Differences in Reward Sensitivity between High and Low Problematic Smartphone Use Adolescents: An ERP Study
Abstract
:1. Introduction
1.1. Problematic Smartphone Use and Reward Sensitivity in Adolescents
1.2. Biomarkers of Reward and Punishment Feedbacks
2. Method
2.1. Participants
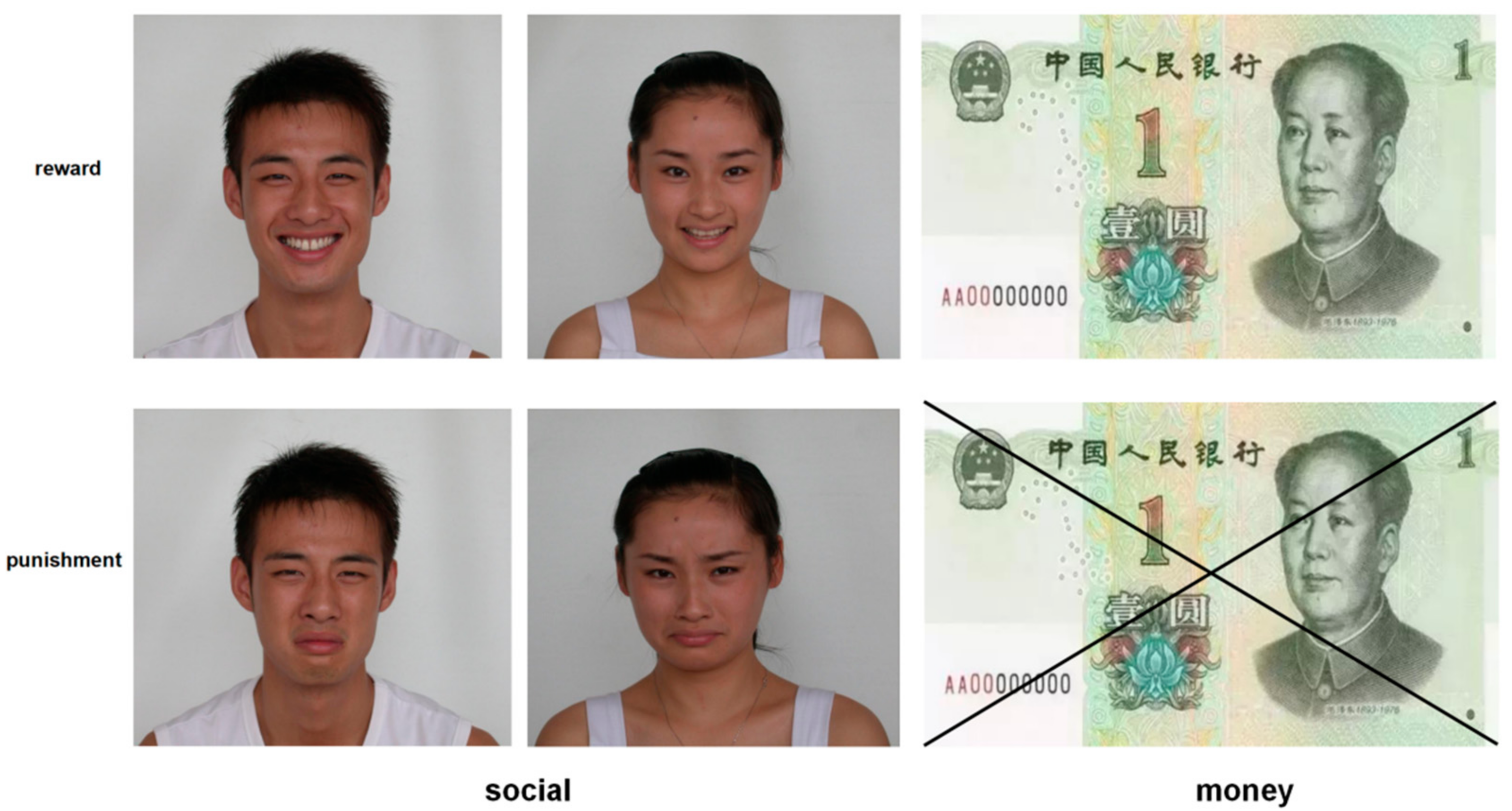
2.2. The Monetary and Social Reward Tasks
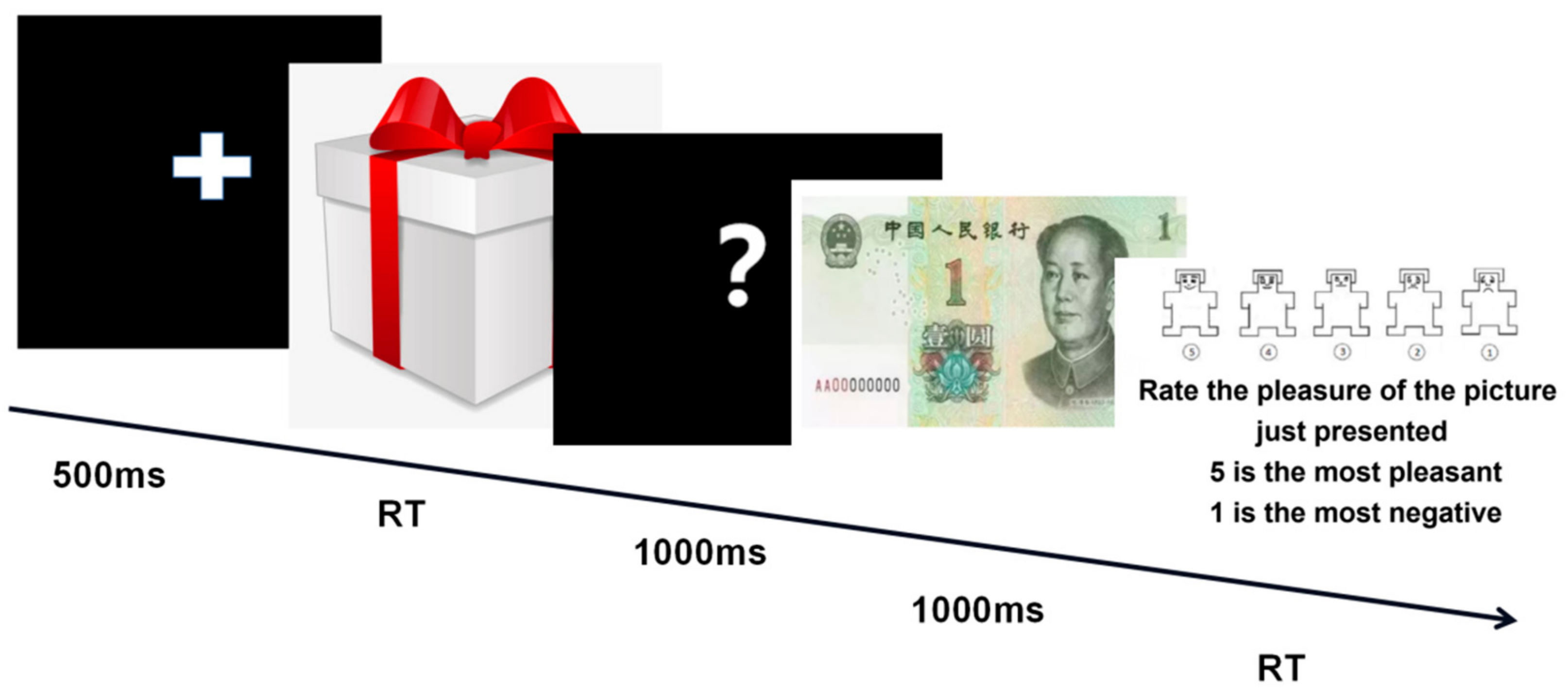
2.3. Procedure
2.4. Psychophysiological Recording, Data Reduction, and Analysis
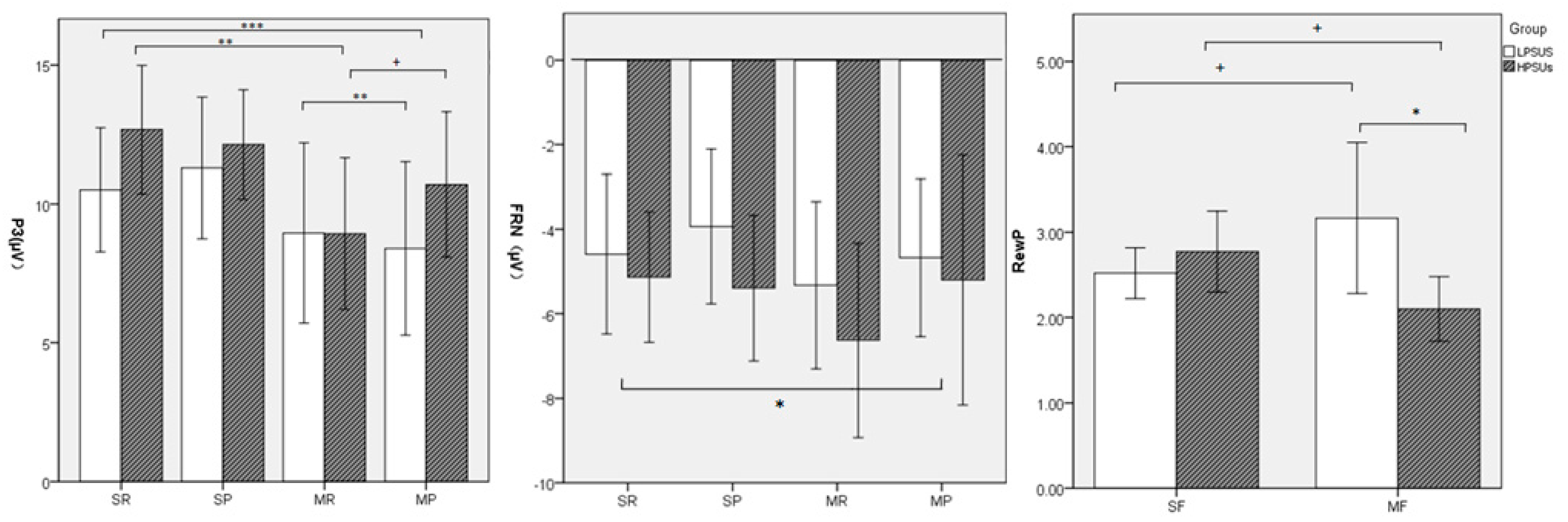
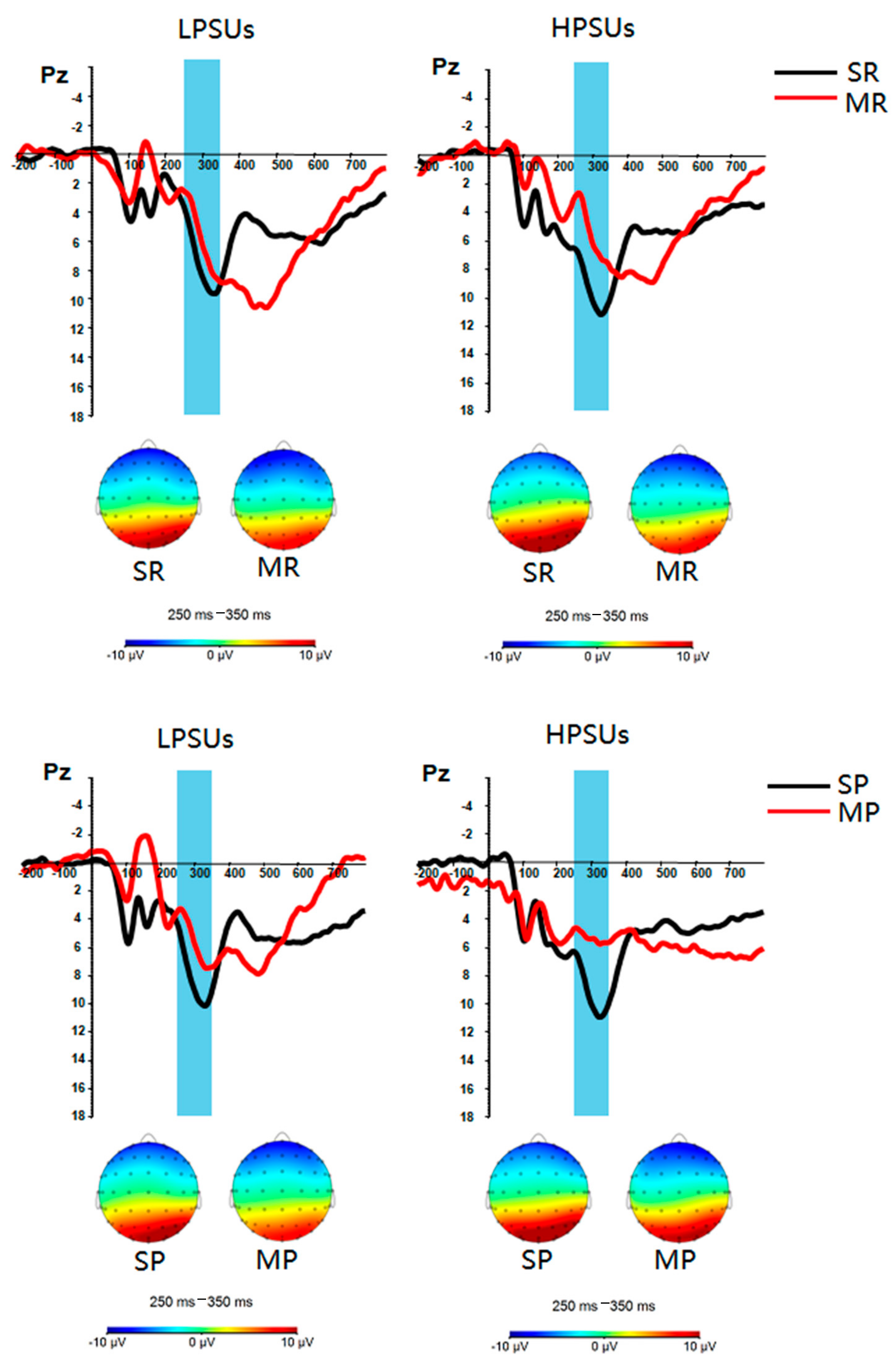
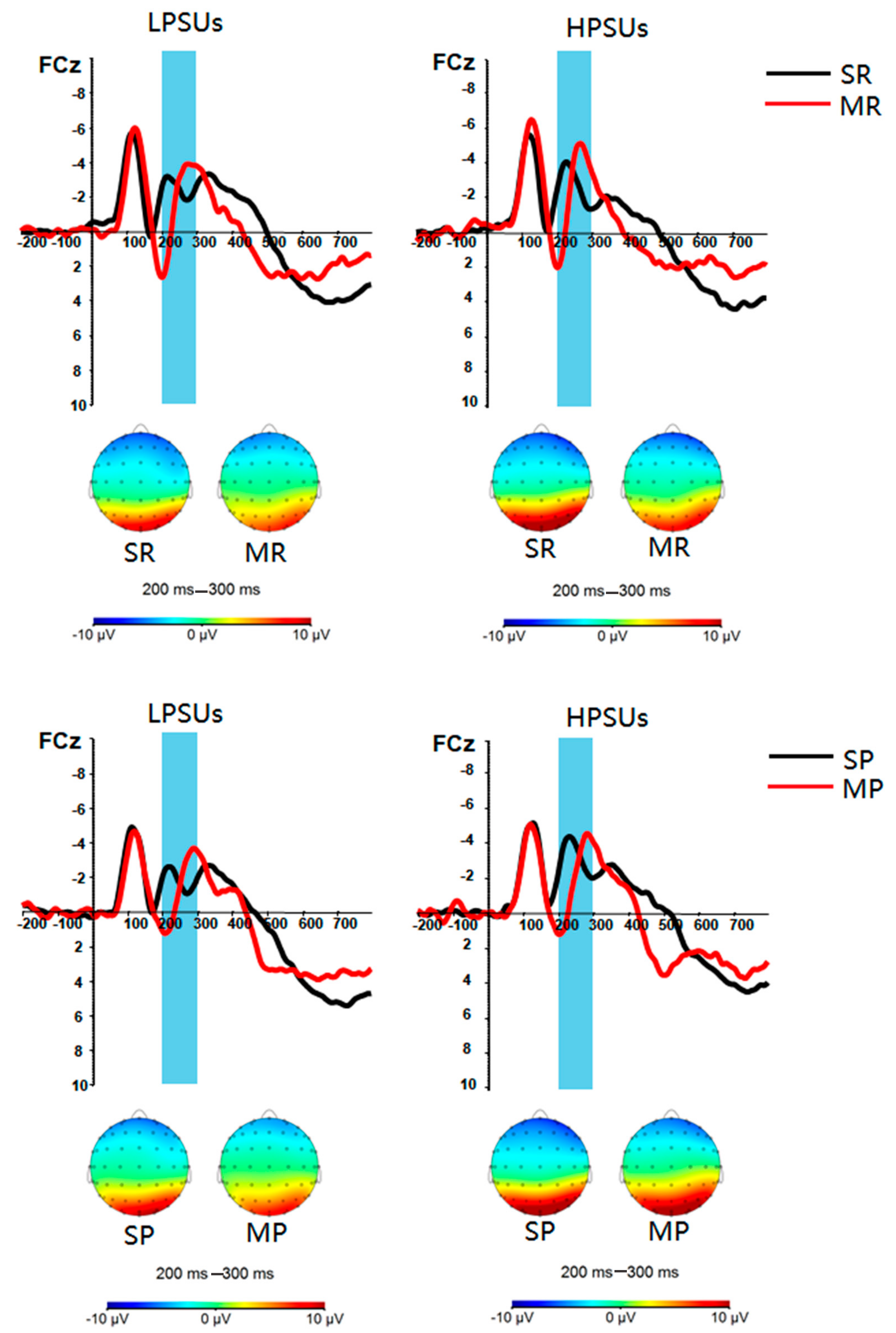
3. Results
Neural Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
References
- Silvers, J.A.; Mcrae, K.; Gabrieli, J.D.; Gross, J.J.; Remy, K.A.; Ochsner, K.N. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion 2012, 12, 1235–1247. [Google Scholar] [CrossRef] [Green Version]
- Chóliz, M. Mobile-phone addiction in adolescence: The test of mobile phone dependence (TMD). Prog. Health Ences 2012, 2, 33–44. [Google Scholar]
- He, J.; Chen, C.; Bao, Y.; Lei, Y. A probe into mobile phone dependence in adolescents: Measurement, harmfulness and genesis mechanism. Chin. J. Clin. Psychol. 2012, 20, 822–825. [Google Scholar]
- Billieux, J.; Maurage, P.; Lopez-Fernandez, O.; Kuss, D.J.; Griffiths, M.D. Can Disordered Mobile Phone Use Be Considered a Behavioral Addiction? An Update on Current Evidence and a Comprehensive Model for Future Research. Curr. Addict. Rep. 2015, 2, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Billieux, J. Problematic Use of the Mobile Phone: A Literature Review and a Pathways Model. Curr. Psychiatry Rev. 2012, 8, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Thomée, S.; Eklöf, M.; Gustafsson, E.; Nilsson, R.; Hagberg, M. Prevalence of perceived stress, symptoms of depression and sleep disturbances in relation to information and communication technology (ICT) use among young adults—An explorative prospective study. Computers Hum. Behav. 2007, 23, 1300–1321. [Google Scholar] [CrossRef]
- Gonçalves, S.; Dias, P.; Correia, A.-P. Nomophobia and lifestyle: Smartphone use and its relationship to psychopathologies. Comput. Hum. Behav. Rep. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Burani, K.; Mulligan, E.M.; Klawohn, J.; Luking, K.R.; Nelson, B.D.; Hajcak, G. Longitudinal increases in reward-related neural activity in early adolescence: Evidence from event-related potentials (ERPs). Dev. Cogn. Neurosci. 2019, 36, 100620. [Google Scholar] [CrossRef]
- Kohls, G.; Peltzer, J.; Herpertz-Dahlmann, B.; Konrad, K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Dev. Sci. 2009, 12, 614–625. [Google Scholar] [CrossRef]
- Balconi, M.; Finocchiaro, R. Deficit in rewarding mechanisms and prefrontal left/right cortical effect in vulnerability for internet addiction. Acta Neuropsychiatr. 2016, 28, 272–285. [Google Scholar] [CrossRef]
- Park, S.M.; Park, Y.A.; Lee, H.W.; Jung, H.Y.; Lee, J.-Y.; Choi, J.-S. The effects of behavioral inhibition/approach system as predictors of Internet addiction in adolescents. Pers. Individ. Differ. 2013, 54, 7–11. [Google Scholar] [CrossRef]
- Balconi, M.; Venturella, I.; Finocchiaro, R. Evidences from rewarding system, FRN and P300 effect in Internet-addiction in young people. Brain Sci. 2017, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.; Huang, J.; Du, X. Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: An fMRI study during a guessing task. J. Psychiatr. Res. 2011, 45, 1525–1529. [Google Scholar] [CrossRef]
- Gao, Q.; Jia, G.; Zhao, J.; Zhang, D. Inhibitory control in excessive social networking users: Evidence from an ERP-based Go-Nogo Task. Front. Psychol. 2019, 10, 1810–1820. [Google Scholar] [CrossRef] [Green Version]
- Izuma, K.; Saito, D.N.; Sadato, N. Processing of Social and Monetary Rewards in the Human Striatum. Neuron 2008, 58, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Somerville, L.H. The Teenage Brain: Sensitivity to Social Evaluation. Curr. Dir. Psychol. Sci. 2013, 22, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, L.; Blakemore, S.-J. Is there heightened sensitivity to social reward in adolescence? Curr. Opin. Neurobiol. 2016, 40, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Cromheeke, S.; Mueller, S.C. The power of a smile: Stronger working memory effects for happy faces in adolescents compared to adults. Cogn. Emot. 2016, 30, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, J.H.; Masten, C.L.; Moore, W.E., III; Oswald, T.M.; Mazziotta, J.C.; Iacoboni, M.; Dapretto, M. Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron 2011, 69, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crone, E.A.; Dahl, R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012, 13, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Van Leijenhorst, L.; Zanolie, K.; Van Meel, C.S.; Westenberg, P.M.; Rombouts, S.A.; Crone, E.A. What Motivates the Adolescent? Brain Regions Mediating Reward Sensitivity across Adolescence. Cereb. Cortex 2010, 20, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-F.; Tang, T.-C.; Yen, J.-Y.; Lin, H.-C.; Huang, C.-F.; Liu, S.-C.; Ko, C.-H. Symptoms of problematic cellular phone use, functional impairment and its association with depression among adolescents in Southern Taiwan. J. Adolesc. 2009, 32, 863–873. [Google Scholar] [CrossRef]
- Hadar, A.; Eliraz, D.; Lazarovits, A.; Alyagon, U.; Zangen, A. Using longitudinal exposure to causally link smartphone usage to changes in behavior, cognition and right prefrontal neural activity. Brain Stimul. 2015, 8, 318. [Google Scholar] [CrossRef]
- Sherman, L.E.; Payton, A.A.; Hernandez, L.M.; Greenfield, P.M.; Dapretto, M. The power of the like in adolescence: Effects of peer influence on neural and behavioral responses to social media. Psychol. Sci. 2016, 27, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Meshi, D.; Morawetz, C.; Heekeren, H.R. Nucleus accumbens response to gains in reputation for the self relative to gains for others predicts social media use. Front. Hum. Neurosci. 2013, 7, 439. [Google Scholar] [CrossRef] [Green Version]
- Lange, S.; Leue, A.; Beauducel, A. Behavioral approach and reward processing: Results on feedback-related negativity and P3 component. Biol. Psychol. 2012, 89, 416–425. [Google Scholar] [CrossRef]
- Boksem, M.A.S.; Kostermans, E.; De Cremer, D. Failing where others have succeeded: Medial Frontal Negativity tracks failure in a social context. Psychophysiology 2010, 48, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Tucker, D.M.; Derryberry, D.; Reed, M.; Poulsen, C. Electrophysiological Responses to Errors and Feedback in the Process of Action Regulation. Psychol. Sci. 2003, 14, 47–53. [Google Scholar] [CrossRef]
- Boksem, M.A.S.; Ruys, K.I.; Aarts, H. Facing disapproval: Performance monitoring in a social context. Soc. Neurosci. 2011, 6, 360–368. [Google Scholar] [CrossRef]
- Tucker, D.M.; Luu, P.; Derryberry, D. Love hurts: The evolution of empathic concern through the encephalization of nociceptive capacity. Dev. Psychopathol. 2005, 17, 699–713. [Google Scholar] [CrossRef]
- Flores, A.; Münte, T.F.; Doñamayor, N. Event-related EEG responses to anticipation and delivery of monetary and social reward. Biol. Psychol. 2015, 109, 10–19. [Google Scholar] [CrossRef]
- Yeung, N.; Sanfey, A.G. Independent coding of reward magnitude and valence in the human brain. J. Neurosci. 2004, 24, 6258–6264. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhou, X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Res. 2009, 1286, 114–122. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Krigolson, O.E.; Lee, S. Reward positivity elicited by predictive cues. NeuroReport 2011, 22, 249–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydari, S.; Holroyd, C.B. Reward positivity: Reward prediction error or salience prediction error? Psychophysiology 2016, 53, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Lukie, C.N.; Montazer-Hojat, S.; Holroyd, C.B. Developmental changes in the reward positivity: An electrophysiological trajectory of reward processing. Dev. Cogn. Neurosci. 2014, 9, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Van Der Molen, M.J.W.; Poppelaars, E.S.; Van Hartingsveldt, C.T.A.; Harrewijn, A.; Moor, B.G.; Westenberg, P.M. Fear of negative evaluation modulates electrocortical and behavioral responses when anticipating social evaluative feedback. Front. Hum. Neurosci. 2014, 7, 936. [Google Scholar] [CrossRef] [Green Version]
- Balconi, M.; Crivelli, D. FRN and P300 ERP effect modulation in response to feedback sensitivity: The contribution of punishment-reward system (BIS/BAS) and Behaviour Identification of action. Neurosci. Res. 2010, 66, 162–172. [Google Scholar] [CrossRef]
- Threadgill, A.H.; Gable, P.A. Approach-motivated pregoal states enhance the reward positivity. Psychophysiology 2016, 53, 733–738. [Google Scholar] [CrossRef]
- Dong, G.; Lu, Q.; Zhou, H.; Zhao, X. Impulse inhibition in people with Internet addiction disorder: Electrophysiological evidence from a Go/NoGo study. Neurosci. Lett. 2010, 485, 138–142. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N.; Mayes, L.C.; Crowley, M.J. Blunted feedback processing during risk-taking in adolescents with features of problematic Internet use. Addict. Behav. 2015, 45, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Frömer, R.; Stürmer, B.; Sommer, W. The better, the bigger: The effect of graded positive performance feedback on the reward positivity. Biol. Psychol. 2016, 114, 61–68. [Google Scholar] [CrossRef]
- Foerster, M.; Roser, K.; Schoeni, A.; Röösli, M. Problematic mobile phone use in adolescents: Derivation of a short scale MPPUS-10. Int. J. Public Health 2015, 60, 277–286. [Google Scholar] [CrossRef] [Green Version]
- De-Sola, J.; Talledo, H.; Rubio, G.; De Fonseca, F.R. Psychological Factors and Alcohol Use in Problematic Mobile Phone Use in the Spanish Population. Front. Psychiatry 2017, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Spreckelmeyer, K.N.; Krach, S.; Kohls, G.; Rademacher, L.; Irmak, A.; Konrad, K.; Kircher, T.; Gründer, G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci. 2009, 4, 158–165. [Google Scholar] [CrossRef]
- Casement, M.D.; Guyer, A.E.; Hipwell, A.E.; McAloon, R.L.; Hoffmann, A.M.; Keenan, K.E.; Forbes, E.E. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev. Cogn. Neurosci. 2014, 8, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Luo, Y. Standardization and Assessment of College Students’ Facial Expression of Emotion. Chin. J. Clin. Psychol. 2005, 4, 21–23. [Google Scholar]
- Yadon, C.A.; Daugherty, T.K. Auditory Sensory Gating and the Big Five Personality Factors. Psychophysiology 2019, 33, 276–285. [Google Scholar] [CrossRef]
- Martin, L.E.; Potts, G.F. Impulsivity in decision-making: An event-related potential investigation. Pers. Individ. Differ. 2009, 46, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Burnett, S.; Sebastian, C.; Kadosh, K.C.; Blakemore, S.-J. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neurosci. Biobehav. Rev. 2011, 35, 1654–1664. [Google Scholar] [CrossRef] [Green Version]
- Przybylski, A.K.; Murayama, K.; DeHaan, C.R.; Gladwell, V. Motivational, emotional, and behavioral correlates of fear of missing out. Comput. Hum. Behav. 2013, 29, 1841–1848. [Google Scholar] [CrossRef]
- Forbes, E.E.; Ryan, N.D.; Phillips, M.L.; Manuck, S.B.; Worthman, C.M.; Moyles, D.L.; Tarr, J.A.; Sciarrillo, S.R.; Dahl, R.E. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child. Adolesc. Psychiatry 2010, 49, 162–172.e5. [Google Scholar] [CrossRef]
- Van Deursen, A.J.; Bolle, C.L.; Hegner, S.M.; Kommers, P.A. Modeling habitual and addictive smartphone behavior: The role of smartphone use types, emotional intelligence, social stress, self-regulation, age, and gender. Comput. Hum. Behav. 2015, 45, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Wilmer, H.H.; Sherman, L.E.; Chein, J.M. Smartphones and Cognition: A Review of Research Exploring the Links between Mobile Technology Habits and Cognitive Functioning. Front. Psychol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Yen, J.-Y.; Ko, C.-H.; Yen, C.-F.; Chen, C.-S. The association between harmful alcohol use and Internet addiction among college students: Comparison of personality. Psychiatry Clin. Neurosci. 2009, 63, 218–224. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Holroyd, C.B.; Mol, N.; Coles, M.G. Reinforcement-related brain potentials from medial frontal cortex: Origins and functional significance. Neurosci. Biobehav. Rev. 2004, 28, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Tan, Ç.; Pamuk, M.; Dönder, A. Loneliness and Mobile Phone. Procedia-Soc. Behav. Sci. 2013, 103, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Lei, L.; Wang, X.; Nie, J.; Chu, X.; Jin, S. The exacerbating role of perceived social support and the “buffering” role of depression in the relation between sensation seeking and adolescent smartphone addiction. Pers. Individ. Differ. 2018, 130, 129–134. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, J.; Hu, L.; Zeng, H. Neurophysiological evidences of the transient effects of mindfulness induction on emotional processing in children: An ERP study. Int. J. Psychophysiol. 2019, 143, 36–43. [Google Scholar] [CrossRef]

| ERPs | Conditions | LPSUs | HPSUs | t | d | p | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| P3 | SR | 10.51 ± 5.41 | 12.68 ± 5.08 | −1.39 | −0.42 | 0.17 | −5.31 | 0.97 |
| MR | 8.95 ± 7.87 | 8.93 ± 6.00 | 0.01 | 0.00 | 0.99 | −4.20 | 4.25 | |
| SP | 11.29 ± 6.18 | 12.14 ± 4.33 | −0.53 | −0.16 | 0.60 | −4.07 | 2.39 | |
| MP | 8.39 ± 7.56 | 10.70 ± 5.74 | −1.15 | −0.35 | 0.26 | −6.36 | 1.75 | |
| FRN | SR | −4.59 ± 4.58 | −5.14 ± 3.40 | 0.45 | 0.14 | 0.66 | −1.89 | 2.98 |
| MR | −5.32 ± 4.79 | −6.63 ± 5.05 | 0.90 | 0.27 | 0.37 | −1.62 | 4.24 | |
| SP | −3.94 ± 4.44 | −5.40 ± 3.79 | 1.19 | 0.36 | 0.24 | −1.02 | 3.94 | |
| MP | −4.67 ± 4.53 | −5.20 ± 6.50 | 0.32 | 0.10 | 0.75 | −2.76 | 3.81 | |
| RewP | SF | 0.88 ± 0.29 | 0.95 ± 0.39 | −0.64 | −0.20 | 0.52 | −0.27 | 0.14 |
| MF | 0.97 ± 0.61 | 0.66 ± 0.43 | 1.94 | 0.59 | 0.06 | −0.01 | 0.63 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Gao, Q.; Hu, L.; Zhang, L.; Li, Y.; Bu, X. Differences in Reward Sensitivity between High and Low Problematic Smartphone Use Adolescents: An ERP Study. Int. J. Environ. Res. Public Health 2021, 18, 9603. https://doi.org/10.3390/ijerph18189603
Deng X, Gao Q, Hu L, Zhang L, Li Y, Bu X. Differences in Reward Sensitivity between High and Low Problematic Smartphone Use Adolescents: An ERP Study. International Journal of Environmental Research and Public Health. 2021; 18(18):9603. https://doi.org/10.3390/ijerph18189603
Chicago/Turabian StyleDeng, Xinmei, Qiufeng Gao, Lijun Hu, Lin Zhang, Yanzhen Li, and Xiangyu Bu. 2021. "Differences in Reward Sensitivity between High and Low Problematic Smartphone Use Adolescents: An ERP Study" International Journal of Environmental Research and Public Health 18, no. 18: 9603. https://doi.org/10.3390/ijerph18189603
APA StyleDeng, X., Gao, Q., Hu, L., Zhang, L., Li, Y., & Bu, X. (2021). Differences in Reward Sensitivity between High and Low Problematic Smartphone Use Adolescents: An ERP Study. International Journal of Environmental Research and Public Health, 18(18), 9603. https://doi.org/10.3390/ijerph18189603





