Association between Hypertriglyceridemic-Waist Phenotype and Risk of Type 2 Diabetes Mellitus in Middle-Aged and Older Chinese Population: A Longitudinal Cohort Study
Abstract
1. Introduction
2. Methods
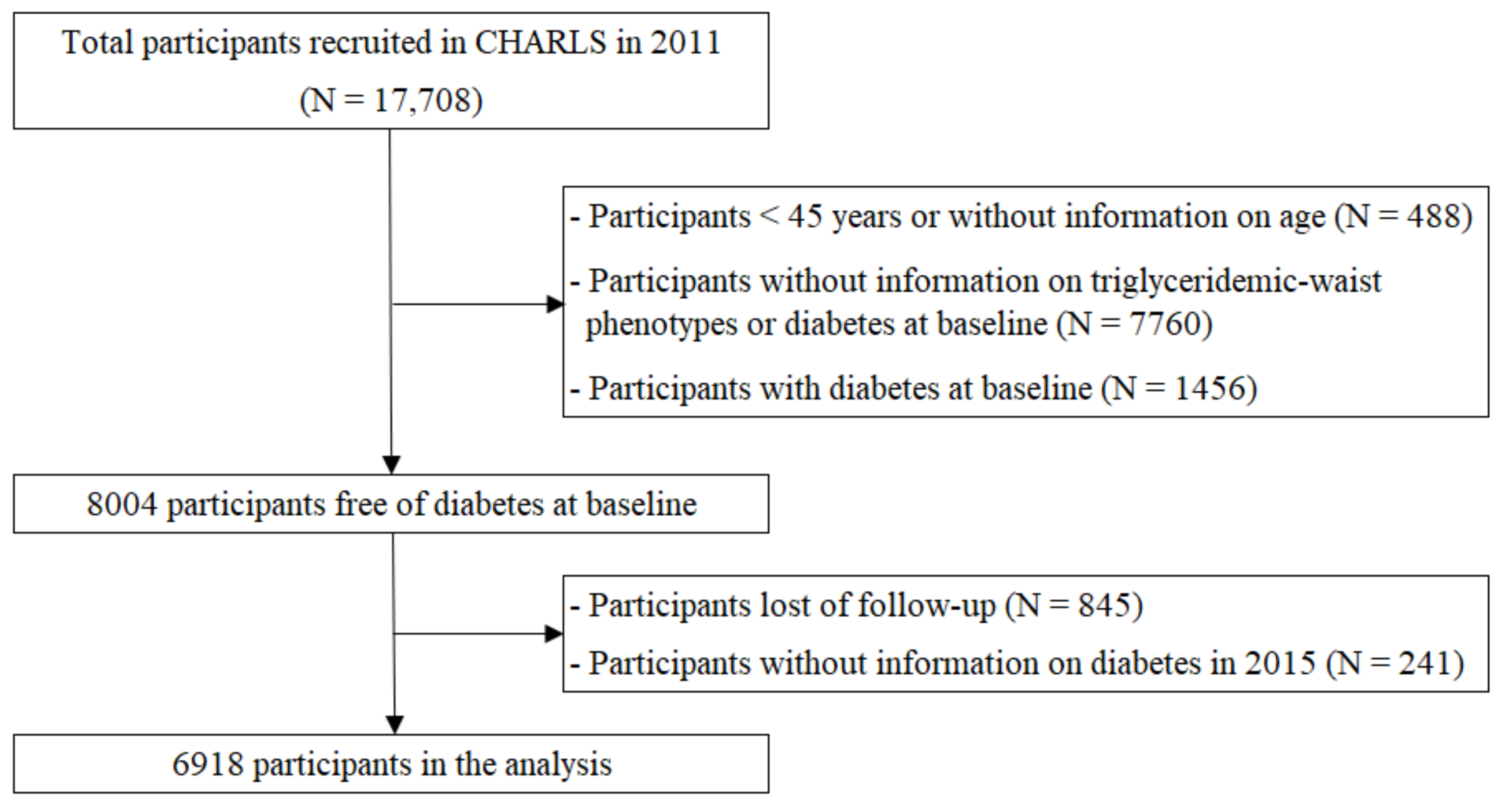
2.1. Study Design and Participant Recruitment
2.2. Outcome Definition
2.3. Exposure Definition
2.4. Measurement of Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.D.; Ley, S.H.; Vasanti, M.; Howard, A.G.; He, Y.; Hu, F.B. Time Trends of Dietary and Lifestyle Factors and Their Potential Impact on Diabetes Burden in China. Diabetes Care 2017, 40, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 9th Edition. Available online: https://www.diabetesatlas.org (accessed on 26 August 2021).
- Obrador, G.T.; Schultheiss, U.T.; Kretzler, M.; Langham, R.G.; Nangaku, M.; Pecoits-Filho, R.; Pollock, C.; Rossert, J.; Correa-Rotter, R.; Stenvinkel, P.; et al. Genetic and environmental risk factors for chronic kidney disease. Kidney Int. Suppl. 2017, 7, 88–106. [Google Scholar] [CrossRef]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [PubMed]
- Cunningham, S.A.; Riosmena, F.; Wang, J.; Boyle, J.P.; Rolka, D.B.; Geiss, L.S. Decreases in diabetes-free life expectancy in the U.S. and the role of obesity. Diabetes Care 2011, 34, 2225–2230. [Google Scholar] [CrossRef]
- Singh, S.; Dhingra, S.; Ramdath, D.D.; Vasdev, S.; Gill, V.; Singal, P.K. Risk factors preceding type 2 diabetes and cardiomyopathy. J. Cardiovasc. Transl. Res. 2010, 3, 580–596. [Google Scholar] [CrossRef]
- Tirosh, A.; Shai, I.; Bitzur, R.; Kochba, I.; Tekes-Manova, D.; Israeli, E.; Shochat, T.; Rudich, A. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care 2008, 31, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, X.; Yu, S.; Sun, G.; Li, Z.; Sun, Y. Association between the Hypertriglyceridemic Waist Phenotype, Prediabetes, and Diabetes Mellitus in Rural Chinese Population: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2016, 13, 368. [Google Scholar] [CrossRef]
- Díaz-Santana, M.V.; Pérez, E.L.S.; Martínez, A.P.O.; Serrano, M.G.; Cardona, C.M.P. Association between the Hypertriglyceridemic Waist Phenotype, Prediabetes, and Diabetes Mellitus among Adults in Puerto Rico. J. Immigr. Minority Health 2016, 18, 102–109. [Google Scholar] [CrossRef]
- Du, T.; Sun, X.; Huo, R.; Yu, X. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: The China Health and Nutrition Survey 2009. Int. J. Obes. Lond. 2014, 38, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Egeland, G.M.; Cao, Z.; Young, T.K. Hypertriglyceridemic-waist phenotype and glucose intolerance among Canadian Inuit: The International Polar Year Inuit Health Survey for Adults 2007–2008. CMAJ 2011, 183, E553–E558. [Google Scholar] [CrossRef][Green Version]
- Gomez-Huelgas, R.; Bernal-López, M.R.; Villalobos, A.; Mancera-Romero, J.; Baca-Osorio, A.J.; Jansen, S.; Guijarro, R.; Salgado, F.; Tinahones, F.J.; Serrano-Ríos, M. Hypertriglyceridemic waist: An alternative to the metabolic syndrome? Results of the IMAP Study (multidisciplinary intervention in primary care). Int. J. Obes. Lond. 2011, 35, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, V.Y.W.; Yu, E.Y.T.; Wong, C.K.H.; Sit, R.W.S.; Wang, J.H.L.; Lam, C.L.K. Hypertriglyceridaemic-waist phenotype and risk of diabetes in people with impaired fasting glucose in primary care: A cohort study. Diabet. Med. 2018, 35, 576–582. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, S.Y.; Kim, N.H.; Chae, H.B.; Lee, T.H.; Jang, C.M.; Yoo, K.M.; Park, H.J.; Lee, M.K.; Jeon, W.S.; et al. Increased risk of diabetes development in subjects with the hypertriglyceridemic waist phenotype: A 4-year longitudinal study. Endocrinol. Metab. Seoul 2014, 29, 514–521. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zheng, Y.; Shu, Y.; He, J.; Wang, Y.; Chen, X. Hypertriglyceridemic waist might be an alternative to metabolic syndrome for predicting future diabetes mellitus. PLoS ONE 2013, 8, e73292. [Google Scholar] [CrossRef]
- Janghorbani, M.; Amini, M. Utility of hypertriglyceridemic waist phenotype for predicting incident type 2 diabetes: The Isfahan Diabetes Prevention Study. J. Diabetes Investig. 2016, 7, 860–866. [Google Scholar] [CrossRef]
- Ma, C.-M.; Liu, X.-L.; Lu, N.; Wang, R.; Lu, Q.; Yin, F.-Z. Hypertriglyceridemic waist phenotype and abnormal glucose metabolism: A system review and meta-analysis. Endocrine 2019, 64, 469–485. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Y.; Sun, X.; Deng, K.; Wang, C.; Li, L.; Zhang, L.; Wang, B.; Zhao, Y.; Zhou, J.; et al. Hypertriglyceridemia-waist and risk of developing type 2 diabetes: The Rural Chinese Cohort Study. Sci. Rep. 2017, 7, 9072. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, M.; Zhao, J.; Wang, C.; Luo, X.; Zhang, J.; Zhu, T.; Li, X.; Yin, L.; Pang, C.; et al. Association of the hypertriglyceridemic waist phenotype and type 2 diabetes mellitus among adults in China. J. Diabetes Investig. 2016, 7, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Huang, J.; Hu, D.; Chen, J.; Cao, J.; Li, J. Is an appropriate cutoff of hypertriglyceridemic waist designated for type 2 diabetes among Chinese adults? Clin. Nutr. 2010, 29, 192–198. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Qi, Q.; Wu, H.; Lu, L.; Liu, C.; Li, H.; Lin, X. Hypertriglyceridemic waist, cytokines and hyperglycaemia in Chinese. Eur J. Clin. Investig. 2012, 42, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, Y.; Chang, H.; Wang, X.; Liu, D.; Zhu, Z.; Huang, G. Hypertriglyceridemic-waist phenotype predicts diabetes: A cohort study in Chinese urban adults. BMC Public Health 2012, 12, 1081. [Google Scholar] [CrossRef]
- Woo, J.; Kwok, T.; Sze, F.; Yuan, H. Ageing in China: Health and social consequences and responses. Int. J. Epidemiol. 2002, 31, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Gustafson, B.; Kirkland, J.L.; Smith, U. Cellular senescence: At the nexus between ageing and diabetes. Diabetologia 2019, 62, 1835–1841. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- American Diabetes Association (ADA). Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Bao, Y.; Lu, J.; Wang, C.; Yang, M.; Li, H.; Zhang, X.; Zhu, J.; Lu, H.; Jia, W.; Xiang, K. Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis 2008, 201, 378–384. [Google Scholar] [CrossRef]
- Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Chin. J. Pract. Intern. Med. 2018, 38, 292–344. [Google Scholar]
- Zhou, B.F.; Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults-study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar]
- Liu, L.-S. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011, 39, 579–615. [Google Scholar] [PubMed]
- Lu, J.; He, J.; Li, M.; Tang, X.; Hu, R.; Shi, L.; Su, Q.; Peng, K.; Xu, M.; Xu, Y.; et al. Predictive Value of Fasting Glucose, Postload Glucose, and Hemoglobin A(1c) on Risk of Diabetes and Complications in Chinese Adults. Diabetes Care 2019, 42, 1539–1548. [Google Scholar] [CrossRef]
- Feller, S.; Boeing, H.; Pischon, T. Body mass index, waist circumference, and the risk of type 2 diabetes mellitus: Implications for routine clinical practice. Dtsch. Arztebl. Int. 2010, 107, 470–476. [Google Scholar]
- Pollex, R.L.; Hanley, A.J.G.; Zinman, B.; Harris, S.B.; Hegele, R.A. Clinical and genetic associations with hypertriglyceridemic waist in a Canadian aboriginal population. Int. J. Obes. Lond. 2006, 30, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Papaetis, G.S.; Papakyriakou, P.; Panagiotou, T.N. Central obesity, type 2 diabetes and insulin: Exploring a pathway full of thorns. Arch. Med. Sci. 2015, 11, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. S3), 14–23. [Google Scholar] [CrossRef]
- Szmuilowicz, E.D.; Stuenkel, C.A.; Seely, E.W. Influence of menopause on diabetes and diabetes risk. Nat. Rev. Endocrinol. 2009, 5, 553–558. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Li, L.; Zhang, L.; Ren, Y.; Zhou, H.; Cui, L.; Mao, Z.; Hu, D.; Wang, C. Prevalence, awareness, treatment, control of type 2 diabetes mellitus and risk factors in Chinese rural population: The RuralDiab study. Sci. Rep. 2016, 6, 31426. [Google Scholar] [CrossRef]
- Guo, V.Y.; Cao, B.; Cai, C.; Cheng, K.K.-Y.; Cheung, B.M.Y. Fetuin-A levels and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2018, 55, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef] [PubMed]
| NWNT | NWHT | EWNT | EWHT | p Value | |
|---|---|---|---|---|---|
| n | 3494 | 672 | 1830 | 922 | |
| Demographic and lifestyle factors | |||||
| Mean age (years) | 59.6 ± 9.3 | 58.0 ± 8.7 | 59.0 ± 9.2 | 58.6 ± 8.8 | <0.001 |
| Gender, n (%) | <0.001 | ||||
| Male | 1966 (56.3%) | 356 (53.0%) | 581 (31.7%) | 288 (31.2%) | |
| Female | 1528 (43.7%) | 316 (47.0%) | 1249 (68.3%) | 634 (68.8%) | |
| Current smoker, n (%) | 1329 (38.2%) | 247 (36.9%) | 336 (18.4%) | 180 (19.6%) | <0.001 |
| Current drinker, n (%) | 1320 (37.8%) | 231 (34.5%) | 468 (25.6%) | 246 (26.7%) | <0.001 |
| Education, n (%) | 0.189 | ||||
| Illiterate/no formal education | 1740 (49.8%) | 302 (44.9%) | 938 (51.3%) | 456 (49.5%) | |
| Primary school | 791 (22.6%) | 162 (24.1%) | 362 (19.8%) | 204 (22.1%) | |
| Middle school | 633 (18.1%) | 151 (22.5%) | 360 (19.7%) | 166 (18.0%) | |
| High school or above | 330 (9.4%) | 57 (8.5%) | 170 (9.3%) | 96 (10.4%) | |
| Current marital status, n (%) | 0.350 | ||||
| Not married | 557 (15.9%) | 90 (13.4%) | 284 (15.5%) | 135 (14.7%) | |
| Married or cohabitated | 2936 (84.1%) | 582 (86.6%) | 1546 (84.5%) | 786 (85.3%) | |
| Area of residence, n (%) | <0.001 | ||||
| Rural | 2539 (72.7%) | 469 (69.8%) | 1139 (62.2%) | 532 (57.7%) | |
| Urban | 955 (27.3%) | 203 (30.2%) | 691 (37.8%) | 390 (42.3%) | |
| Clinical/biochemical measures | |||||
| BMI (kg/m2) | 21.3 ± 2.7 | 22.2 ± 2.6 | 25.9 ± 3.3 | 26.8 ± 3.0 | <0.001 |
| Waist circumference (cm) | |||||
| Male | 79.3 ± 5.9 | 81.8 ± 5.7 | 96.1 ± 5.5 | 97.0 ± 5.3 | <0.001 |
| Female | 76.6 ± 5.6 | 78.2 ± 4.7 | 92.6 ± 6.4 | 94.4 ± 6.9 | <0.001 |
| Systolic BP (mmHg) | 125.9 ± 20.7 | 128.3 ± 20.3 | 133.8 ± 21.4 | 135.8 ± 21.9 | <0.001 |
| Diastolic BP (mmHg) | 73.1 ± 11.9 | 75.1 ± 11.8 | 77.9 ± 12.0 | 79.5 ± 12.4 | <0.001 |
| Plasma glucose (mmol/L) | 5.5 ± 0.8 | 5.8 ± 0.8 | 5.6 ± 0.7 | 5.9 ± 0.8 | <0.001 |
| HbA1c (%) | 5.1 ± 0.4 | 5.1 ± 0.4 | 5.1 ± 0.4 | 5.2 ± 0.4 | <0.001 |
| Total cholesterol (mmol/L) | 4.8 ± 0.9 | 5.3 ± 1.0 | 5.0 ± 0.9 | 5.4 ± 1.1 | <0.001 |
| Triglycerides (mmol/L) | 0.9 (0.7–1.2) | 2.2 (1.9–2.8) | 1.1 (0.9–1.4) | 2.3 (2.0–3.0) | <0.001 |
| HDL-cholesterol (mmol/L) | 1.5 ± 0.4 | 1.1 ± 0.3 | 1.3 ± 0.3 | 1.0 ± 0.3 | <0.001 |
| LDL-cholesterol (mmol/L) | 2.9 ± 0.8 | 3.0 ± 1.0 | 3.2 ± 0.9 | 3.0 ± 1.0 | <0.001 |
| History of chronic diseases | |||||
| Hypertension, n (%) | 1176 (33.8%) | 270 (40.4%) | 967 (53.2%) | 561 (61.1%) | <0.001 |
| Dyslipidemia, n (%) | 277 (8.7%) | 78 (13.1%) | 292 (17.8%) | 249 (30.1%) | <0.001 |
| NWNT | NWHT | EWNT | EWHT | ||||
|---|---|---|---|---|---|---|---|
| RR (95%CI) | RR (95%CI) | p Value | RR (95%CI) | p Value | RR (95%CI) | p Value | |
| Crude model | Ref | 1.253 (0.923–1.703) | 0.148 | 2.153 (1.781–2.602) | <0.001 | 3.179 (2.568–3.934) | <0.001 |
| Model 1 | Ref | 1.093 (0.817–1.462) | 0.550 | 1.998 (1.662–2.400) | <0.001 | 2.423 (1.979–2.966) | <0.001 |
| Model 2 | Ref | 1.063 (0.793–1.425) | 0.682 | 1.580 (1.265–1.972) | <0.001 | 1.909 (1.499–2.447) | <0.001 |
| NWNT | NWHT | EWNT | EWHT | ||||
|---|---|---|---|---|---|---|---|
| RR (95%CI) | RR (95%CI) | p Value | RR (95%CI) | p Value | RR (95%CI) | p Value | |
| Gender | |||||||
| Male | Ref | 0.914 (0.604–1.382) | 0.670 | 1.603 (1.131–2.271) | 0.008 | 1.977 (1.334–2.928) | 0.001 |
| Female | Ref | 1.285 (0.842–1.960) | 0.245 | 1.652 (1.222–2.234) | 0.001 | 1.947 (1.394–2.719) | <0.001 |
| Age group | |||||||
| <60 years | Ref | 0.907 (0.576–1.429) | 0.675 | 1.631 (1.174–2.266) | 0.004 | 1.937 (1.343–2.793) | <0.001 |
| ≥60 years | Ref | 1.224 (0.836–1.790) | 0.299 | 1.526 (1.128–2.064) | 0.006 | 1.854 (1.323–2.599) | <0.001 |
| Area of residence | |||||||
| Rural | Ref | 1.052 (0.747–1.481) | 0.773 | 1.557 (1.197–2.024) | 0.001 | 2.081 (1.547–2.801) | <0.001 |
| Urban | Ref | 1.083 (0.613–1.914) | 0.784 | 1.525 (1.008–2.307) | <0.046 | 1.564 (0.997–2.455) | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Liang, Z.; Sun, H.; Lu, C.; Chen, W.; Wang, H.H.X.; Guo, V.Y. Association between Hypertriglyceridemic-Waist Phenotype and Risk of Type 2 Diabetes Mellitus in Middle-Aged and Older Chinese Population: A Longitudinal Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 9618. https://doi.org/10.3390/ijerph18189618
Chen D, Liang Z, Sun H, Lu C, Chen W, Wang HHX, Guo VY. Association between Hypertriglyceridemic-Waist Phenotype and Risk of Type 2 Diabetes Mellitus in Middle-Aged and Older Chinese Population: A Longitudinal Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(18):9618. https://doi.org/10.3390/ijerph18189618
Chicago/Turabian StyleChen, Dezhong, Ziyun Liang, Huimin Sun, Ciyong Lu, Weiqing Chen, Harry H. X. Wang, and Vivian Yawei Guo. 2021. "Association between Hypertriglyceridemic-Waist Phenotype and Risk of Type 2 Diabetes Mellitus in Middle-Aged and Older Chinese Population: A Longitudinal Cohort Study" International Journal of Environmental Research and Public Health 18, no. 18: 9618. https://doi.org/10.3390/ijerph18189618
APA StyleChen, D., Liang, Z., Sun, H., Lu, C., Chen, W., Wang, H. H. X., & Guo, V. Y. (2021). Association between Hypertriglyceridemic-Waist Phenotype and Risk of Type 2 Diabetes Mellitus in Middle-Aged and Older Chinese Population: A Longitudinal Cohort Study. International Journal of Environmental Research and Public Health, 18(18), 9618. https://doi.org/10.3390/ijerph18189618







