Validation of an Index for Functionally Important Respiratory Symptoms among Adults in the Nationally Representative Population Assessment of Tobacco and Health Study, 2014–2016
Abstract
:1. Introduction
2. Materials and Methods
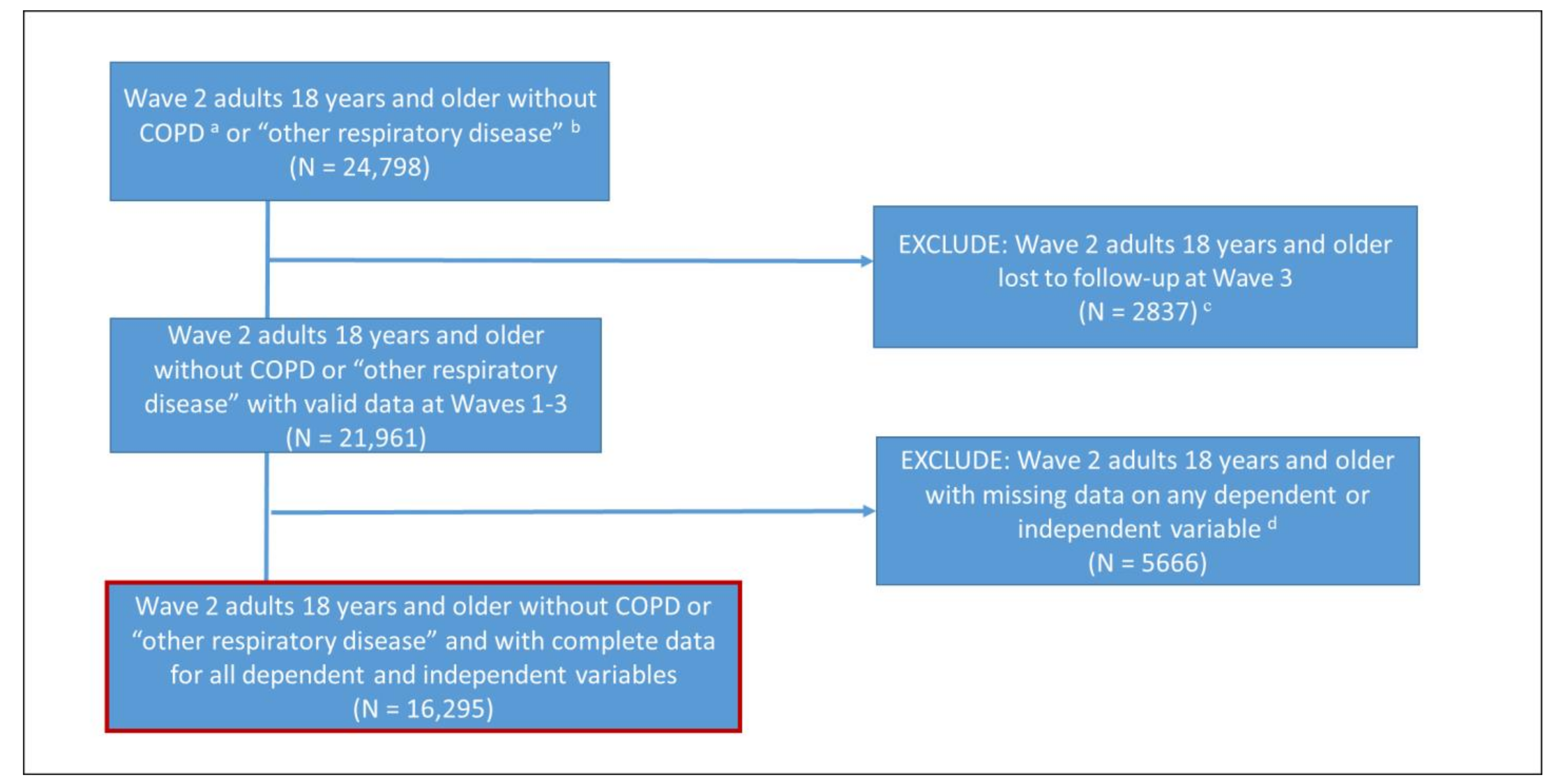
2.1. Study Population
2.2. Measures for Respiratory Symptoms in the PATH Study
2.3. Item-Level Validation against Similar NHANES Items
2.4. Creating a Respiratory Symptoms Index
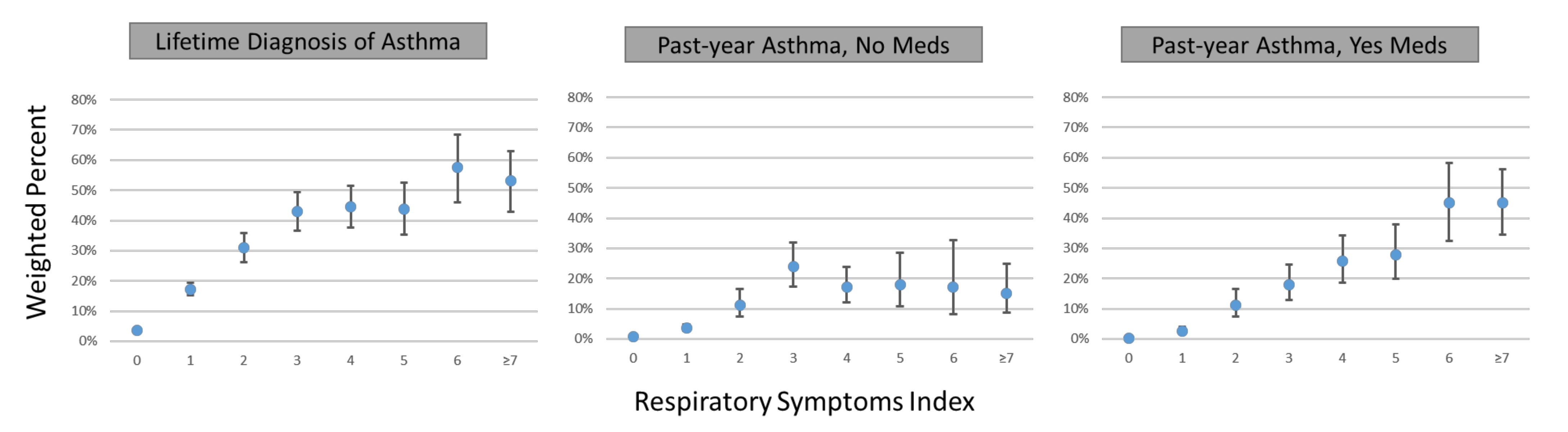
2.5. Validating the Respiratory Symptoms Index against Asthma
2.6. Determining Cut-Off Values for Functionally Important Respiratory Symptoms among Those with Asthma
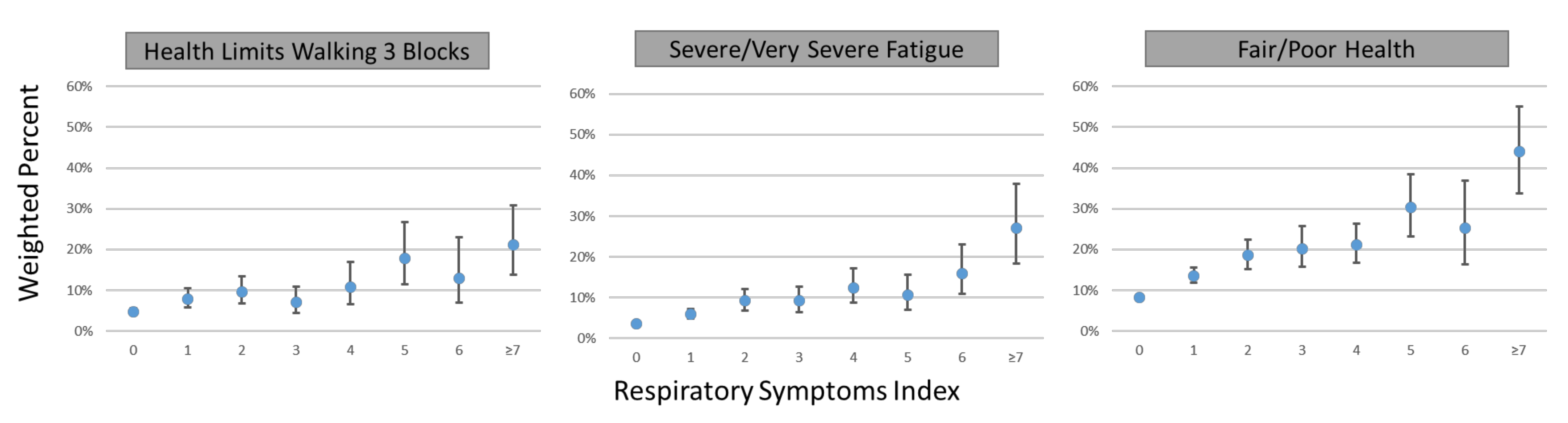
2.7. Association between Respiratory Symptoms and Functional Outcomes in the General Population
2.8. Analytical Approach
3. Results
3.1. Descriptive Statistics, Internal Consistency, and Test–Retest Reliability
3.2. Item-Level Validation against Similar NHANES Questions
3.3. Association of Respiratory Symptoms Index with Self-Reported Asthma
3.4. Association of Various Respiratory Symptom Score Cut-Off Values with Self-Reported Asthma
3.5. Association of Respiratory Symptom Score Cut-Off Values with Functional Outcomes in the General Population
3.6. Association of Various Respiratory Symptoms Score Cut-Off Values with Cigarette Smoke Exposure in the General Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Disclaimer
References
- Mannino, D.M.; Gagnon, R.C.; Petty, T.L.; Lydick, E. Obstructive lung disease and low lung function in adults in the United States: Data from the National Health and Nutrition Examination Survey, 1988-1994. Arch. Intern. Med. 2000, 160, 1683–1689. [Google Scholar] [CrossRef]
- Silvestri, M.; Franchi, S.; Pistorio, A.; Petecchia, L.; Rusconi, F. Smoke exposure, wheezing, and asthma development: A systematic review and meta-analysis in unselected birth cohorts. Pediatric Pulmonol. 2015, 50, 353–362. [Google Scholar] [CrossRef]
- Santanello, N.C.; Davies, G.; Galant, S.P.; Pedinoff, A.; Sveum, R.; Seltzer, J.; Seidenberg, B.C.; Knorr, B.A. Validation of an asthma symptom diary for interventional studies. Arch. Dis. Child. 1999, 80, 414–420. [Google Scholar] [CrossRef]
- Bursch, B.; Schwankovsky, L.; Gilbert, J.; Zeiger, R. Construction and validation of four childhood asthma self-management scales: Parent barriers, child and parent self-efficacy, and parent belief in treatment efficacy. J. Asthma 1999, 36, 115–128. [Google Scholar] [CrossRef]
- Wasserfallen, J.-B.; Gold, K.; Schulman, K.A.; Baraniuk, J.N. Development and validation of a rhinoconjunctivitis and asthma symptom score for use as an outcome measure in clinical trials. J. Allergy Clin. Immunol. 1997, 100, 16–22. [Google Scholar] [CrossRef]
- Hyland, A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Lambert, E.; Carusi, C.; Taylor, K.; Crosse, S.; Fong, G.T.; Cummings, K.M.; et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob. Control 2017, 26, 371–378. [Google Scholar] [CrossRef]
- Asher, M.; Keil, U.; Anderson, H.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.; Pearce, N.; Sibbald, B.; Stewart, A. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Aït-Khaled, N.; Beasley, R.; Mallol, J.; Keil, U.; Mitchell, E.; Robertson, C. Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007, 62, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Weiland, S.; Keil, U.; Langridge, P.; Anderson, H.R.; Strachan, D.; Bauman, A.; Young, L.; Gluyas, P.; Ruffin, D. Self-reported prevalence of asthma symptoms in children in Australia, England, Germany and New Zealand: An international comparison using the ISAAC protocol. Eur. Respir. J. 1993, 6, 1455–1461. [Google Scholar]
- Ellwood, P.; Asher, M.I.; Billo, N.E.; Bissell, K.; Chiang, C.-Y.; Ellwood, E.M.; El-Sony, A.; García-Marcos, L.; Mallol, J.; Marks, G.B.; et al. The Global Asthma Network rationale and methods for Phase I global surveillance: Prevalence, severity, management and risk factors. Eur. Respir. J. 2017, 49, 1601605. [Google Scholar] [CrossRef]
- Ellwood, P.; Ellwood, E.; Rutter, C.; Perez-Fernandez, V.; Morales, E.; García-Marcos, L.; Pearce, N.; Asher, M.I.; Strachan, D.; GAN Phase I Study Group. Global Asthma Network Phase I surveillance: Geographical coverage and response rates. J. Clin. Med. 2020, 9, 3688. [Google Scholar] [CrossRef]
- Ford, E.S.; Mannino, D.M.; Wheaton, A.G.; Giles, W.H.; Presley-Cantrell, L.; Croft, J.B. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: Findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest 2013, 143, 1395–1406. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Most Recent National Asthma Data. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 15 February 2021).
- McHugh, M.K.; Symanski, E.; Pompeii, L.A.; Delclos, G.L. Prevalence of asthma among adult females and males in the United States: Results from the National Health and Nutrition Examination Survey (NHANES), 2001–2004. J. Asthma 2009, 46, 759–766. [Google Scholar]
- Sargent, J.D.; Halenar, M.J.; Woloshin, S.; Edwards, K.C.; Schwartz, L.; Emond, J.; Tanski, S.; Taylor, K.A.; Liu, J.; Goniewicz, M.L.; et al. Tobacco use and respiratory symptoms among US adults: Findings from the Longitudinal Population Assessment of Tobacco and Health (PATH) Study 2014–2016. 2021; in preparation. [Google Scholar]
- Tourangeau, R.; Yan, T.; Sun, H.; Hyland, A.; Stanton, C.A. Population Assessment of Tobacco and Health (PATH) reliability and validity study: Selected reliability and validity estimates. Tob. Control 2019, 28, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Piesse, A.; Opsomer, J.; Dohrmann, S.; Di Gaetano, R.; Morganstein, D.; Taylor, K.; Carusi, C.; Hyland, A. Longitudinal Uses of the Population Assessment of Tobacco and Health Study. Tob. Regul. Sci. 2021, 7, 3–16. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Health and Human Services; National Institutes of Health National Institute on Drug Abuse; United States Department of Health and Human Services Food and Drug Administration Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Restricted-Use Files, User Guide. ICPSR36231-v21 Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]. Available online: https://www.icpsr.umich.edu/files/NAHDAP/documentation/ug36231-all_REST.pdf (accessed on 23 February 2021).
- Allen, M.J.; Yen, W.M. Introduction to Measurement Theory; Waveland Press: Long Grove, IL, USA, 2001. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Jensen, R.E.; Potosky, A.L.; Reeve, B.B.; Hahn, E.; Cella, D.; Fries, J.; Smith, A.W.; Keegan, T.H.M.; Wu, X.-C.; Paddock, L.; et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual. Life Res. 2015, 24, 2333–2344. [Google Scholar] [CrossRef]
- McCarthy, P.J. Pseudoreplication Further Evaluation and Application of the Balanced Half-Sample Technique. Vital Health Stat. Ser. 2 Data Eval. Methods Res. 1969, 31, 1–24. [Google Scholar]
- Judkins, D.R. Fay’s method for variance estimation. J. Off. Stat. 1990, 6, 223. [Google Scholar]
- Rivest, L.-P. Statistical properties of Winsorized means for skewed distributions. Biometrika 1994, 81, 373–383. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC.: College Station, TX, USA, 2019. [Google Scholar]
- Martinez, C.H.; Mannino, D.M.; Jaimes, F.A.; Curtis, J.L.; Han, M.K.; Hansel, N.N.; Diaz, A.A. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann. Am. Thorac. Soc. 2015, 12, 1788–1795. [Google Scholar] [CrossRef]
| Wave 2 PATH Study 2014–2015 | NHANES 2011–2012 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Question a | Response Options | PATH Study Variable | Prevalence b (SE) 18+ Years | Question | Response Options | NHANES Variable | Prevalence c (SE) 18+ Years | ||
| Have you ever had wheezing or whistling in the chest at any time in the past? | Yes, No | R02_ AX0046 | Yes | 26.5% (0.4) | None equivalent | ||||
| Have you had wheezing or whistling in the chest in the past 12 months? | Yes, No | R02_ AX0047 | Yes | 16.8% (0.3) | In the past 12 months, have you had wheezing or whistling in your chest? | Yes, No | RDQ070 | Yes | 13.1% (0.9) |
| How many attacks of wheezing have you had in the past 12 months? | None, 1–3, 4–12, more than 12 | R02_ AX0048 | None d 1–3 4–12 >12 | 84.6% (0.3) 9.7% (0.3) 3.2% (0.2) 2.5% (0.1) | In the past 12 months, how many attacks of wheezing or whistling have you had? | Count, if 12 or more episodes enter 12 | RDQ080 | None e 1–3 4–12+ | 87.3% (0.9) 7.3% (0.7) 5.4% (0.6) |
| In the past 12 months, how often, on average has your sleep been disturbed due to wheezing? | None, less than one night/week, one or more nights/week | R02_ AX0049 | None d <1/w ≥1/w | 93.4% (0.2) 4.1% (0.2) 2.5% (0.1) | In the past 12 months, how often, on average has your sleep been disturbed because of wheezing? | None, less than 1 night/week, one or more nights per week | RDQ090 | None e <1/w ≥1/w | 93.9 (0.6) 2.9% (0.4) 3.2% (0.4) |
| In the past 12 months, has wheezing ever been severe enough to limit your speech to only one or two words between breaths? | Yes, No | R02_ AX0050 | Yes | 2.3% (0.1) | None equivalent | ||||
| In the past 12 months, has your chest sounded wheezy during or after exercise? | Yes, No | R02_ AX0052 | Yes | 11.5% (0.3) | In the past 12 months, has your chest sounded wheezy during or after exercise or physical activity? | Yes, No | RDQ100 | Yes e | 6.7% (0.7) |
| In the past 12 months, have you had a dry cough at night, apart from a cough associated with a cold or chest infection? | Yes, No | R02_ AX0053 | Yes | 16.5% (0.3) | In the past 12 months, have you had a dry cough at night not counting a cough associated with a cold or chest infection for at least 14 days? | Yes, No | RDQ140 | Yes | 5.0% (0.4) |
| Predictive Validity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lifetime Asthma n = 1829/16,295 AROC = 0.7517 | Past-Year Asthma, No Meds n = 436/14,902 AROC = 0.8033 | Past-Year Asthma, On Meds n = 371/14,837 AROC = 0.9057 | ||||||||
| Respiratory Symptom Index Score Cut-Off Level | Weighted Prevalence (SE) | Unweighted Sensitivity | Unweighted False Positive Rate | Weighted Unadjusted Odds Ratio (95% CI) | Unweighted Sensitivity | Unweighted False Positive Rate | Weighted Unadjusted Odds Ratio (95% CI) | Unweighted Sensitivity | Unweighted False Positive Rate | Weighted Unadjusted Odds Ratio (95% CI) |
| ≥1 | 22.8% (0.5) | 68.3% | 22.3% | 10.66 (9.28, 12.24) | 77.1% | 22.3% | 14.19 (9.53, 21.13) | 91.9% | 22.3% | 57.14 (35.55, 91.85) |
| ≥2 | 10.7% (0.3) | 47.1% | 10.2% | 12.08 (10.59, 13.78) | 58.7% | 10.2% | 18.89 (13.86, 25.74) | 80.1% | 10.2% | 53.75 (38.50, 75.04) |
| ≥3 | 7.2% (0.3) | 35.2% | 6.5% | 12.37 (10.55, 14.49) | 44.7% | 6.5% | 17.85 (13.13, 24.28) | 67.7% | 6.5% | 43.62 (31.24, 60.90) |
| ≥4 | 4.6% (0.2) | 23.8% | 4.0% | 11.27 (9.40, 13.52) | 26.6% | 4.0% | 10.87 (7.66, 15.44) | 54.2% | 4.0% | 38.66 (27.86, 53.63) |
| Question | Response Options | PATH Study Variable | Weighted Prevalence (Standard Error) | |
|---|---|---|---|---|
| Does your health limit you in any of the following activities? | Choose all that apply. | |||
| Walking up 10 steps | Yes, No | R02_ AX0097_02 | Yes | 4.2% (0.3) |
| Walking 3 blocks | Yes, No | R02_ AX0097_03 | Yes | 5.8% (0.4) |
| Walking a mile | Yes, No | R02_ AX0097_04 | Yes | 9.4% (0.4) |
| In the past 7 days, how would you rate your fatigue on average? By fatigue, we mean feeling unrested or overly tired during the day, no matter how many hours of sleep you’ve had. | 1 = None | R02_ AX0101 | None | 23.8%(0.6) |
| 2 = Mild | Mild | 47.3% (0.6) | ||
| 3 = Moderate | Moderate | 24.1% (0.5) | ||
| 4 = Severe | Severe | 4.0% (0.2) | ||
| 5 = Very severe | Very Severe | 0.8 (0.1) | ||
| In general, how would you rate your physical health? | 1 = Excellent | R02_ AX0090 | Excellent | 17.4% (0.5) |
| 2 = Very good | Very good | 39.7% (0.5) | ||
| 3= Good | Good | 32.5% (0.5) | ||
| 4 = Fair | Fair | 9.3% (0.3) | ||
| 5= Poor | Poor | 1.1% (0.1) | ||
| Respiratory Symptom Index Cut-Off Value | |||||
|---|---|---|---|---|---|
| Functional Outcome | Continuous | ≥1 | ≥2 | ≥3 | ≥4 |
| Adjusted b Odds Ratio (95% CI) | |||||
| Physical limitations c | |||||
| Walking up 10 steps | 1.19 (1.11, 1.28) | 1.46 (1.12, 1.89) | 1.96 (1.42, 2.68) | 2.00 (1.45, 2.76) | 2.45 (1.56, 3.22) |
| Walking 3 blocks | 1.25 (1.18, 1.33) | 1.77 (1.41, 2.22) | 2.10 (1.55, 2.83) | 2.19 (1.60, 3.01) | 2.89 (2.13, 3.93) |
| Walking a mile | 1.31 (1.25, 1.38) | 2.00 (1.65, 2.43) | 2.84 (2.31, 3.49) | 2.71 (2.14, 3.43) | 3.13 (2.39, 4.10) |
| Adjusted b Relative Risk Ratio (95% CI) | |||||
| Fatigue d | |||||
| None | Ref | Ref | Ref | Ref | Ref |
| Mild | 1.24 (1.16, 1.33) | 1.76 (1.51, 2.07) | 1.85 (1.51, 2.29) | 1.83 (1.41, 2.37) | 1.99 (1.41, 2.82) |
| Moderate | 1.45 (1.34, 1.56) | 2.79 (2.35, 3.31) | 3.04 (2.40, 3.86) | 2.85 (2.18, 3.72) | 3.76 (2.68, 5.27) |
| Severe | 1.67 (1.54, 1.81) | 4.19 (3.37, 5.21) | 5.35 (4.02, 7.12) | 5.25 (3.78, 7.29) | 7.04 (4.66, 10.62) |
| Very severe | 1.74 (1.54, 1.96) | 3.82 (2.37, 6.17) | 6.31 (3.94, 10.11) | 7.69 (4.73, 12.49) | 10.00 (5.52, 18.11) |
| Adjusted b Relative Risk Ratio (95% CI) | |||||
| Physical health e | |||||
| Excellent | Ref | Ref | Ref | Ref | Ref |
| Very good | 1.19 (1.11, 1.28) | 1.48 (1.24, 1.76) | 1.78 (1.41, 2.26) | 1.48 (1.12, 1.94) | 1.58 (1.05, 2.36) |
| Good | 1.46 (1.35, 1.58) | 2.53 (2.09, 3.05) | 3.55 (2.71, 4.64) | 3.14 (2.34, 4.20) | 3.64 (2.52, 5.26) |
| Fair | 1.69 (1.54, 1.84) | 3.53 (2.82, 4.42) | 5.87 (4.39, 7.85) | 5.55 (4.07, 7.57) | 6.90 (4.42, 10.79) |
| Poor | 1.91 (1.73, 2.11) | 6.70 (4.46, 10.07) | 8.64 (5.78, 12.93) | 9.02 (5.88, 13.83) | 11.66 (7.21, 18.83) |
| Respiratory Symptom Index Cut-Off Value | |||||
|---|---|---|---|---|---|
| Variable | Continuous | ≥1 | ≥2 | ≥3 | ≥4 |
| Relative risk (95% CI) b | |||||
| Current cigarette smoking c | |||||
| Never | Ref | Ref | Ref | Ref | Ref |
| Former | 1.34 (1.16, 1.55) | 1.28 (1.13, 1.45) | 1.37 (1.14, 1.65) | 1.32 (1.04, 1.69) | 1.49 (1.09, 2.06) |
| Current nondaily | 1.96 (1.67, 2.29) | 1.89 (1.65, 2.15) | 2.08 (1.70, 2.55) | 2.02 (1.58, 2.59) | 2.12 (1.48, 3.03) |
| Current Daily | 3.11 (2.75, 3.52) | 2.29 (2.08, 2.53) | 3.73 (3.22, 4.31) | 4.02 (3.36, 4.81) | 4.31 (3.36, 5.52) |
| Pack-years of cigarette smoking (per each additional 5 years) | 1.05 (1.03, 1.07) | 1.05 (1.03, 1.07) | 1.05 (1.03, 1.07) | 1.05 (1.02, 1.08) | 1.04 (1.01, 1.08) |
| Second-hand smoke exposure (per each additional 5 h) | 1.03 (1.02, 1.04) | 1.02 (1.01, 1.03) | 1.03 (1.02, 1.04) | 1.03 (1.02, 1.04) | 1.04 (1.03, 1.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halenar, M.J.; Sargent, J.D.; Edwards, K.C.; Woloshin, S.; Schwartz, L.; Emond, J.; Tanski, S.; Pierce, J.P.; Taylor, K.A.; Lauten, K.; et al. Validation of an Index for Functionally Important Respiratory Symptoms among Adults in the Nationally Representative Population Assessment of Tobacco and Health Study, 2014–2016. Int. J. Environ. Res. Public Health 2021, 18, 9688. https://doi.org/10.3390/ijerph18189688
Halenar MJ, Sargent JD, Edwards KC, Woloshin S, Schwartz L, Emond J, Tanski S, Pierce JP, Taylor KA, Lauten K, et al. Validation of an Index for Functionally Important Respiratory Symptoms among Adults in the Nationally Representative Population Assessment of Tobacco and Health Study, 2014–2016. International Journal of Environmental Research and Public Health. 2021; 18(18):9688. https://doi.org/10.3390/ijerph18189688
Chicago/Turabian StyleHalenar, Michael J., James D. Sargent, Kathryn C. Edwards, Steven Woloshin, Lisa Schwartz, Jennifer Emond, Susanne Tanski, John P. Pierce, Kristie A. Taylor, Kristin Lauten, and et al. 2021. "Validation of an Index for Functionally Important Respiratory Symptoms among Adults in the Nationally Representative Population Assessment of Tobacco and Health Study, 2014–2016" International Journal of Environmental Research and Public Health 18, no. 18: 9688. https://doi.org/10.3390/ijerph18189688
APA StyleHalenar, M. J., Sargent, J. D., Edwards, K. C., Woloshin, S., Schwartz, L., Emond, J., Tanski, S., Pierce, J. P., Taylor, K. A., Lauten, K., Goniewicz, M. L., Niaura, R., Anic, G., Chen, Y., Callahan-Lyon, P., Gardner, L. D., Thekkudan, T., Borek, N., Kimmel, H. L., ... Brunette, M. F. (2021). Validation of an Index for Functionally Important Respiratory Symptoms among Adults in the Nationally Representative Population Assessment of Tobacco and Health Study, 2014–2016. International Journal of Environmental Research and Public Health, 18(18), 9688. https://doi.org/10.3390/ijerph18189688







