Stress and Inflammatory Responses to Repeated Days High-Intensity Stochastic Cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2009, 20, 843–852. [Google Scholar] [CrossRef]
- Martin, K.R.; Bopp, J.; Burrell, L.; Hook, G. The effect of 100% tart cherry juice on serum uric acid levels, biomarkers of inflammation and cardiovascular disease risk factors. FASEB 2011, 339–340. [Google Scholar]
- Cook, M.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef]
- Robinson, S.L.; Hattersley, J.; Frost, G.S.; Chambers, E.S.; Wallis, G.A. Maximal fat oxidation during exercise is positively associated with 24-hour fat oxidation and insulin sensitivity in young, healthy men. J. Appl. Physiol. 2015, 118, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; David, V.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.M.; Robinson, E.; Soumpasi, V.; Barker, M. Effect of a Tart Cherry Juice Supplement on Arterial Stiffness and Inflammation in Healthy Adults: A Randomised Controlled Trial. Plant Foods Hum. Nutr. 2014, 69, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.R.; Burrell, L.; Bopp, J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: A randomized, crossover pilot study. Food Funct. 2018, 9, 5290–5300. [Google Scholar] [CrossRef] [PubMed]
- Lear, R.; O’Leary, M.; O’Brien Andersen, L.; Holt, C.C.; Stensvold, C.R.; Giezen, M.; Bowtell, J.L. Tart cherry concentrate does not alter the gut microbiome, glycaemic control or systemic inflammation in a middle-aged population. Nutrients 2019, 11, 1063. [Google Scholar] [CrossRef] [Green Version]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. The Influence of Tart Cherry (Prunus cerasus, cv Montmorency) Concentrate Supplementation for 3 Months on Cardiometabolic Risk Factors in Middle-Aged Adults: A Randomised, Placebo-Controlled Trial. Nutrients 2021, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.A.; Navaei, N.; Pourafshar, S.; Jaime, S.J.; Akhavan, N.S.; Alvarez-Alvarado, S.; Proaño, G.; Litwin, N.S.; Clark, E.A.; Foley, E.M.; et al. Effects of Montmorency Tart Cherry Juice Consumption on Cardiometabolic Biomarkers in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Trial. J. Med. Food 2020, 23, 1238–1247. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.; Champagne, C.M.; Cefalu, W.T. Bioactives in Blueberries Improve Insulin Sensitivity in Obese, Insulin-Resistant Men and Women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, A.M.; Wang, N.; Palmisano, J.; E Milbury, P.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.-Y.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2018, 58, 1223–1235. [Google Scholar] [CrossRef]
- Boldaji, R.B.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. J. Sci. Food Agric. 2019, 100, 846–854. [Google Scholar] [CrossRef]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free. Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef]
- Stote, K.S.; Sweeney, M.I.; Kean, T.; Baer, D.J.; Novotny, J.A.; Shakerley, N.; Chandrasekaran, A.; Carrico, P.M.; Melendez, J.A.; Gottschall-Pass, K.T. The effects of 100% wild blueberry (Vaccinium angustifolium) juice consumption on cardiometablic biomarkers: A randomized, placebo-controlled, crossover trial in adults with increased risk for type 2 diabetes. BMC Nutr. 2017, 3, 45. [Google Scholar] [CrossRef]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of Pomegranate Juice Consumption on Myocardial Perfusion in Patients With Coronary Heart Disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, M.; Martínez-Abundis, E.; Espinel-Bermúdez, M.C.; Pérez-Rubio, K.G. Effect of Pomegranate Juice on Insulin Secretion and Sensitivity in Patients with Obesity. Ann. Nutr. Metab. 2011, 58, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charrois, T.L.; McAlister, A.F.; Cooney, D.; Lewanczuk, R.; Kolber, M.R.; Campbell, N.R.; Rosenthal, M.; Houle, S.K.; Tsuyuki, R.T. Improving hypertension management through pharmacist prescribing; the rural alberta clinical trial in optimizing hypertension (Rural RxACTION): Trial design and methods. Implement. Sci. 2011, 6, 94. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Wang, J.S. Systolic blood pressure trajectory and cardiovascular outcomes: An analysis using data in the Systolic Blood Pressure Intervention Trial. Int. J. Clin. Pr. 2020, 74. [Google Scholar] [CrossRef]
- Sinclair, J.; Stainton, P.; Dillon, D.; Taylor, P.J.; Richardson, C.; Bottoms, L.; Hobbs, S.J.; Liles, H.; Allan, R. The efficacy of a tart cherry drink for the treatment of patellofemoral pain in recreationally active individuals; a placebo randomized control trial. Sport Sci. Health 2021, 53, 449. [Google Scholar]
- Bosy-Westphal, A.; Jensen, B.; Braun, W.; Pourhassan, M.; Gallagher, D.; Müller, M.J. Quantification of whole-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur. J. Clin. Nutr. 2017, 71, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Kengne, A.-P.; Huxley, R.; Batty, G.; De Galan, B.; Grobbee, D.; Pillai, A.; Zoungas, S.; Marre, M.; Woodward, M.; et al. Comparison of waist-to-hip ratio and other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: A prospective cohort study from ADVANCE. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; King, J.A.; Goerlach, J.; Nimmo, M.A. The impact of high-intensity intermittent exercise on resting metabolic rate in healthy males. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 113, 3039–3047. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of Substrate Oxidation During Exercise by Means of Gas Exchange Measurements. Int. J. Sports Med. 2004, 26, S28–S37. [Google Scholar] [CrossRef]
- Sinclair, J.; Shore, H.; Dillon, S. The effect of minimalist, maximalist and energy return footwear of equal mass on running economy and substrate utilisation. Comp. Exerc. Physiol. 2016, 12, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Tudor-Locke, C.; Han, H.; Aguiar, E.J.; Barreira, T.V.; Schuna, J.M., Jr.; Kang, M.; Rowe, D.A. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: A narrative review. Br. J. Sports Med. 2015, 52, 776–788. [Google Scholar] [CrossRef]
- Anandaraja, S.; Narang, R.; Godeswar, R.; Laksmy, R.; Talwar, K.K. Low-density lipoprotein cholesterol estimation by a new formula in Indian population. Int. J. Cardiol. 2005, 102, 117–120. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Pan, B.; Jin, X.; Yao, H.; Chen, B.; Zou, Y.; Ge, J.; Chen, H. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis. 2010, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Pedro-Botet, J. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- O’brien, E.; Asmar, R.; Beilin, L.; Imai, Y.; Mallion, J.M.; Mancia, G. European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J. Hypertens. 2003, 21, 821–848. [Google Scholar] [CrossRef]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005, 45, 142–161. [Google Scholar] [PubMed]
- Matricciani, L.; Paquet, C.; Fraysse, F.; Grobler, A.; Wang, Y.; Baur, L.; Juonala, M.; Nguyen, M.T.; Ranganathan, S.; Burgner, D.; et al. Sleep and cardiometabolic risk: A cluster analysis of actigraphy-derived sleep profiles in adults and children. Sleep 2021. [Google Scholar] [CrossRef]
- Godos, J.; Ferri, R.; Castellano, S.; Angelino, D.; Mena, P.; Del Rio, D.; Caraci, F.; Galvano, F.; Grosso, G. Specific Dietary (Poly)phenols Are Associated with Sleep Quality in a Cohort of Italian Adults. Nutrients 2020, 12, 1226. [Google Scholar] [CrossRef]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2011, 51, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, W.R.; Carr, M.; Gorman, C.; Perlis, M.L. Effects of a Tart Cherry Juice Beverage on the Sleep of Older Adults with Insomnia: A Pilot Study. J. Med. Food 2010, 13, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Smith, S.S.; Oei, T.P.; Douglas, J.A.; Brown, I.; Jorgensen, G.; Andrews, J. Confirmatory factor analysis of the Epworth Sleepiness Scale (ESS) in patients with obstructive sleep apnoea. Sleep Med. 2008, 9, 739–744. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanei-Gheshlagh, R.; Parizad, N.; Sayehmiri, K. The Relationship Between Depression and Metabolic Syndrome: Systematic Review and Meta-Analysis Study. Iran. Red Crescent Med. J. 2016, 18, e26523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 2020, 12, 2445. [Google Scholar] [CrossRef] [PubMed]
- Bentsen, B.G.; Natvig, B.; Winnem, M. Questions you didn’t ask? COOP/WONCA Charts in clinical work and research. Fam. Pract. 1999, 16, 190–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Rev. Bras. Psiquiatr. 2013, 35, 416–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinclair, J.; Shadwell, G.; Dillon, S.; Allan, R.; Butters, B.; Bottoms, L. Effects of Montmorency Tart Cherry and Blueberry Juice on Cardiometabolic Outcomes in Healthy Individuals: Protocol for a 3-Arm Placebo Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 9759. https://doi.org/10.3390/ijerph18189759
Sinclair J, Shadwell G, Dillon S, Allan R, Butters B, Bottoms L. Effects of Montmorency Tart Cherry and Blueberry Juice on Cardiometabolic Outcomes in Healthy Individuals: Protocol for a 3-Arm Placebo Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(18):9759. https://doi.org/10.3390/ijerph18189759
Chicago/Turabian StyleSinclair, Jonathan, Gareth Shadwell, Stephanie Dillon, Robert Allan, Bobbie Butters, and Lindsay Bottoms. 2021. "Effects of Montmorency Tart Cherry and Blueberry Juice on Cardiometabolic Outcomes in Healthy Individuals: Protocol for a 3-Arm Placebo Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 18: 9759. https://doi.org/10.3390/ijerph18189759
APA StyleSinclair, J., Shadwell, G., Dillon, S., Allan, R., Butters, B., & Bottoms, L. (2021). Effects of Montmorency Tart Cherry and Blueberry Juice on Cardiometabolic Outcomes in Healthy Individuals: Protocol for a 3-Arm Placebo Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(18), 9759. https://doi.org/10.3390/ijerph18189759






