Antitumor and Antioxidant Activity of S-Methyl Methionine Sulfonium Chloride against Liver Cancer Induced in Wistar Albino Rats by Diethyl Nitrosamine and Carbon Tertrachloride
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Animals
2.3. Preparation of DEN and CCl4
2.4. Experimental Design
2.5. Blood Samples
2.6. Tissue Sample
2.7. Serum Biochemical Assays
2.8. Assessment of Hepaticoxidative/Antioxidant Status
2.9. Histopathological Examination
2.10. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.11. Data Statistical Analysis
3. Result
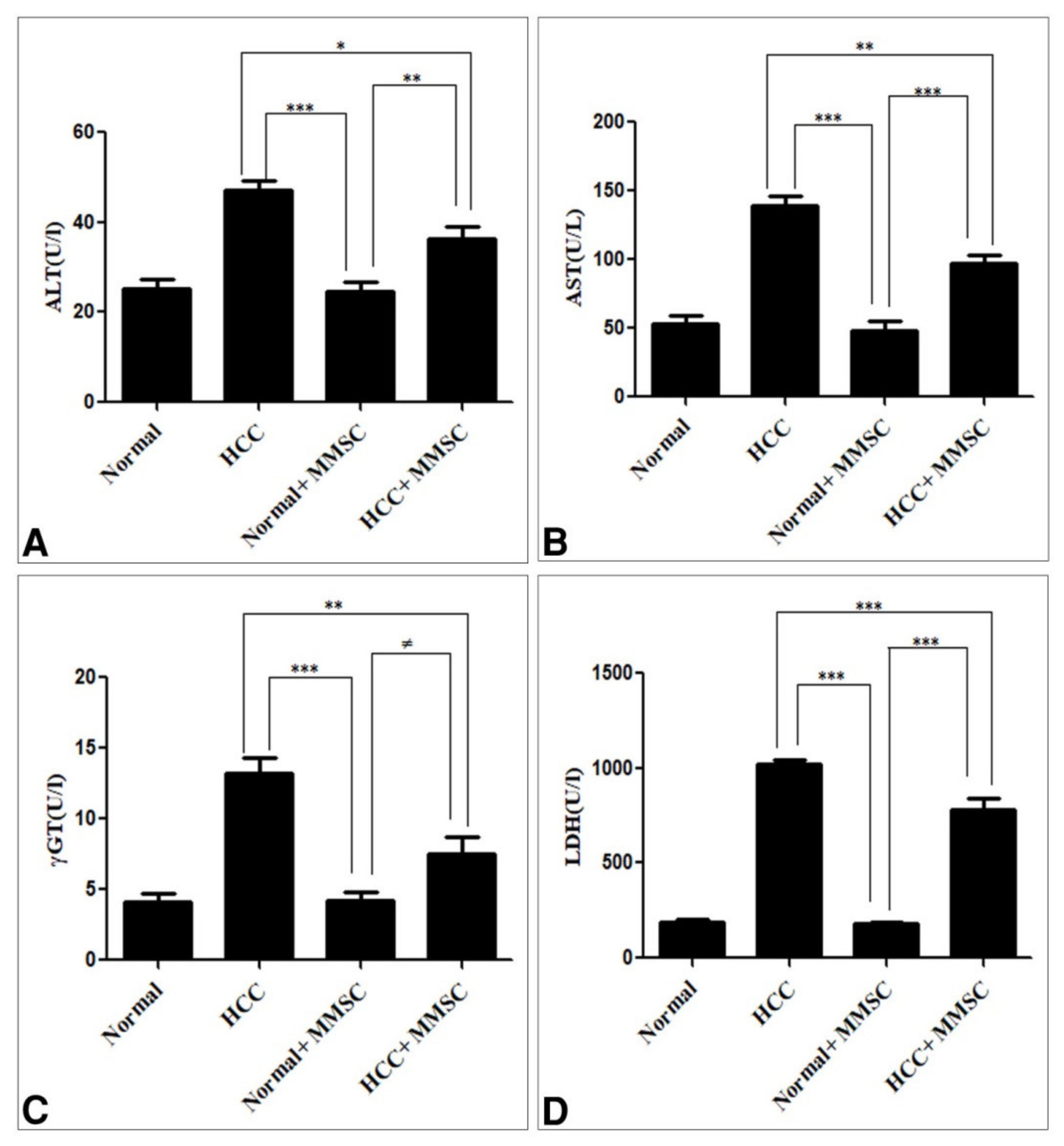
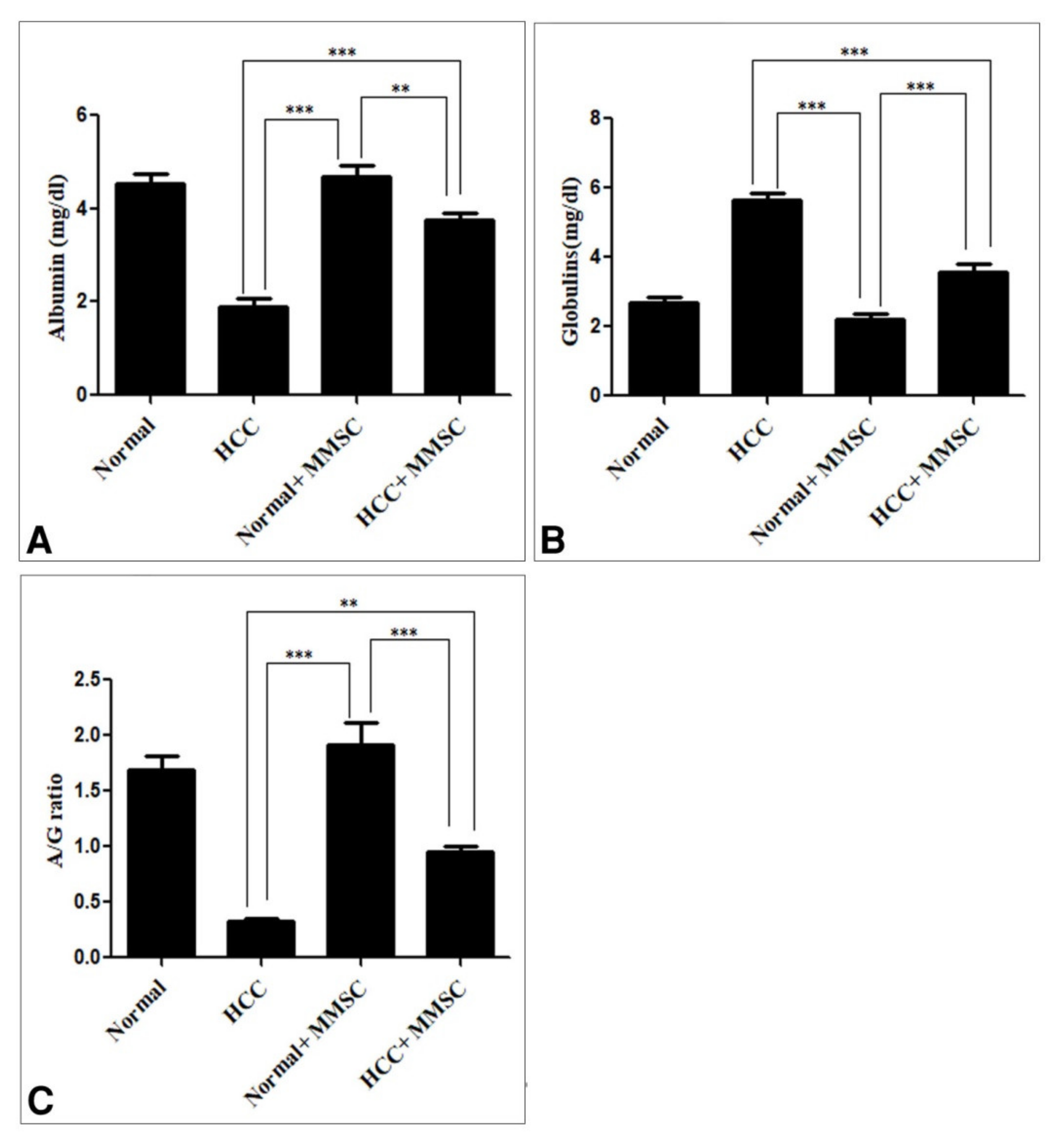
3.1. Biochemical Findings
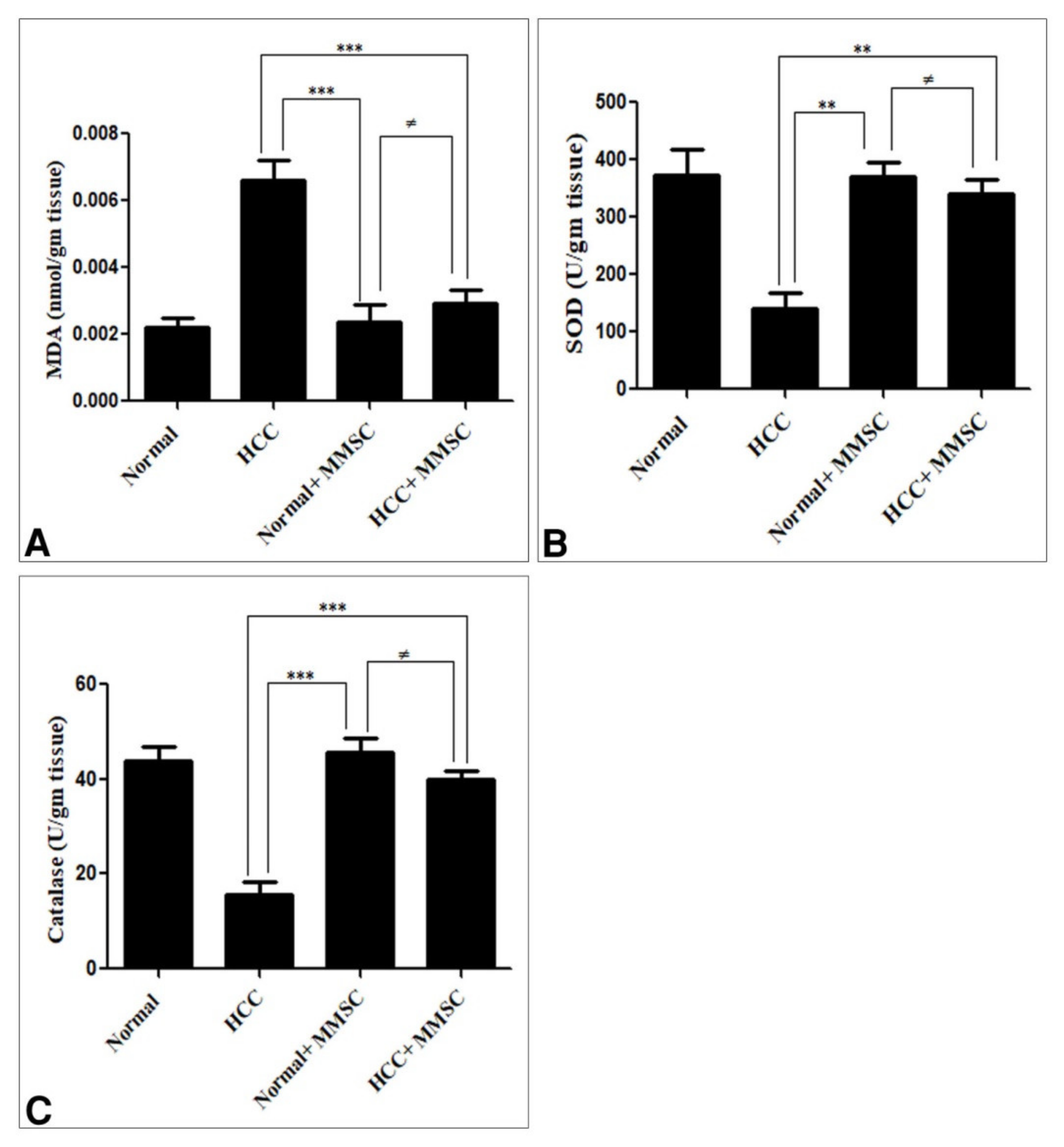
3.2. Oxidative/Antioxidant Activity
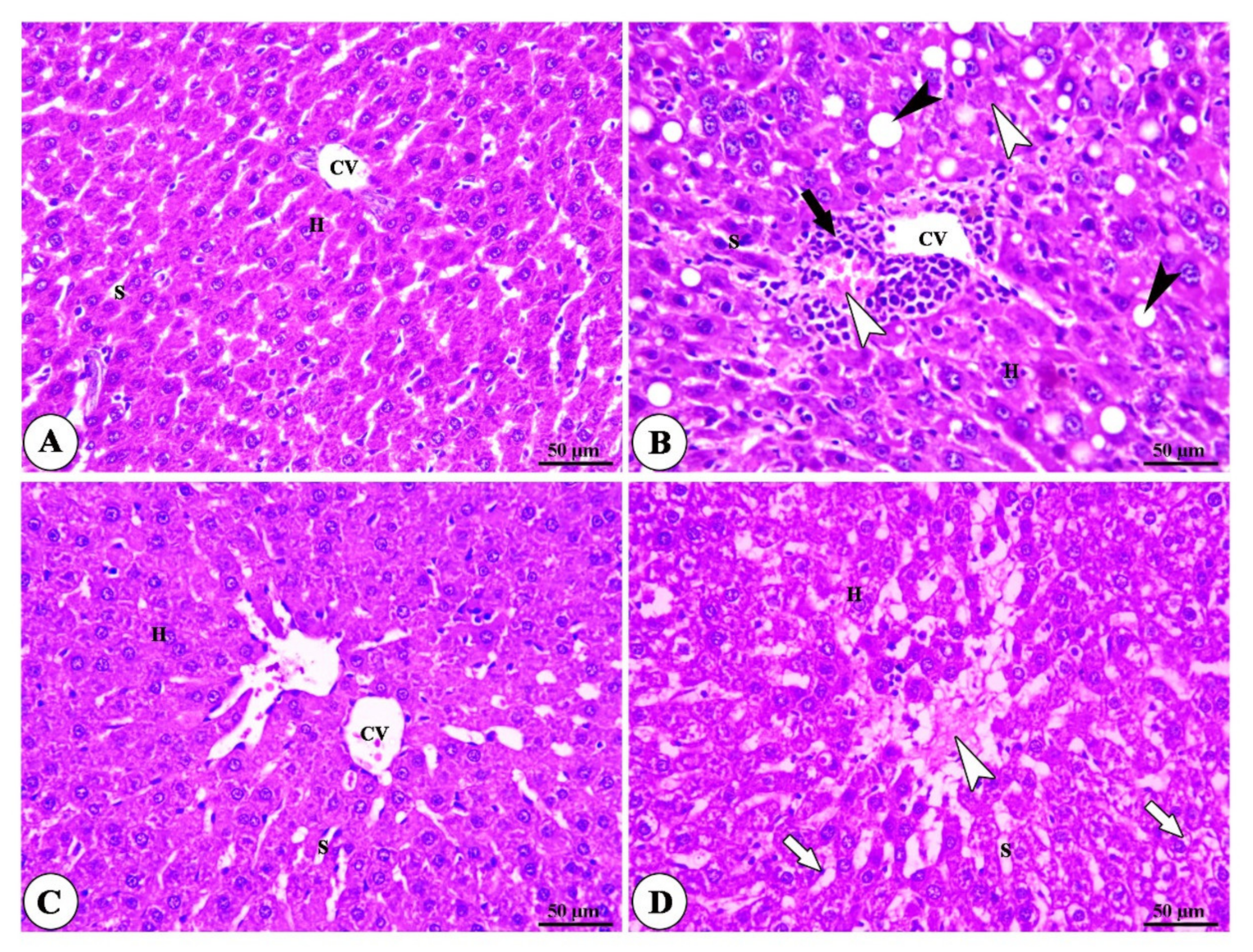
3.3. Histopathogy Findings
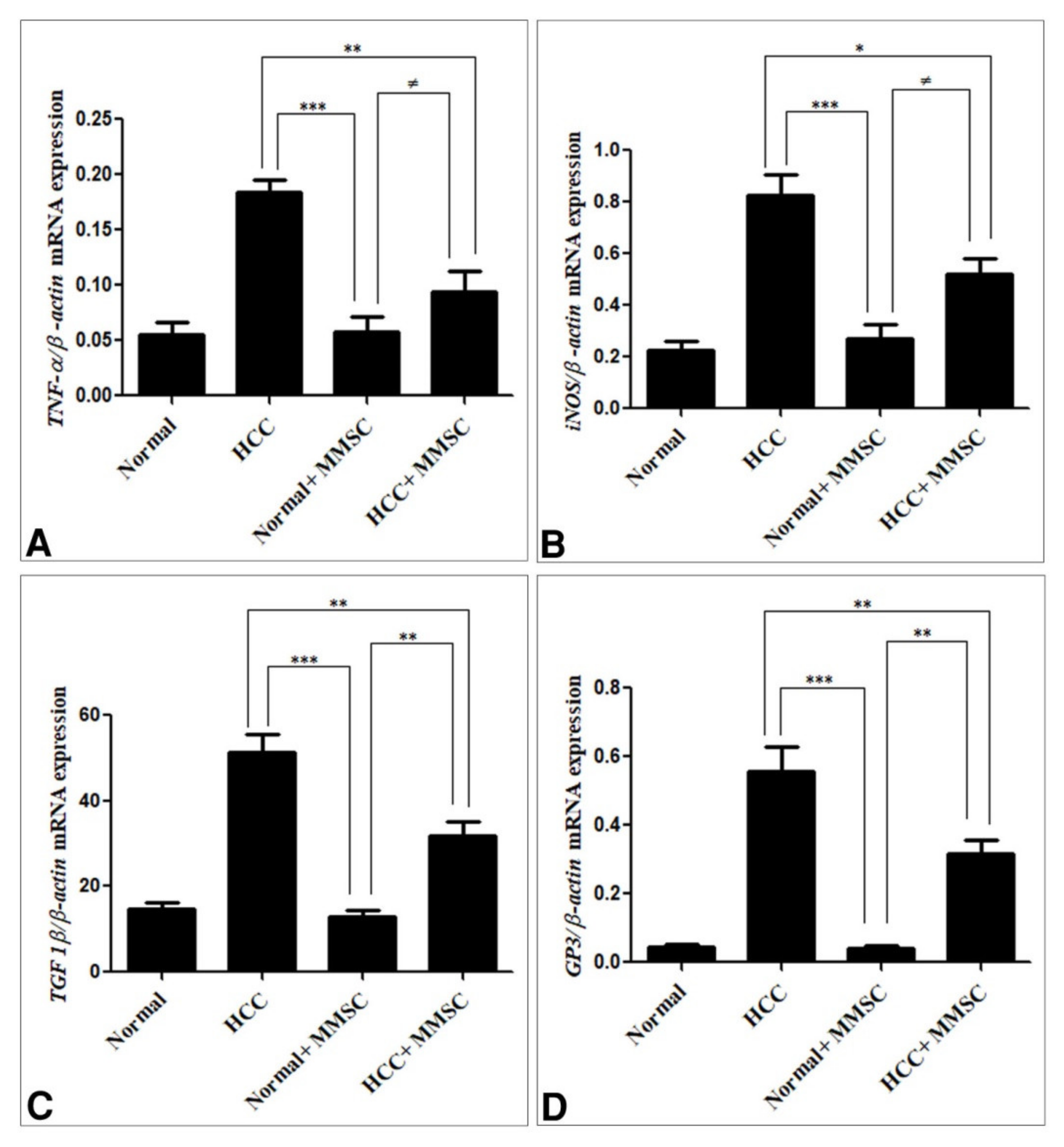
3.4. Inflammatory Cytokines, and Immunoregulatory Cytokines Expression by qRT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complementary Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef]
- Farazi, P.; DePinho, R. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.-S.; Le Tourneau, C.; O’Neil, B.; Ott, P.A.; Piha-Paul, S.; Gomez-Roca, C.; Van Brummelen, E.M.; Rugo, H.S.; Thomas, S.; Saraf, S.; et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1–Positive Cervical Cancer: Results from the Phase Ib KEYNOTE-028 Trial. J. Clin. Oncol. 2017, 35, 4035–4041. [Google Scholar] [CrossRef]
- Anwar, W.; Khaled, H.M.; Amra, H.A.E.S.; El-Nezami, H.; Loffredo, C. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: Possibilities for prevention. Mutat. Res. Rev. Mutat. Res. 2008, 659, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Hietanen, E.; Malaveille, C. Carcinogenic nitrosamines: Free radical aspects of their action. Free Radic. Biol. Med. 1989, 7, 637–644. [Google Scholar] [CrossRef]
- Bosnir, J.; Smit, Z.; Puntarić, D.; Horvat, T.; Klarić, M.; Simić, S.; Zorić, I. Presence of N-nitrosamines in canned liver patty. Coll. Antropol. 2003, 27 (Suppl. S1), 67–70. [Google Scholar]
- Chuang, S.E.; Kuo, M.-L.; Hsu, C.H.; Chen, C.R.; Lin, J.K.; Lai, G.M.; Hsieh, C.Y.; Cheng, A.-L. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 2000, 21, 331–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Maeda, S.; Chang, L.; Karin, M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc. Natl. Acad. Sci. USA 2006, 103, 10544–10551. [Google Scholar] [CrossRef] [Green Version]
- Filmus, J.; Capurro, M. Glypican-3: A marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013, 280, 2471–2476. [Google Scholar] [CrossRef]
- Iglesias, B.V.; Centeno, G.; Pascuccelli, H.; Ward, F.; Peters, M.G.; Filmus, J.; Puricelli, L.; Joffé, E.B.D.K. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol. Histopathol. 2008, 23, 11. [Google Scholar]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Yamauchi, N.; Watanabe, A.; Hishinuma, M.; Ohashi, K.-I.; Midorikawa, Y.; Morishita, Y.; Niki, T.; Shibahara, J.; Mori, M.; Makuuchi, M.; et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod. Pathol. 2005, 18, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, Y.; Kataoka, H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.; Filmus, J. Glypican-3 as a serum marker for hepatocellular carcinoma. Cancer Res. 2005, 65, 372–373. [Google Scholar]
- Zittermann, S.I.; Capurro, M.I.; Shi, W.; Filmus, J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int. J. Cancer 2010, 126, 1291–1301. [Google Scholar]
- Wu, Y.; Liu, H.; Weng, H.; Zhang, X.; Li, P.; Fan, C.-L.; Li, B.; Dong, P.-L.; Li, L.; Dooley, S.; et al. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int. J. Oncol. 2015, 46, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jin, R.; Zhang, X.; Lv, F.; Liu, L.; Liu, D.; Liu, K.; Li, N.; Chen, D. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology 2012, 56, 1380–1390. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, W.-K.; Choi, N.; Suh, W.; Lee, J.; Kim, D.-D.; Kim, I.; Sung, J.-H. Development of S-Methylmethionine Sulfonium Derivatives and Their Skin-Protective Effect against Ultraviolet Exposure. Biomol. Ther. 2018, 26, 306–312. [Google Scholar] [CrossRef]
- Kopinski, J.; Fogarty, R.; McVeigh, J. Effect of s-methylmethionine sulphonium chloride on oesophagogastric ulcers in pigs. Aust. Veter. J. 2007, 85, 362–367. [Google Scholar] [CrossRef]
- Lee, N.Y.; Park, K.Y.; Min, H.J.; Song, K.Y.; Lim, Y.Y.; Park, J.; Kim, B.J.; Kim, M.N. Inhibitory effect of vitamin U (S-methylmethionine sulfonium chloride) on differentiation in 3T3-L1 pre-adipocyte cell lines. Ann. Dermatol. 2012, 24, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Abass, S.A.; Abdel-Hamid, N.M.; Abouzed, T.K.; El-Shishtawy, M.M. Chemosensitizing effect of Alpinia officinarum rhizome extract in cisplatin-treated rats with hepatocellular carcinoma. Biomed. Pharmacother. 2018, 101, 710–718. [Google Scholar] [CrossRef]
- Elaidy, S.M.; Moghazy, A.; El-Kherbetawy, M.K. Evaluation of the Therapeutic Effects of Polyvinylpyrrolidone-Capped Silver Nanoparticles on the Diethylnitrosamine/Carbon Tetrachloride-Induced Hepatocellular Carcinoma in Rats. Egypt. J. Basic Clin. Pharmacol. 2017, 7, 9–24. [Google Scholar]
- Chatterjee, T. Handbook of Laboratory Mice and Rats; Department of Pharmaceutical Technology, Jadavpur University: Calcutta, India, 1993; p. 157. [Google Scholar]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dito, W. Lactate Dehydrogenase: A Brief Review. Clin. Enzymol. 1979, 1, 1–8. [Google Scholar]
- Szasz, G. New substrates for measuring gamma-glutamyl transpeptidase activity. Z. Klin. Chem. Klin. Biochem. 1974, 12, 228. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Bancroft, J.; Layton, C.; Suvarna, S. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: New York, NY, USA, 2013. [Google Scholar]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef]
- Yasin, B.R.; El-Fawal, H.A.; Mousa, S.A. Date (Phoenix dactylifera) Polyphenolics and Other Bioactive Compounds: A Traditional Islamic Remedy’s Potential in Prevention of Cell Damage, Cancer Therapeutics and beyond. Int. J. Mol. Sci. 2015, 16, 30075–30090. [Google Scholar] [CrossRef] [Green Version]
- Choo, S.P.; Tan, W.L.; Goh, B.K.P.; Tai, W.M.; Zhu, A.X. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016, 122, 3430–3446. [Google Scholar] [CrossRef] [PubMed]
- Chinelo, N.; Uzoma, N.O. Effect of methanol extract of Synsepalum dulcificum pulp on some biochemical parameters in albino rats. J. Coast. Life Med. 2015, 3, 233–240. [Google Scholar]
- Baş, S.S. The influence of vitamin U supplementation on liver injury of amiodarone-administered rats. Eur. J. Biol. 2016, 75, 1–10. [Google Scholar]
- Sokmen, B.B.; Tunali, S.; Yanardag, R. Effects of vitamin U (S-methyl methionine sulphonium chloride) on valproic acid induced liver injury in rats. Food Chem. Toxicol. 2012, 50, 3562–3566. [Google Scholar] [CrossRef]
- Jayakumar, S.; Madankumar, A.; Asokkumar, S.; Raghunandhakumar, S.; Gokula Dhas, K.; Kamaraj, S.; Divya, M.G.J.; Devaki, T. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 2012, 360, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, D.H.; Ali, S.A. The protective effect of Phoenix dactylifera L. seeds against CCl4-induced hepatotoxicity in rats. J. Ethnopharmacol. 2014, 155, 736–743. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, H.; Zheng, J.; Liu, Y. Glypican-3: A new target for diagnosis and treatment of hepatocellular carcinoma. J. Cancer 2020, 11, 2008. [Google Scholar] [CrossRef]
- Aglan, H.A.; Ahmed, H.H.; El-Toumy, S.A.; Mahmoud, N.S. Gallic acid against hepatocellular carcinoma: An integrated scheme of the potential mechanisms of action from in vivo study. Tumor Biol. 2017, 39, 1010428317699127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, M.G.; Ranocha, P.; Gage, D.A.; Simon, E.S.; Rhodes, D.; Peel, G.J.; Mellema, S.; Saito, K.; Awazuhara, M.; Li, C.; et al. Insertional Inactivation of the Methionine S-Methyltransferase Gene Eliminates the S-Methylmethionine Cycle and Increases the Methylation Ratio. Plant Physiol. 2003, 131, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Barbier-Torres, L.; Fan, W.; Mato, J.; Lu, S.C. Methionine adenosyltransferases in liver cancer. World J. Gastroenterol. 2019, 25, 4300–4319. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Rosas, M.G.; Chávez, E.; Velasco-Loyden, G.; Domínguez-López, M.; Martínez-Pérez, L.; De Sánchez, V.C. Diminished S-adenosylmethionine biosynthesis and its metabolism in a model of hepatocellular carcinoma is recuperated by an adenosine derivative. Cancer Biol. Ther. 2019, 21, 81–94. [Google Scholar] [CrossRef] [PubMed]
| Sense | Antisense | Annealing Temperature | |
|---|---|---|---|
| TNF-α | GACCCTCACACTCAGATCATCTTCT | TTGTCTTTGAGATCCATGCCATT | 60 |
| iNOS | TCTTCAAGGACCTACCTCAGGC | GCTAAGGCAAAGCTGCTAGGTC | 60 |
| TGF B-1 | TCACTTGTTTTGGTGGATGC | TTCTGTCTCTCAAGTCCCCC | 60 |
| GP3 | GTGCTGGAACGGACAAGAG | TTCTTCATCCCATTCCTTGC | 60 |
| β-actin | TGTTGTCCCTGTATGCCTCT | TAATGTCACGCACGATTTCC | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouzed, T.K.; Althobaiti, F.; Abdelkhlek, N.A.; Eldomany, E.B.; Nasr, N.E.; Sadek, K.M.; El-Shazly, S.A.; Kahilo, K.A.; Dorghamm, D.A. Antitumor and Antioxidant Activity of S-Methyl Methionine Sulfonium Chloride against Liver Cancer Induced in Wistar Albino Rats by Diethyl Nitrosamine and Carbon Tertrachloride. Int. J. Environ. Res. Public Health 2021, 18, 9726. https://doi.org/10.3390/ijerph18189726
Abouzed TK, Althobaiti F, Abdelkhlek NA, Eldomany EB, Nasr NE, Sadek KM, El-Shazly SA, Kahilo KA, Dorghamm DA. Antitumor and Antioxidant Activity of S-Methyl Methionine Sulfonium Chloride against Liver Cancer Induced in Wistar Albino Rats by Diethyl Nitrosamine and Carbon Tertrachloride. International Journal of Environmental Research and Public Health. 2021; 18(18):9726. https://doi.org/10.3390/ijerph18189726
Chicago/Turabian StyleAbouzed, Tarek Kamal, Fayez Althobaiti, Nesreen Adel Abdelkhlek, Ehab Bedir Eldomany, Nasr Elsayed Nasr, Kadry Mohamed Sadek, Samir Ahmed El-Shazly, Khaled A. Kahilo, and Doaa Abdallha Dorghamm. 2021. "Antitumor and Antioxidant Activity of S-Methyl Methionine Sulfonium Chloride against Liver Cancer Induced in Wistar Albino Rats by Diethyl Nitrosamine and Carbon Tertrachloride" International Journal of Environmental Research and Public Health 18, no. 18: 9726. https://doi.org/10.3390/ijerph18189726
APA StyleAbouzed, T. K., Althobaiti, F., Abdelkhlek, N. A., Eldomany, E. B., Nasr, N. E., Sadek, K. M., El-Shazly, S. A., Kahilo, K. A., & Dorghamm, D. A. (2021). Antitumor and Antioxidant Activity of S-Methyl Methionine Sulfonium Chloride against Liver Cancer Induced in Wistar Albino Rats by Diethyl Nitrosamine and Carbon Tertrachloride. International Journal of Environmental Research and Public Health, 18(18), 9726. https://doi.org/10.3390/ijerph18189726






