Effects of Training with Different Modes of Strength Intervention on Psychosocial Disorders in Adolescents: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search
2.3. Data Extraction
2.4. Risk of Publication Bias between Studies
2.5. Methodological Quality and Risk of Bias of Individual Studies
2.6. Summary Measures and Synthesis of Results in Studies
3. Results
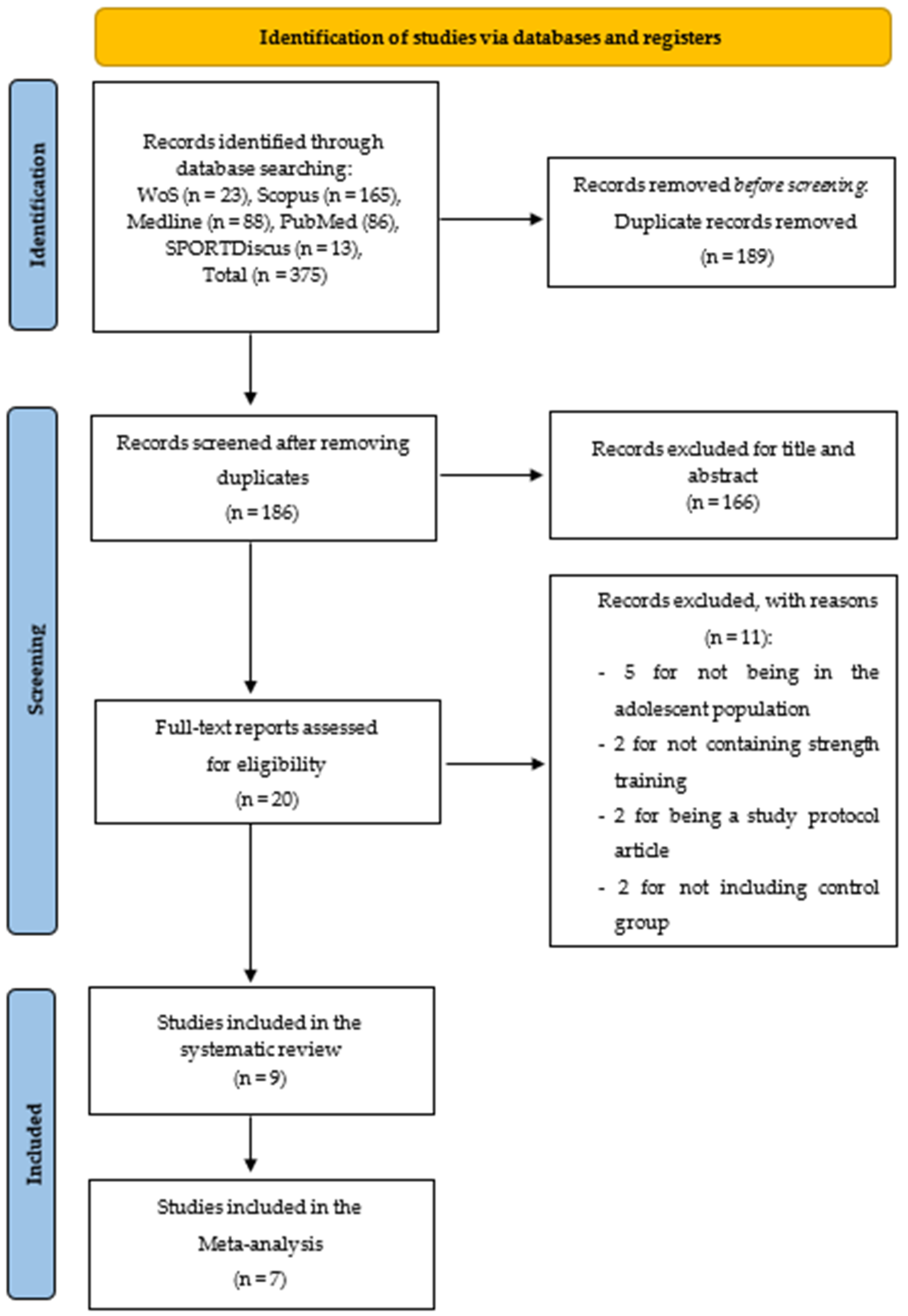
3.1. Studies Selection
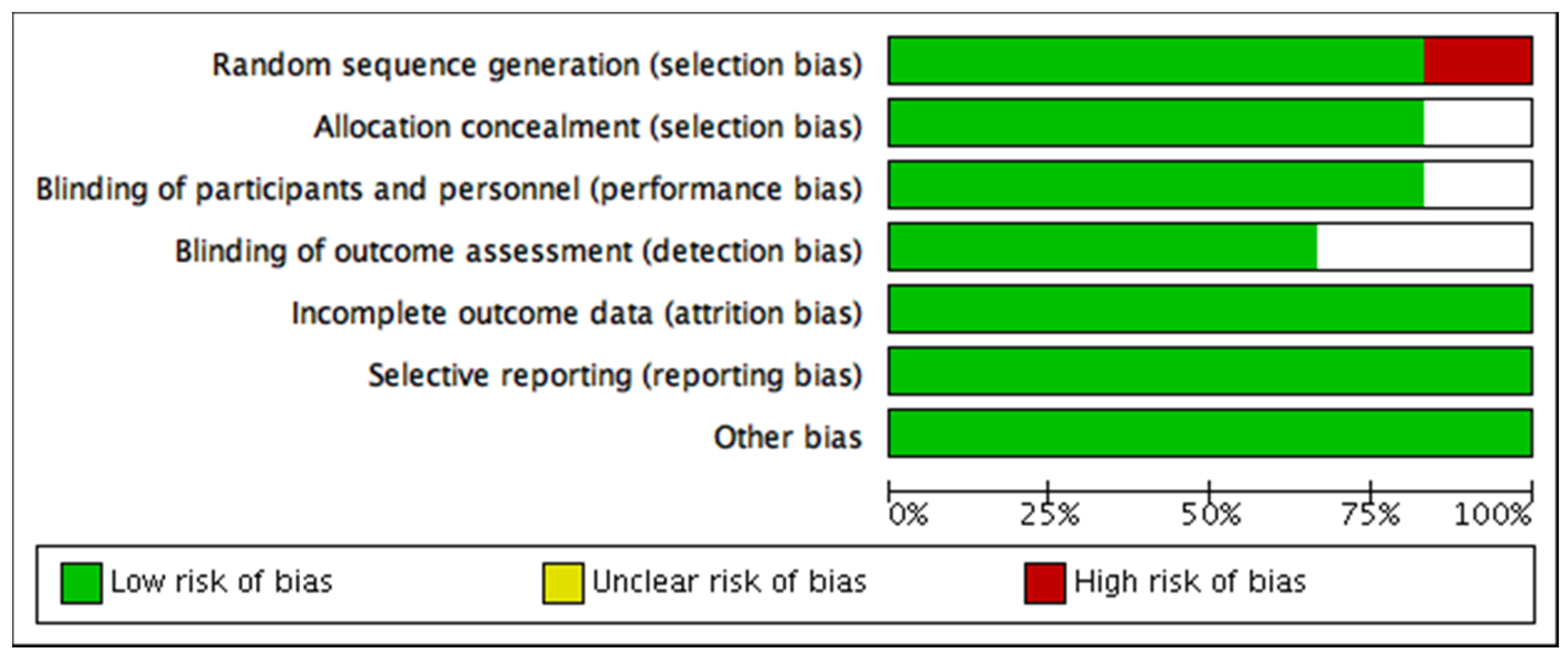
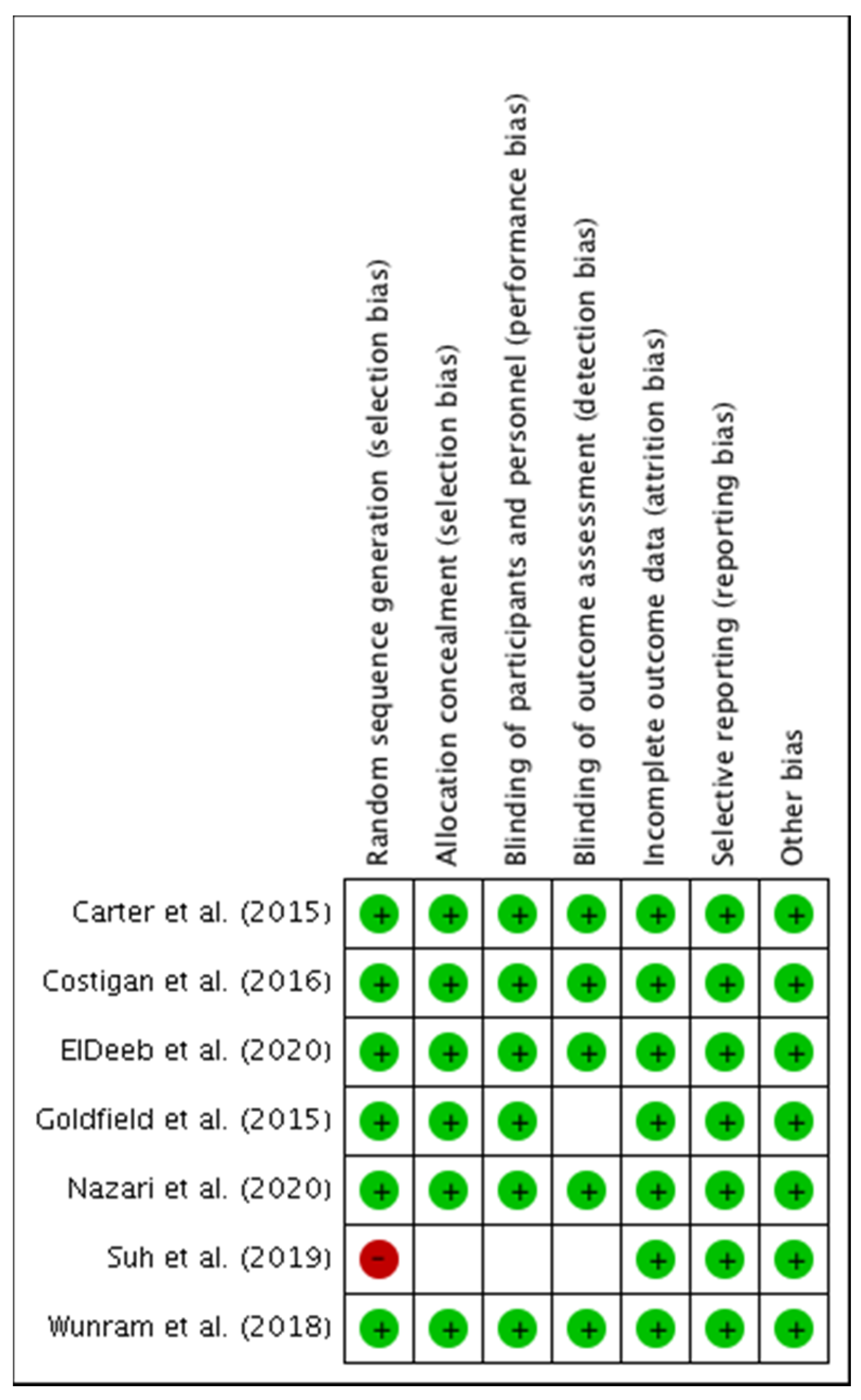
3.1.1. Risk of Bias among Studies
3.1.2. Assessment of Methodological Quality and Risk of Bias of Individual Studies
3.2. Meta-Analysis
3.2.1. Effects of Different Strength Training Methods on Anxiety Levels
3.2.2. Effects of Training with Different Modes of Strength Intervention on Depression Levels
3.2.3. Effects of Concurrent Training on Depression
3.2.4. Effects of Conventional and Vibration Platform Strength Training on Depression
4. Discussion
4.1. Physical Performance and Psychosocial Disorders of Anxiety, Stress, and Depression
4.2. Effects of Different Methods of Strength Training Associated with Psychosocial Disorders of Anxiety, Stress, and Depression
4.3. Strength Training and Brain Development Associated with Psychosocial Disorders of Anxiety, Stress, and Depression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crempien, C.; De La Parra, G.; Grez, M.; Valdés, C.; López, M.J.; Krause, M. Características sociodemográficas y clínicas de pacientes diagnosticados con depresión en Centros Comunitarios de Salud Mental (COSAM) de Santiago, Chile. Rev. Chil. Neuro-Psiquiatr. 2017, 55, 26–35. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory: Bibliography; Consulting Psychologists Press: Santa Clara, CA, USA, 1989. [Google Scholar]
- Kent, M. Diccionario Oxford de Medicina y Ciencias del Deporte, 2nd ed.; Editorial Paidotribo: Barcelona, Spain, 2003. [Google Scholar]
- Assoc, A.P. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; Volume 21. [Google Scholar]
- Othman, N.; Ahmad, F.; El Morr, C.; Ritvo, P. Perceived impact of contextual determinants on depression, anxiety and stress: A survey with university students. Int. J. Ment. Health Syst. 2019, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Barahona-Fuentes, G.D.; Lagos, R.S.; Ojeda, Á.C.H. The influence of self-talk on levels of stress and anxiety in tennis players: A systematic review. Rev. Bras. Cienc. Esporte 2019, 41, 135–141. [Google Scholar] [CrossRef]
- Huerta, Á.; Barahona-Fuentes, G.; Galdames, S.; Cáceres, P.; Ortiz, P. Efectos de un programa de Zumba® sobre niveles de ansiedad-rasgo, ansiedad-estado y condición física en estudiantes universitarias chilenas. Cuad. Psicol. Deport. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Dedding, C.; Pham, T.T.; Wright, P.; Bunders, J. Depression, anxiety, and suicidal ideation among Vietnamese secondary school students and proposed solutions: A cross-sectional study. BMC Public Health 2013, 13, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, N.E.; Hadjistavropoulos, H.D. Is anxiety about health associated with desire to exercise, physical activity, and exercise dependence? Personal. Individ. Differ. 2011, 51, 1059–1062. [Google Scholar] [CrossRef]
- Gerber, M.; Brand, S.; Elliot, C.; Holsboer-Trachsler, E.; Pühse, U. Aerobic exercise, ball sports, dancing, and weight lifting as moderators of the relationship between stress and depressive symptoms: An exploratory cross-sectional study with Swiss university students. Percept. Mot. Ski. 2014, 119, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ensari, I.; Greenlee, T.A.; Motl, R.W.; Petruzzello, S.J. Meta-analysis of acute exercise effects on state anxiety: An update of randomized controlled trials over the past 25 years. Depress. Anxiety 2015, 32, 624–634. [Google Scholar] [CrossRef]
- Stonerock, G.L.; Hoffman, B.M.; Smith, P.J.; Blumenthal, J.A. Exercise as Treatment for Anxiety: Systematic Review and Analysis. Ann. Behav. Med. 2015, 49, 542–556. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, B.; Vancampfort, D.; Rosenbaum, S.; Firth, J.; Cosco, T.; Veronese, N.; Salum, G.A.; Schuch, F.B. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: A meta-analysis. Psychiatry Res. 2017, 249, 102–108. [Google Scholar] [CrossRef]
- Wegner, M.; Amatriain-Fernández, S.; Kaulitzky, A.; Murillo-Rodriguez, E.; Machado, S.; Budde, H. Systematic Review of Meta-Analyses: Exercise Effects on Depression in Children and Adolescents. Front. Psychiatry 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Wittfeld, K.; Jochem, C.; Dörr, M.; Schminke, U.; Gläser, S.; Bahls, M.; Markus, M.R.P.; Felix, S.B.; Leitzmann, M.F.; Ewert, R.; et al. Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population. Mayo Clin. Proc. 2020, 95, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Herting, M.M.; Keenan, M.F. Exercise and the developing brain in children and adolescents. In Physical Activity and the Aging Brain; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–19. [Google Scholar]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; VanPatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, M.C.; Yucel, K.; Nazarov, A.; MacQueen, G.M. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci. JPN 2009, 34, 41–54. [Google Scholar]
- Norbury, R.; Godlewska, B.; Cowen, P.J. When less is more: A functional magnetic resonance imaging study of verbal working memory in remitted depressed patients. Psychol. Med. 2014, 44, 1197–1203. [Google Scholar] [CrossRef]
- Rao, U.; Chen, L.-A.; Bidesi, A.S.; Shad, M.U.; Thomas, M.A.; Hammen, C.L. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol. Psychiatry 2010, 67, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Botteron, K.N.; Luby, J.L.; Belden, A.C.; Gaffrey, M.S.; Babb, C.M.; Nishino, T.; Miller, M.I.; Ratnanather, J.T.; Barch, D.M. Structural-functional correlations between hippocampal volume and cortico-limbic emotional responses in depressed children. Cogn. Affect. Behav. Neurosci. 2013, 13, 135–151. [Google Scholar] [CrossRef] [Green Version]
- Gorham, L.S.; Jernigan, T.; Hudziak, J.; Barch, D.M. Involvement in Sports, Hippocampal Volume, and Depressive Symptoms in Children. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 484–492. [Google Scholar] [CrossRef]
- Gordon, B.R.; McDowell, C.P.; Lyons, M.; Herring, M.P. The Effects of Resistance Exercise Training on Anxiety: A Meta-Analysis and Meta-Regression Analysis of Randomized Controlled Trials. Sports. Med. 2017, 47, 2521–2532. [Google Scholar] [CrossRef]
- Winett, R.A.; Carpinelli, R.N. Potential health-related benefits of resistance training. Prev. Med. 2001, 33, 503–513. [Google Scholar] [CrossRef]
- Dias, C.P.; Toscan, R.; de Camargo, M.; Pereira, E.P.; Griebler, N.; Baroni, B.M.; Tiggemann, C.L. Effects of eccentric-focused and conventional resistance training on strength and functional capacity of older adults. AGE 2015, 37, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Marin, P.J.; Rhea, M.R.; Wilson, S.M.C.; Loenneke, J.P.; Anderson, J.C. Concurrent training: A meta-analysis examining interference of aerobic and resistance exercises. J. Strength Cond. Res. 2012, 26, 2293–2307. [Google Scholar] [CrossRef]
- Nordlund, M.M.; Thorstensson, A. Strength training effects of whole-body vibration? Scand. J. Med. Sci. Sports 2007, 17, 12–17. [Google Scholar] [CrossRef]
- Butcher, S.J.; Neyedly, T.J.; Horvey, K.J.; Benko, C.R. Do physiological measures predict selected CrossFit® benchmark performance? Open Access J. Sports. Med. 2015, 6, 241. [Google Scholar] [CrossRef] [Green Version]
- de Hoyo, M.; Gonzalo-Skok, O.; Sañudo, B.; Carrascal, C.; Plaza-Armas, J.R.; Camacho-Candil, F.; Otero-Esquina, C. Comparative effects of in-season full-back squat, resisted sprint training, and plyometric training on explosive performance in U-19 elite soccer players. J. Strength Cond. Res. 2016, 30, 368–377. [Google Scholar] [CrossRef]
- Barahona-Fuentes, G.D.; Ojeda, Á.H.; Jerez-Mayorga, D. Effects of different methods of strength training on indicators of muscle fatigue during and after strength training: A systematic review. Mot. Rev. Educ. Física 2020, 26. [Google Scholar] [CrossRef]
- Ojeda, Á.H.; Chirosa, L.J.; Barrilao, R.G.; Rios, I.J.C.; Serrano, P.A.C. Efecto de la resistencia variable sobre la potenciación post activación: Una revisión sistemática. Arch. Med. Deporte Rev. Fed. Española Med. Deporte Confed. Iberoam. Med. Deporte 2016, 33, 338–345. [Google Scholar]
- Barahona-Fuentes, G.D.F.; Huerta Ojeda, Á.; Galdames Maliqueo, S.A. Influencia de la pliometría basada en un Entrenamiento Intervalado de Alta Intensidad sobre la altura de salto y pico de potencia en futbolistas Sub-17. Educ. Física Y Cienc. 2019, 21, e080. [Google Scholar] [CrossRef] [Green Version]
- Sabido, R.; Peñaranda, M.; Hernández-Davó, J.L. Comparison of Acute Responses to Four Different Hypertrophy-Oriented Resistance Training Methodologies. Eur. J. Hum. Mov. 2016, 37, 109–121. [Google Scholar]
- Barbat-Artigas, S.; Rolland, Y.; Zamboni, M.; Aubertin-Leheudre, M. How to assess functional status: A new muscle quality index. J. Nutr. Health Aging 2012, 16, 67–77. [Google Scholar] [CrossRef]
- Fragala, M.S.; Kenny, A.M.; Kuchel, G.A. Muscle Quality in Aging: A Multi-Dimensional Approach to Muscle Functioning with Applications for Treatment. Sports Med. 2015, 45, 641–658. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Peterson, M.D.; Liu, D.; Gordish-Dressman, H.; Hubal, M.J.; Pistilli, E.; Angelopoulos, T.J.; Clarkson, P.M.; Moyna, N.M.; Pescatello, L.S.; Seip, R.L.; et al. Adiposity attenuates muscle quality and the adaptive response to resistance exercise in non-obese, healthy adults. Int. J. Obes. 2011, 35, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Chiles Shaffer, N.; Gonzalez-Freire, M.; Shardell, M.D.; Zoli, M.; Studenski, S.A.; Ferrucci, L. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J. Cachex Sarcopenia Muscle 2017, 8, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Waddington, H.; Masset, E.; Jimenez, E. What have we learned after ten years of systematic reviews in international development? J. Dev. Eff. 2018, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- da Silva, F.C.; Iop, R.d.R.; de Oliveira, L.C.; Boll, A.M.; de Alvarenga, J.G.S.; Gutierres Filho, P.J.B.; de Melo, L.M.A.B.; Xavier, A.J.; da Silva, R. Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: A systematic review of randomized controlled trials of the last 10 years. PLoS ONE 2018, 13, e0193113. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, M. Children’s Depression Inventory: Manual; Multi-Health Systems: North Tonawanda, NY, USA, 1992. [Google Scholar]
- Terry, P.C.; Lane, A.M.; Fogarty, G.J. Construct validity of the Profile of Mood States—Adolescents for use with adults. Psychol. Sport Exerc. 2003, 4, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Wallenstein, G.V.; Blaisdell-Gross, B.; Gajria, K.; Guo, A.; Hagan, M.; Kornstein, S.G.; Yonkers, K.A. Development and validation of the Premenstrual Symptoms Impact Survey (PMSIS): A disease-specific quality of life assessment tool. J. Women’s Health 2008, 17, 439–450. [Google Scholar] [CrossRef]
- Speilberger, C.D.; Vagg, P.R. Psychometric properties of the STAI: A reply to Ramanaiah, Franzen, and Schill. J. Personal. Assess. 1984, 48, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.R.; Richmond, B.O. What I think and feel: A revised measure of children’s manifest anxiety. J. Abnorm. Child Psychol. 1978, 6, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.; Slade, T. Interpreting scores on the Kessler psychological distress scale (K10). Aust. N. Z. J. Public Health 2001, 25, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Muris, P.; Meesters, C.; Fijen, P. The self-perception profile for children: Further evidence for its factor structure, reliability, and validity. Personal. Individ. Differ. 2003, 35, 1791–1802. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013; ISBN 1483276481. [Google Scholar]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Nazari, M.; Shabani, R.; Dalili, S. The effect of concurrent resistance-aerobic training on serum cortisol level, anxiety, and quality of life in pediatric type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2020, 33, 599–604. [Google Scholar] [CrossRef]
- Carter, T.; Guo, B.; Turner, D.; Morres, I.; Khalil, E.; Brighton, E.; Armstrong, M.; Callaghan, P. Preferred intensity exercise for adolescents receiving treatment for depression: A pragmatic randomised controlled trial. BMC Psychiatry 2015, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Goldfield, G.S.; Alberga, A.S.; Hadjiyannakis, S.; Phillips, P.; Malcolm, J.; Wells, G.A.; Kenny, G.P.; Prud’homme, D.; Gougeon, R.; Tulloch, H.; et al. Effects of Aerobic Training, Resistance Training, or Both on Psychological Health in Adolescents with Obesity: The HEARTY Randomized Controlled Trial. J. Consult. Clin. Psychol. 2015, 83, 1123–1135. [Google Scholar] [CrossRef]
- Gordon, B.A.; Knapman, L.M.; Lubitz, L. Graduated exercise training and progressive resistance training in adolescents with chronic fatigue syndrome: A randomized controlled pilot study. Clin. Rehabil. 2010, 24, 1072–1079. [Google Scholar] [CrossRef]
- Wunram, H.L.; Hamacher, S.; Hellmich, M.; Volk, M.; Jänicke, F.; Reinhard, F.; Bloch, W.; Zimmer, P.; Graf, C.; Schönau, E.; et al. Whole body vibration added to treatment as usual is effective in adolescents with depression: A partly randomized, three-armed clinical trial in inpatients. Eur. Child Adolesc. Psychiatry 2018, 27, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Costigan, S.A.; Eather, N.; Plotnikoff, R.C.; Hillman, C.H.; Lubans, D.R. High-Intensity Interval Training for Cognitive and Mental Health in Adolescents. Med. Sci. Sports Exerc. 2016, 48, 1985–1993. [Google Scholar] [CrossRef]
- ElDeeb, A.; Atta, H.; Osman, D. Effect of whole body vibration versus resistive exercise on premenstrual symptoms in adolescents with premenstrual syndrome. Bull. Fac. Phys. Ther. 2020, 25, 1–6. [Google Scholar] [CrossRef]
- Suh, J.; Choi, H.S.; Kwon, A.; Chae, H.W.; Eom, S.; Kim, H.S. Once-weekly supervised combined training improves neurocognitive and psychobehavioral outcomes in young patients with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2019, 32, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Eather, N.; Morgan, P.J.; Lubans, D.R. Effects of exercise on mental health outcomes in adolescents: Findings from the CrossFit™ teens randomized controlled trial. Psychol. Sport Exerc. 2016, 26, 14–23. [Google Scholar] [CrossRef]
- Goodman, R.; Meltzer, H.; Bailey, V. The Strengths and Difficulties Questionnaire: A pilot study on the validity of the self-report version. Eur. Child Adolesc. Psychiatry 1998, 7, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.; Afonso, J.; Ramirez-Campillo, R.; Murawska-Ciałowciz, E.; Marques, A.; Clemente, F.M. The effects of exclusively resistance training-based supervised programs in people with depression: A systematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2020, 17, 6715. [Google Scholar] [CrossRef]
- Wu, J.; Tong, H.; Liu, Z.; Tao, J.; Chen, L.; Chan, C.C.H.; Lee, T.M.C. Neurobiological effects of perceived stress are different between adolescents and middle-aged adults. Brain Imaging Behav. 2021, 15, 846–854. [Google Scholar] [CrossRef]
- Lacomba-Trejo, L.; Valero-Moreno, S.; Montoya-Castilla, I.; Pérez-Marín, M. Psychosocial Factors and Chronic Illness as Predictors for Anxiety and Depression in Adolescence. Front. Psychol. 2020, 11, 2529. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.G.; Hawley, J.A. Concurrent exercise training: Do opposites distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, W.J.; Ratamess, N.A.; Nindl, B.C. Recovery responses of testosterone, growth hormone, and IGF-1 after resistance exercise. J. Appl. Physiol. 2017, 122, 549–558. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [Green Version]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.L.; Lehti, M.; Hulmi, J.J. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. 2019, 126, 30–43. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [Green Version]
- Levada, O.A.; Troyan, A.S.; Pinchuk, I.Y. Serum insulin-like growth factor-1 as a potential marker for MDD diagnosis, its clinical characteristics, and treatment efficacy validation: Data from an open-label vortioxetine study. BMC Psychiatry 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Sharma, A.N.; Soares, J.C.; Carvalho, A.F.; Quevedo, J. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J. Affect. Disord. 2016, 197, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Troyan, A.S.; Levada, O.A. The Diagnostic Value of the Combination of Serum Brain-Derived Neurotrophic Factor and Insulin-Like Growth Factor-1 for Major Depressive Disorder Diagnosis and Treatment Efficacy. Front. Psychiatry 2020, 11, 800. [Google Scholar] [CrossRef]
- de Alcantara Borba, D.; da Silva Alves, E.; Rosa, J.P.P.; Facundo, L.A.; Costa, C.M.A.; Silva, A.C.; Narciso, F.V.; Silva, A.; de Mello, M.T. Can IGF-1 Serum Levels Really be Changed by Acute Physical Exercise? A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2020, 17, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Köhler, C.A.; McIntyre, R.S.; Knöchel, C.; Brunoni, A.R.; Thase, M.E.; Quevedo, J.; Fernandes, B.S.; Berk, M. Peripheral vascular endothelial growth factor as a novel depression biomarker: A meta-analysis. Psychoneuroendocrinology 2015, 62, 18–26. [Google Scholar] [CrossRef]
- Smyth, J.; Ockenfels, M.C.; Porter, L.; Kirschbaum, C.; Hellhammer, D.H.; Stone, A.A. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology 1998, 23, 353–370. [Google Scholar] [CrossRef]
- Fiksdal, A.; Hanlin, L.; Kuras, Y.; Gianferante, D.; Chen, X.; Thoma, M.V.; Rohleder, N. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology 2019, 102, 44–52. [Google Scholar] [CrossRef]
- Burke, H.M.; Davis, M.C.; Otte, C.; Mohr, D.C. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology 2005, 30, 846–856. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, J.; Chai, P.; Li, H.; Luo, C.; Yang, T.; Li, L.; Shan, B.; Xu, X.; Xu, L. Brain volume alteration and the correlations with the clinical characteristics in drug-naive first-episode MDD patients: A voxel-based morphometry study. Neurosci. Lett. 2010, 480, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Crichton, J.P.; Esteban-Cornejo, I.; Solis-Urra, P.; Mora-Gonzalez, J.; Cadenas-Sanchez, C.; Rodriguez-Ayllon, M.; Migueles, J.H.; Molina-Garcia, P.; Verdejo-Roman, J.; Kramer, A.F.; et al. Association of Sedentary Behavior with Brain Structure and Intelligence in Children with Overweight or Obesity: The ActiveBrains Project. J. Clin. Med. 2020, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Feter, N.; Penny, J.C.; Freitas, M.P.; Rombaldi, A.J. Effect of physical exercise on hippocampal volume in adults: Systematic review and meta-analysis. Sci. Sports 2018, 33, 327–338. [Google Scholar] [CrossRef]
- Morel, G.R.; León, M.L.; Uriarte, M.; Reggiani, P.C.; Goya, R.G. Therapeutic potential of IGF-I on hippocampal neurogenesis and function during aging. Neurogenesis 2017, 4, e1259709. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, Y.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 2003, 60, 804–815. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, K.P.M.; de Oliveira, V.H.; Medeiros, G.C.B.S.; Mata, Á.N.d.S.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef]
- Hung, C.-L.; Tseng, J.-W.; Chao, H.-H.; Hung, T.-M.; Wang, H.-S. Effect of acute exercise mode on serum brain-derived neurotrophic factor (BDNF) and task switching performance. J. Clin. Med. 2018, 7, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinus, N.; Hansen, D.; Feys, P.; Meesen, R.; Timmermans, A.; Spildooren, J. The Impact of Different Types of Exercise Training on Peripheral Blood Brain-Derived Neurotrophic Factor Concentrations in Older Adults: A Meta-Analysis. Sports Med. 2019, 49, 1529–1546. [Google Scholar] [CrossRef]
- Polyakova, M.; Stuke, K.; Schuemberg, K.; Mueller, K.; Schoenknecht, P.; Schroeter, M.L. BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. J. Affect. Disord. 2015, 174, 432–440. [Google Scholar] [PubMed]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscience 2012, 18, 82–97. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Scarr, E.; Millan, M.J.; Bahn, S.; Bertolino, A.; Turck, C.W.; Kapur, S.; Möller, H.-J.; Dean, B. Biomarkers for psychiatry: The journey from fantasy to fact, a report of the 2013 CINP think tank. Int. J. Neuropsychopharmacol. 2015, 18, pyv042. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Author | Objective | Sample | Variables | Results | Physical Performance |
|---|---|---|---|---|---|
| Effects of Strength and Concurrent Training on Anxiety and Depression | |||||
| Costigan et al. [61] | To evaluate the efficacy of two high-intensity interval training (HIIT) protocols for improving cognitive and mental health outcomes (executive function, psychological well-being, psychological distress, and physical self-concept) in adolescents | 65 adolescents, 45 M and 20 W CG: 22 (15.6 ± 0.6) EG1: 21 (15.7 ± 0.7) EG2: 22 (15.5 ± 0.6) | IV: HIIT (EG1); HIIT + ST (EG2) DV: Cognitive and mental health (evaluated through the Kessler Psychological Distress Scale). | ns | -- |
| Eather et al. [64] | To investigate the effectiveness of the CrossFit Teens resistance training program for improving mental health outcomes in adolescents and to explore potential moderators and mediators. | 96 adolescents (15.5 ± 0.50) CG: 45 EG: 51 | IV: CrossFit Teens. DV: Mental health (evaluated through the Strength and Difficulties Questionnaire) | ns | -- |
| Suh et al. [63] | To evaluate the effect of combined aerobic and resistance exercise in adolescents with type l diabetic. | 30 adolescents with Diabetes Mellitus l EG: 20 (17.10 ± 4.54) CG: 10 (21.80 ± 3.05) | IV: CT. DV: Anxiety, depression (evaluated through the Beck’s Depression Inventory, Children’s Depression Inventory, State-trait Anxiety Inventory and Revised Children’s Manifest Anxiety Scale). Glycemic control, cardiovascular function, and physical fitness | EG ↑ (p < 0.05) in Vo2 max and maximal force. Anxiety and depression were ns. | ↑ in both groups |
| ElDeeb et al. [62] | To compare the effect of whole-body vibration and resistive exercise on premenstrual symptoms in adolescents with premenstrual syndrome. | 60 young sedentary W CG: 20 (17.9 ± 1.16) EG1: 20 (17.7 ± 1.17) EG2: 20 (17.3 ± 1.41) | IV: VPT (EG1); ST (EG2). DV: Premenstrual symptoms, anxiety, and depression (evaluated through the Premenstrual Symptoms Impact Survey) | EG1 and EG2, ↓ (p < 0.05) their levels of anxiety and depression, significantly. | -- |
| Effects of Strength and Concurrent Training on Anxiety | |||||
| Nazari et al. [56] | To explore the effect of concurrent resistance-aerobic training on serum cortisol level, anxiety, and quality of life among pediatric type l diabetic. | 40 children adolescents with type 1 diabetes. EG: 20 (11.22 ± 1.90) CG: 20 (11.00 ± 2.67) | IV: CT. DV: Anxiety, serum cortisol level, and quality of life (evaluated through the Revised Children’s Manifest Anxiety Scale). | EG ↓ (p = 0.001) anxiety, significantly. Quality of life ↑ significantly (p = 0.003). Cortisol was ns. | -- |
| Effects of Strength and Concurrent Training on Depression | |||||
| Goldfield et al. [58] | To determine the effects of aerobic training, resistance training, and combined training on mood, body image, and self-esteem in adolescents with obesity. | 304 adolescents with obesity. 91 M y 213 W CG: 76 (15.6 ± 1.3) EG1: 75 (15.5 ± 1.4) EG2: 78 (15.9 ± 1.5) EG3: 75 (15.5 ± 1.3) | IV: AT (EG1); ST (EG2); CT [AT + ST] (EG3) DV: Mood with depression, fatigue and anger (evaluated through the Brunel Mood Scale), body image and self-esteem. | EG2 ↓ (p 0.02) their depression levels, significantly. | -- |
| Carter et al. [57] | To determine the effectiveness of a preferred intensity exercise intervention on the depressive symptoms of adolescents with depression. | 87 youngsters with depression. 9 M y 68 W CG: 43 (15.4 ± 0.9) EG: 44 (15.4 ± 1.0) | IV: CT. DV: Depression (evaluated through the Children’s depression inventory 2), and quality of life | ns | -- |
| Gordon et al. [59] | To investigate the differential effects of graded aerobic exercise and progressive resistance training on exercise tolerance, fatigue, and quality of life in adolescent patients with chronic fatigue syndrome. | 22 adolescents with chronic fatigue. EG1: 11 (16.2 ± 0.8) EG2: 11 (15.6 ± 1.6) | IV: AT (EG1); ST (EG2). DV: Exercise tolerance, fatigue, and depression (evaluated through the Becks Depression Index). | EG1 and EG2 ↓ (p 0.02 and p 0.03) depression levels significantly. | ↑ in both groups |
| Wunram et al. [60] | To investigate the feasibility and effectiveness of a high-frequency whole-body vibration (WBV) training as add-on anti-depressive treatment in medication-naive inpatient adolescents with diagnosed major depression compared to an endurance cycling condition. | 64 teenagers with depression. EG1: 20 (16.1 ± 1.2) EG2: 21 (15.9 ± 1.2) CG: 23 (15.7 ± 1.1) | IV: Ergometer (EG1); VPT (EG2). DV: Depressive symptoms (evaluated through the Depressionsinventar für Kinder und Jugendliche). | Depression was ns after 6w. Depression ↓ after 26w EG1 (p = 0.037) y EG2 (p = 0.042), significantly. | EG1: ↑ EG2: = |
| Author | W | S/w | Methodology | Reps (n) | Sets (n) | Intensity/Load | Rest Between Sets |
|---|---|---|---|---|---|---|---|
| Costigan et al. [61] | 8 | 3 | AT HIIT (EG1); Gross motor cardiorespiratory exercises (shuttle runs, jumping jacks, and skipping) | Maximum number of repetitions in 30 s for 8–10 min | NR | 92.4% (HR max) | 30 s |
| 8 | 3 | CT [HIIT + ST] (EG2); (shuttle runs, jumping jacks, skipping, combined with body weight squats, push-ups) | Maximum number of repetitions in 30 s for 8–10 min | NR | 91.8% (HR max) | 30 s | |
| Eather et al. [64] | 8 | 2 | CrossFit (squat jumps, lunges, medicine ball toss, push-ups, deadlifts and shoulder press) | Depending on the performance obtained W previous | NR | NR | NR |
| Suh et al. [63] | 12 | 1 | CT [AT + ST] (10 min of leg extension and leg press, and 40 min of cycling and walking on the treadmill) | ST = 12 AT = 1 | ST = 5 AT = 1 | ST = 70% (1-MR) AT = 70–80% (HR max) | NR |
| ElDeeb et al. [62] | 12 | 3 | VPT (with a knee angle of 150° and vibration amplitude of 1 mm). | 1-min | 3–10 | 20 Hz | 1-min |
| 12 | 3 | ST (exercises for shoulder, elbow, hip, and knee joints). | 3–12 | 1 for shoulder | 60–70% (1-MR) | 2-min | |
| Nazari et al. [56] | 16 | 3 | CT [ST + AT] (20-min Pilates exercises + 20-min bodyweight exercises. Then, 20-min AT including 10-min of V-forward, V-back and 10-min of march) | ST = 8–12 | ST = 2–3 | ST = NR AT = 50–75% (HR max) | ST = 30 s AT = 2-min |
| Goldfield et al. [58] | 22 | 3 | AT (EG1); (45-min of Treadmill, elliptical, and/or bicycle) | 1 | 1 | 65–85% (HR max) | NR |
| 22 | 3 | ST (EG2); (Seven exercises with weight machines or free weights) | 8–15 | 2–3 | 80% (1-MR) | NR | |
| 22 | 3 | CT [AT + ST] (EG3) | AT = 1 ST = 8–15 | AT = 1 ST = 2–3 | AT = 65–85% (HR max) ST = 80% (1-MR) | NR | |
| Carter et al. [57] | 6 | 2 | CT [AT + ST] (abdomen and back exercises; two medicine ball arm exercises from the supine position; rebound, static and dynamic balance exercises on a trampoline; bodyweight squatting exercise against a wall and stationary bicycle) | NR | NR | NR | NR |
| Gordon et al. [59] | 4 | 5 | AT (20–40 min of stationary bicycle, and treadmill) | NR | NR | 40–60% (of reserve HR) | NR |
| 4 | 5 | ST (16 exercises combine upper and lower body and core stability | 10–15 | 1 | NR | NR | |
| Wunram et al. [60] | 6 | 4 | AT (Ergometer) | NR | NR | ||
| 6 | 4 | ST [VPT] (arm and shoulder contractions, trunk rotation, variety of leg and squat positions with 2–3 min for exercise and amplitude of 2 mm) | NR | NR | 20 Hz | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barahona-Fuentes, G.; Huerta Ojeda, Á.; Chirosa-Ríos, L. Effects of Training with Different Modes of Strength Intervention on Psychosocial Disorders in Adolescents: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 9477. https://doi.org/10.3390/ijerph18189477
Barahona-Fuentes G, Huerta Ojeda Á, Chirosa-Ríos L. Effects of Training with Different Modes of Strength Intervention on Psychosocial Disorders in Adolescents: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(18):9477. https://doi.org/10.3390/ijerph18189477
Chicago/Turabian StyleBarahona-Fuentes, Guillermo, Álvaro Huerta Ojeda, and Luis Chirosa-Ríos. 2021. "Effects of Training with Different Modes of Strength Intervention on Psychosocial Disorders in Adolescents: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 18: 9477. https://doi.org/10.3390/ijerph18189477








