Influence of Residence Area and Basic Livelihood Conditions on the Prevalence and Diagnosis Experience of Osteoporosis in Postmenopausal Women Aged over 50 Years: Evaluation Using Korea National Health and Nutrition Examination Survey Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data and Study Population
2.2. Variables
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
3.2. Osteoporosis Prevalence and Diagnosis
3.3. Influence of the Factors on the Osteoporosis Prevalence and Diagnosis Rate in the Urban and Rural Areas
3.4. Influence of the Basic Livelihood Condition on the Osteoporosis Prevalence and Diagnosis Rate in the Rural and Urban Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouillon, R.; Burckhardt, P.; Christiansen, C.; Fleisch, H.A.; Fujita, T.; Gennari, C.; Marin, T.J.; Mazzuoli, G.; Melton, L.J.; Ringe, J.D.; et al. Consensus development conference: Prophylaxis and treatment of osteoporosis. Am. J. Med. 1991, 90, 107–110. [Google Scholar] [CrossRef]
- Meeta, M.; Harinarayan, C.V.; Marwah, R.; Sahay, R.; Kalra, S.; Babhulkar, S. Clinical practice guidelines on postmenopausal osteoporosis: An executive summary and recommendations–Update 2019–2020. J. Midlife Health 2020, 11, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Oh, H.J.; Kim, D.J.; Lee, Y.; Chung, Y.S. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: The Korea National Health and Nutrition Examination Survey 2008–2009. J. Bone Miner. Res. 2012, 27, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.P.; Liao, E.Y.; Zhang, H.; Dai, R.C.; Shan, P.F.; Cao, X.Z.; Liu, S.P.; Jiang, Y. Determination of age-specific bone mineral density and comparison of diagnosis and prevalence of primary osteoporosis in Chinese women based on both Chinese and World Health Organization criteria. J. Bone Miner. Metab. 2004, 22, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Iki, M.; Kagamimori, S.; Kagawa, Y.; Matsuzaki, T.; Yoneshima, H.; Marumo, F. Bone mineral density of the spine, hip and distal forearm in representative samples of the Japanese female population: Japanese Population-Based Osteoporosis (JPOS) Study. Osteoporos. Int. 2001, 12, 529–537. [Google Scholar] [CrossRef]
- Kiebzak, G.M.; Beinart, G.A.; Perser, K.; Ambrose, C.G.; Siff, S.J.; Heggeness, M.H. Undertreatment of osteoporosis in men with hip fracture. Arch. Intern. Med. 2002, 162, 2217–2222. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.A.; Cabral, D.; Cheng, H.; Katz, J.N.; Finkelstein, J.S.; Avorn, J.; Solomon, D.H. Patterns of bone mineral density testing: Current guidelines, testing rates, and interventions. J. Gen. Intern. Med. 2004, 19, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehlbach, S.H.; Fournier, M.; Bigelow, C. Recognition of osteoporosis by primary care physicians. Am. J. Public Health 2002, 92, 271–273. [Google Scholar] [CrossRef]
- Choi, M.H.; Yang, J.H.; Seo, J.S.; Kim, Y.J.; Kang, S.W. Prevalence and diagnosis experience of osteoporosis in postmenopausal women over 50: Focusing on socioeconomic factors. PLoS ONE 2021, 16, e0248020. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Roh, Y.H.; Lee, E.S.; Ahn, J.; Kim, H.S.; Gong, H.S.; Baek, K.H.; Chung, H.Y. Factors affecting willingness to get assessed and treated for osteoporosis. Osteoporos. Int. 2019, 30, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.C.; Sosa, M.; Saavedra, P.; Lainez, P.; Marrero, M.; Torres, M.; Medina, C.D. Poverty is a risk factor for osteoporotic fractures. Osteoporos. Int. 2009, 20, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Bae, K.H.; Seo, J.B.; Jeon, J.H.; Lee, W.K.; Lee, I.K.; Kim, J.G.; Park, K.G. Association between household size, residential area, and osteoporosis: Analysis of 2008 to 2011 Korea National Health and Nutrition Examination Survey. Korean J. Intern. Med. 2016, 31, 712–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, L.; Judge, A.; Javaid, M.K.; Cooper, C.; Vestergaard, P.; Abrahamsen, B.; Harvey, N.C. Social inequality and fractures—Secular trends in the Danish population: A case-control study. Osteoporos. Int. 2018, 29, 2243–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, M.M.Y.; Ang, W.M.; McGuiness, M.; Larke, A.B. Undertreatment of osteoporosis in regional Western Australia. Australas. J. Ageing 2012, 31, 110–114. [Google Scholar] [CrossRef]
- Lim, D.; Bahk, J.; Ock, M.; Kim, I.; Kang, H.Y.; Kim, Y.Y.; Park, J.H.; Khang, Y.H. Income-related inequality in quality-adjusted life expectancy in Korea at the national and district levels. Health Qual. Life Outcomes 2020, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.K.; Beatty, K.; Leider, J.P.; Knudson, A.; Anderson, B.L.; Meit, M. The double disparity facing rural local health departments. Annu. Rev. Public Health 2016, 37, 167–184. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.W.; Noh, J.H.; Kim, D.J. The prevalence of and demographic factors associated with radiographic knee osteoarthritis in Korean adults aged ≥50 years: The 2010–2013 Korea national health and nutrition examination survey. PLoS ONE 2020, 15, e0230613. [Google Scholar] [CrossRef]
- Garcia, M.C.; Rossen, L.M.; Bastian, B.A.; Faul, M.; Dowling, N.F.; Thomas, C.C.; Schieb, L.; Hong, Y.; Yoon, P.W.; Iademarco, M.F. Potentially excess deaths from the five leading causes of death in metropolitan and nonmetropolitan counties—United States, 2010–2017. MMWR Surveill. Summ. 2019, 68, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Jo, E.H.; Son, J.A.; So, E.S. Factors association national health screening programparticipation according to sex in Korean:Using the fifth Korea national health and nutrition examinationsurvey(KNHANES Ⅵ, 2013). Korean J. Health Educ. Prompt. 2016, 33, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Vanasse, A.; Courteau, J.; Cohen, A.A.; Orzanco, M.G.; Drouin, C. Rural-urban disparities in the management and health issues of chronic diseases in Quebec (Canada) in the early 2000s. Rural Remote Health 2010, 10, 1548. [Google Scholar]
- Ministry of Health and Welfare. Korea Health Statistics. The Number of Beneficiaries of National Basic Livelihood Security System in Korea 2019. Available online: http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd (accessed on 5 April 2021).
- Jeon, B.; Noguchi, H.; Kwon, S.; Ito, T.; Tamiya, N. Disability, poverty, and role of the basic livelihood security system on health services utilization among the elderly in South Korea. Soc. Sci. Med. 2017, 178, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.H. The impact of basic livelihoods condition on the current smoking: Applying the counterfactual model. Korean J. Health Educ. Promot. 2019, 36, 53–67. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Korea Health Statistics. 2019 Korea National Health and Nutrition Examination Survey (KNHANHES VIII-1). Available online: https://knhanes.kdca.go.kr/knhanes/main.do (accessed on 5 April 2021).
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health-Care Level: Technical Report; University of Sheffield Medical School and the World Health Organization Collaborating Centre for Metabolic Bone Diseases: Sheffield, UK, 2007; pp. 1–339. Available online: https://www.sheffield.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf (accessed on 5 April 2021).
- Hong, J.E.; Yoon, S.Y. A Study on factors associated with the patterns of alcohol use in recipients of national basic livelihood guarantees. J. Soc. Sci. 2016, 33, 73–104. [Google Scholar]
- Eom, J.R.; Moon, J.W. Relationship between depression and life satisfaction of social assistance recipients—Focusing on mediating effect of self-esteem and coping strategies for family conflict. Health Soc. Sci. 2016, 43, 85–113. [Google Scholar] [CrossRef]
- Jang, I.Y.; Jung, H.W.; Lee, C.K.; Lee, Y.S.; Kim, K.I.; Kim, K.W.; Oh, H.J.; Ji, M.Y.; Lee, E.J.; Kim, D.H. Rural and urban disparities in frailty and aging-related health conditions in Korea. J. Am. Geriatr. Soc. 2016, 64, 908–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.H. Socioeconomic differences among community-dwelling diabetic adults screened for diabetic retinopathy and nephropathy: The 2015 Korean Community Health Survey. PLoS ONE 2018, 13, e0191496. [Google Scholar] [CrossRef] [PubMed]
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Report of a WHO study group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129. [Google Scholar]
- Cauley, J.A. Public health impact of osteoporosis. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, W.; Wang, X.; Li, C.; Li, J.; Zhao, Y.; Chen, L.; Qi, X.; Qiao, L.; Da, W.; et al. Urban–rural differences in bone mineral density and its association with reproductive and menstrual factors among older women. Calcif. Tissue Int. 2020, 106, 637–645. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Shin, J.Y.; Park, B.J. Socioeconomic disparities in osteoporosis prevalence: Different results in the overall Korean adult population and single-person households. J. Prev. Med. Public Health 2015, 48, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewald, D.P.; Eisman, J.A.; Ewald, B.D.; Winzenberg, T.M.; Seibel, M.J.; Ebeling, P.R.; Flicker, L.; Nash, P.T. Population rates of bone densitometry use in Australia, 2001–2005, by sex and rural versus urban location. Med. J. Aust. 2009, 190, 126–128. [Google Scholar] [CrossRef]
- Nguyen, J.; Anandasivam, N.S.; Cooperman, D.; Pelker, R.; Wiznia, D.H. Does Medicaid insurance provide sufficient access to pediatric orthopedic care under the affordable care act? Glob. Pediatr. Health 2019, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiznia, D.H.; Nwachuku, E.; Roth, A.; Kim, C.Y.; Save, A.; Anandasivam, N.S.; Medvecky, M.; Pelker, R. The influence of medical insurance on patient access to orthopaedic surgery sports medicine appointments under the Affordable Care Act. Orthop. J. Sports Med. 2017, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, I.R. Relationships between fat and bone. Osteoporos. Int. 2008, 19, 595–606. [Google Scholar] [CrossRef]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—A meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total | Urban | Rural | p-Values a | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Total | 1476 | 100.0 | 1042 | 100.0 | 434 | 100.0 | ||
| Age group (year) | 50–59 | 552 | 37.4 | 420 | 40.3 | 132 | 30.4 | <0.001 |
| 60–69 | 505 | 34.2 | 364 | 34.9 | 141 | 32.5 | ||
| ≥70 | 419 | 28.4 | 258 | 24.8 | 161 | 37.1 | ||
| Educational level | ≤Middle school | 1183 | 80.1 | 781 | 75.0 | 402 | 92.6 | <0.001 |
| ≥High school | 293 | 19.9 | 261 | 25.0 | 32 | 7.4 | ||
| Basic livelihood condition | Non-beneficiaries | 1355 | 91.8 | 976 | 93.7 | 379 | 87.3 | <0.001 |
| Beneficiaries | 121 | 8.2 | 66 | 6.3 | 55 | 12.7 | ||
| Physical activity b | No | 1179 | 79.9 | 850 | 81.6 | 329 | 75.8 | 0.012 |
| Yes | 297 | 20.1 | 192 | 18.4 | 105 | 24.2 | ||
| Obesity c | No | 1406 | 95.3 | 998 | 95.8 | 408 | 94.0 | 0.145 |
| Yes | 70 | 4.7 | 44 | 4.2 | 26 | 6.0 | ||
| Hypertension d | No | 720 | 48.8 | 520 | 49.9 | 200 | 46.1 | 0.181 |
| Yes | 756 | 51.2 | 522 | 50.1 | 234 | 53.9 | ||
| Diabetes e | No | 1277 | 86.5 | 890 | 85.4 | 387 | 89.2 | 0.054 |
| Yes | 199 | 13.5 | 152 | 14.6 | 47 | 10.8 | ||
| Residence area | Urban | 1042 | 70.6 | |||||
| Rural | 434 | 29.4 | ||||||
| Variables | Prevalence | Diagnosis | |||||
|---|---|---|---|---|---|---|---|
| n | % | χ2 Test p-Values | n | % | χ2 Test p-Values | ||
| Total | 513 | 34.8 | 325 | 22.0 | |||
| Age group (year) | 50–59 | 82 | 14.9 | <0.001 | 61 | 11.1 | <0.001 |
| 60–69 | 165 | 32.7 | 126 | 25.0 | |||
| ≥70 | 266 | 63.5 | 138 | 32.9 | |||
| Educational level | ≤Middle school | 472 | 39.9 | <0.001 | 325 | 23.2 | 0.033 |
| ≥High school | 41 | 14.0 | 51 | 17.4 | |||
| Basic livelihood condition | Non-beneficiaries | 448 | 33.1 | <0.001 | 294 | 21.9 | 0.318 |
| Beneficiaries | 65 | 53.7 | 31 | 25.6 | |||
| Physical activity | No | 426 | 36.1 | 0.027 | 252 | 21.4 | 0.234 |
| Yes | 87 | 29.3 | 73 | 24.6 | |||
| Obesity | No | 500 | 35.6 | 0.004 | 314 | 22.3 | 0.192 |
| Yes | 13 | 18.6 | 11 | 15.7 | |||
| Hypertension | No | 224 | 31.1 | 0.004 | 151 | 21.0 | 0.344 |
| Yes | 289 | 38.2 | 174 | 23.0 | |||
| Diabetes | No | 453 | 35.5 | 0.142 | 272 | 21.3 | 0.091 |
| Yes | 60 | 30.2 | 53 | 26.6 | |||
| Residence area | Urban | 331 | 31.8 | <0.001 | 222 | 21.3 | 0.305 |
| Rural | 182 | 41.9 | 103 | 23.7 | |||
| Variables | Prevalence | Diagnosis Rate | |||||
|---|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | p-Values | Adjusted OR | 95% CI | p-Values | ||
| Age group (year) | 50–59 | Ref. | Ref. | ||||
| 60–69 | 2.62 | (1.92–3.57) | <0.001 | 2.81 | (1.99–3.98) | <0.001 | |
| ≥70 | 8.83 | (6.33–12.32) | <0.001 | 4.33 | (3.00–6.24) | <0.001 | |
| Educational level | ≥High school | Ref. | Ref. | ||||
| ≤Middle school | 2.47 | (1.69–3.62) | <0.001 | 0.92 | (0.64–1.32) | 0.637 | |
| Basic livelihood condition | Non-beneficiaries | Ref. | Ref. | ||||
| Beneficiaries | 1.51 | (0.99–2.30) | 0.057 | 0.96 | (0.61–1.50) | 0.847 | |
| Physical activity | No | Ref. | Ref. | ||||
| Yes | 0.77 | (0.56–1.04) | 0.091 | 1.33 | (0.97–1.81) | 0.072 | |
| Obesity | No | Ref. | Ref. | ||||
| Yes | 0.33 | (0.17–0.63) | 0.001 | 0.60 | (0.31–1.18) | 0.139 | |
| Hypertension | No | Ref. | Ref. | ||||
| Yes | 0.92 | (0.72–1.19) | 0.529 | 0.86 | (0.66–1.12) | 0.269 | |
| Diabetes | No | Ref. | Ref. | ||||
| Yes | 0.54 | (0.37–0.77) | 0.001 | 1.17 | (0.82–1.67) | 0.396 | |
| Residence area | Urban | Ref. | Ref. | ||||
| Rural | 1.41 | (1.11–1.71) | 0.001 | 1.01 | (0.76–1.34) | 0.927 | |
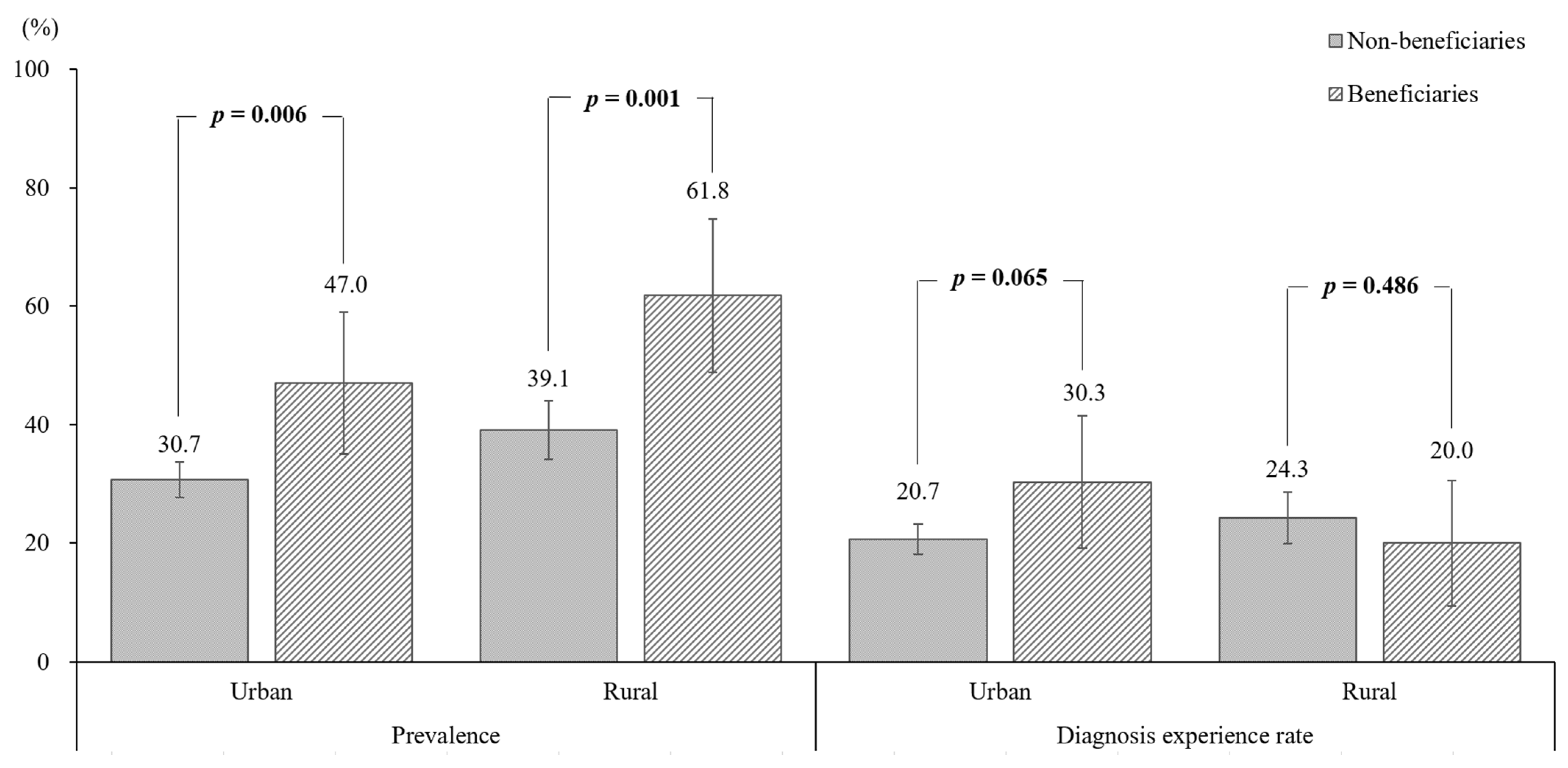
| Models a | Residence Area | Dependent Variables | Independent Variable (Basic Livelihood Condition) | Adjusted OR | 95% CI | p-Values |
|---|---|---|---|---|---|---|
| Model 1 | Urban | Prevalence | Non-beneficiaries | Ref. | ||
| Beneficiaries | 1.24 | (0.71–2.17) | 0.447 | |||
| Model 2 | Diagnosis rate | Non-beneficiaries | Ref. | |||
| Beneficiaries | 1.34 | (0.76–2.37) | 0.305 | |||
| Model 3 | Rural | Prevalence | Non-beneficiaries | Ref. | ||
| Beneficiaries | 2.08 | (1.06–4.10) | 0.033 | |||
| Model 4 | Diagnosis rate | Non-beneficiaries | Ref. | |||
| Beneficiaries | 0.56 | (0.27–1.17) | 0.120 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-W.; Yang, J.-H.; Shin, W.-C.; Kim, Y.-J.; Choi, M.-H. Influence of Residence Area and Basic Livelihood Conditions on the Prevalence and Diagnosis Experience of Osteoporosis in Postmenopausal Women Aged over 50 Years: Evaluation Using Korea National Health and Nutrition Examination Survey Data. Int. J. Environ. Res. Public Health 2021, 18, 9478. https://doi.org/10.3390/ijerph18189478
Kang S-W, Yang J-H, Shin W-C, Kim Y-J, Choi M-H. Influence of Residence Area and Basic Livelihood Conditions on the Prevalence and Diagnosis Experience of Osteoporosis in Postmenopausal Women Aged over 50 Years: Evaluation Using Korea National Health and Nutrition Examination Survey Data. International Journal of Environmental Research and Public Health. 2021; 18(18):9478. https://doi.org/10.3390/ijerph18189478
Chicago/Turabian StyleKang, Suk-Woong, Ji-Hee Yang, Won-Chul Shin, Yoon-Ji Kim, and Min-Hyeok Choi. 2021. "Influence of Residence Area and Basic Livelihood Conditions on the Prevalence and Diagnosis Experience of Osteoporosis in Postmenopausal Women Aged over 50 Years: Evaluation Using Korea National Health and Nutrition Examination Survey Data" International Journal of Environmental Research and Public Health 18, no. 18: 9478. https://doi.org/10.3390/ijerph18189478
APA StyleKang, S.-W., Yang, J.-H., Shin, W.-C., Kim, Y.-J., & Choi, M.-H. (2021). Influence of Residence Area and Basic Livelihood Conditions on the Prevalence and Diagnosis Experience of Osteoporosis in Postmenopausal Women Aged over 50 Years: Evaluation Using Korea National Health and Nutrition Examination Survey Data. International Journal of Environmental Research and Public Health, 18(18), 9478. https://doi.org/10.3390/ijerph18189478







