The Effects of Electrical Stimulation Program on Navicular Height, Balance, and Fear of Falling in Community-Dwelling Elderly
Abstract
:1. Introduction
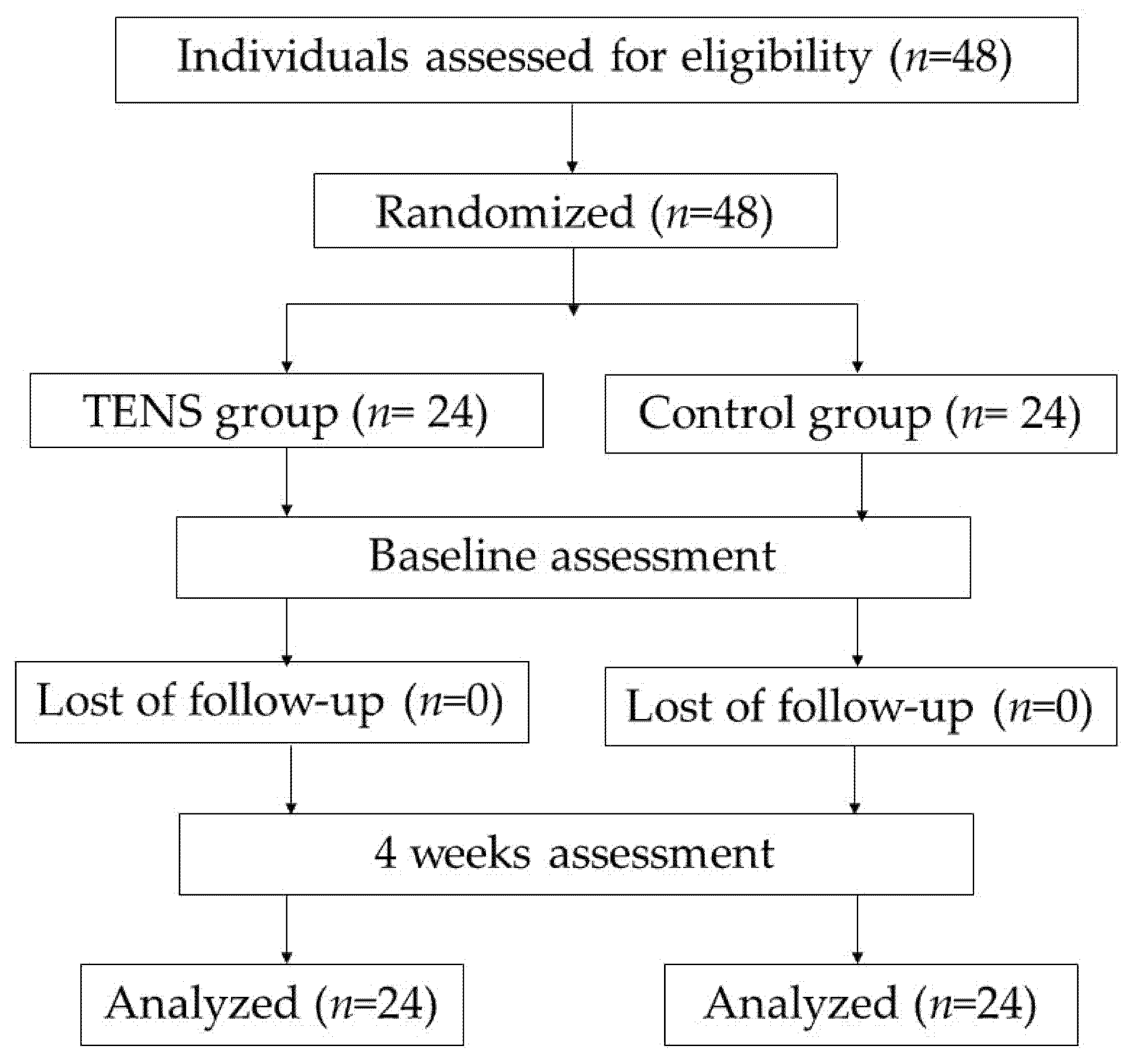
2. Materials and Methods
2.1. Study Design
2.2. Participants
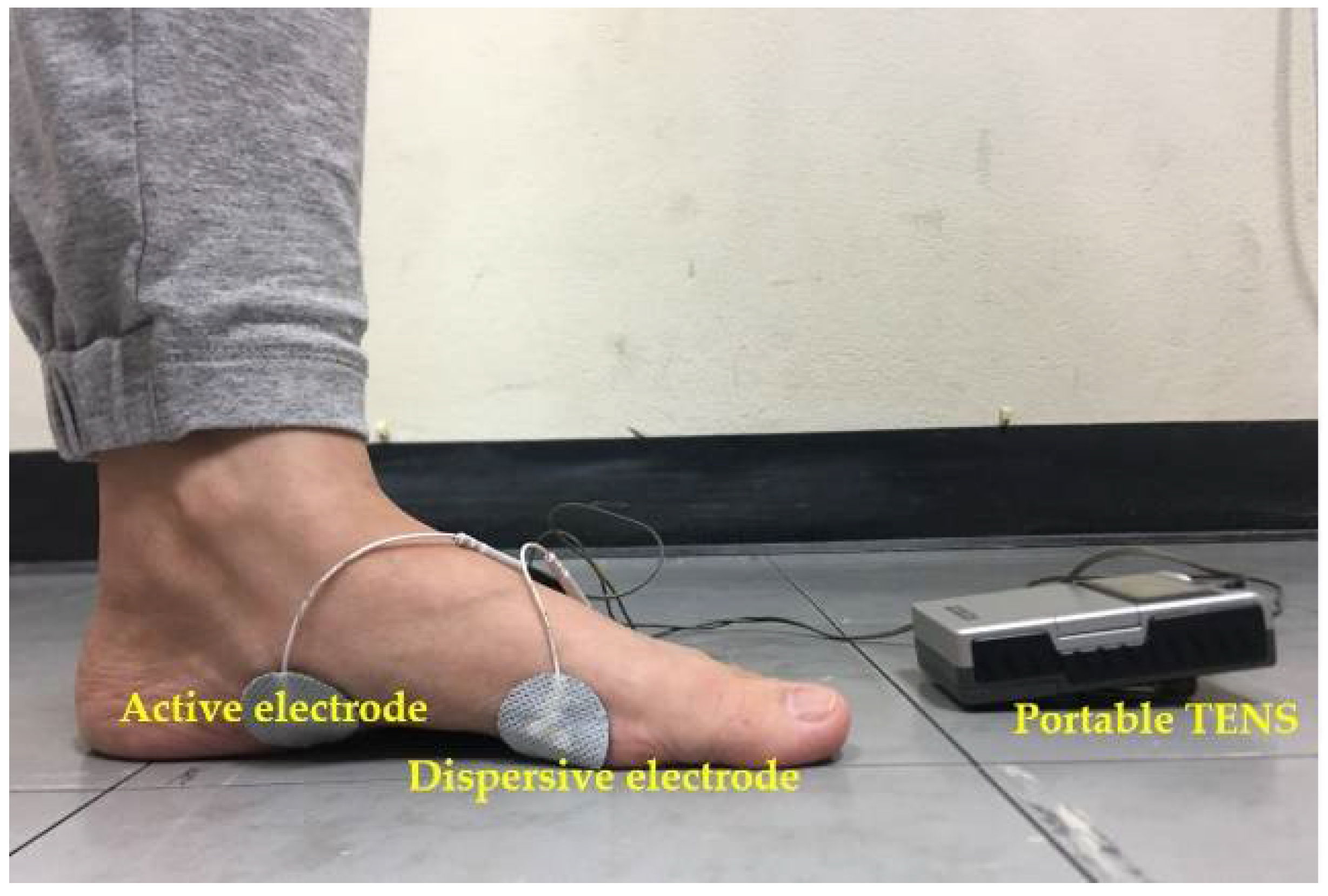
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwenk, M.; Jordan, E.D.; Honarvararaghi, B.; Mohler, J.; Armstrong, D.G.; Najafi, B. Effectiveness of foot and ankle exercise programs on reducing the risk of falling in older adults: A systematic review and meta-analysis of randomized controlled trials. J. Am. Podiatr. Med. Assoc. 2013, 103, 534–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiamwong, L.; Suwanno, J. Fear of Falling and Related Factors in a Community-based Studyof People 60 Years and Older in Thailand. Int. J. Gerontol. 2017, 11, 80–84. [Google Scholar] [CrossRef]
- Talley, K.M.; Wyman, J.F.; Gross, C.R.; Lindquist, R.A.; Gaugler, J.E. Change in Balance Confidence and Its Associations With Increasing Disability in Older Community-Dwelling Women at Risk for Falling. J. Aging Health 2014, 26, 616–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, O.T.; Jullamate, P.; Piphatvanitcha, N.; Rosenberg, E. Factors related to fear of falling among community-dwelling older adults. J. Clin. Nurs. 2017, 26, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Al-Momani, M.; Al-Momani, F.; Alghadir, A.H.; Alharethy, S.; Gabr, S.A. Factors related to gait and balance deficits in older adults. Clin. Interv. Aging 2016, 11, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Hwang, Y.I. Comparison of the Intrinsic Foot Muscle Activities between Therapeutic and Three-Dimensional Foot-Ankle Exercises in Healthy Adults: An Explanatory Study. Int. J. Environ. Res. Public Health 2020, 17, 7189. [Google Scholar] [CrossRef]
- Menz, H.B. Biomechanics of the Ageing Foot and Ankle: A Mini-Review. Gerontology 2015, 61, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Headlee, D.L.; Leonard, J.L.; Hart, J.M.; Ingersoll, C.D.; Hertel, J. Fatigue of the plantar intrinsic foot muscles increases navicular drop. J. Electromyogr. Kinesiol. 2008, 18, 420–425. [Google Scholar] [CrossRef]
- Fourchet, F.; Gojanovic, B. Foot core strengthening: Relevance in injury prevention and rehabilitation for runners. Swiss Sport Exerc. Med. 2016, 64, 26–30. [Google Scholar] [CrossRef]
- Kelly, L.A.; Cresswell, A.G.; Racinais, S.; Whiteley, R.; Lichtwark, G. Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J. R. Soc. Interface 2014, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulligan, E.P.; Cook, P.G. Effect of plantar intrinsic muscle training on medial longitudinal arch morphology and dynamic function. Man. Ther. 2013, 18, 425–430. [Google Scholar] [CrossRef]
- Kelly, L.A.; Kuitunen, S.; Racinais, S.; Cresswell, A.G. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. Clin. Biomech. 2012, 27, 46–51. [Google Scholar] [CrossRef]
- McKeon, P.O.; Fourchet, F. Freeing the foot: Integrating the foot core system into rehabilitation for lower extremity injuries. Clin. Sports Med. 2015, 34, 347–361. [Google Scholar] [CrossRef] [PubMed]
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2015, 249, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namsawang, J.; Eungpinichpong, W.; Vichiansiri, R.; Rattanathongkom, S. Effects of the Short Foot Exercise With Neuromuscular Electrical Stimulation on Navicular Height in Flexible Flatfoot in Thailand: A Randomized Controlled Trial. J. Prev. Med. Public Health 2019, 52, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddocks, M.; Halliday, V.; Chauhan, A.; Taylor, V.; Nelson, A.; Sampson, C.; Byrne, A.; Griffiths, G.; Wilcock, A. Neuromuscular electrical stimulation of the quadriceps in patients with non-small cell lung cancer receiving palliative chemotherapy: A randomized phase II study. PLoS ONE 2013, 8, e86059. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, K.A.; Silvian, P.; Ahmad, I.; Reddy, R.S.; Tedla, J.S.; Kakaraparthi, V.N.; Rengaramanujam, K. Effectiveness of Low-Frequency Stimulation in Proprioceptive Neuromuscular Facilitation Techniques for Post Ankle Sprain Balance and Proprioception in Adults: A Randomized Controlled Trial. Biomed. Res. Int. 2020, 2020, 9012930. [Google Scholar] [CrossRef]
- Jung, K.S.; Jung, J.H.; In, T.S.; Cho, H.Y. Effectiveness of Heel-Raise-Lower Exercise after Transcutaneous Electrical Nerve Stimulation in Patients with Stroke: A Randomized Controlled Study. J. Clin. Med. 2020, 9, 3532. [Google Scholar] [CrossRef] [PubMed]
- Laufer, Y.; Elboim-Gabyzon, M. Does sensory transcutaneous electrical stimulation enhance motor recovery following a stroke? A systematic review. Neurorehabil. Neural Repair 2011, 25, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.S.; In, T.S.; Cho, H.Y. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: A randomized controlled study. Gait Posture 2017, 54, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Brincks, J.; Nielsen, J.F. Increased power generation in impaired lower extremities correlated with changes in walking speeds in sub-acute stroke patients. Clin. Biomech. 2012, 27, 138–144. [Google Scholar] [CrossRef]
- Fourchet, F.; Kilgallon, M.; Loepelt, H.; Millet, G.P. Plantar muscles electrostimulation and navicular drop. Sci. Sport 2009, 24, 262–264. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, S.H.; In, T.S.; Lee, K.J.; Song, C.H. Effects of Transcutaneous Electrical Nerve Stimulation (TENS) on Changes in Postural Balance and Muscle Contraction following Muscle Fatigue. J. Phys. Ther. Sci. 2011, 23, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.A.; Lee, E.; Lee, S. The effect of intrinsic foot muscle training on medial longitudinal arch and ankle stability in patients with chronic ankle sprain accompanied by foot pronation. Phys. Ther. Rehabil. Sci. 2016, 5, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vieira, C.M.A.; Sousa, L.M.M.; Severino, S.; Sousa, L.; Caldeira, S. Cross-cultural validation of the falls efficacy scale international in elderly: Systematic literature review. J. Clin. Gerontol. Geriatr. 2016, 7, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.Y.; Jung, J.H.; Hahm, S.C.; Jung, K.; Kim, S.J.; Suh, H.R.; Cho, H.Y. The effect of single trial transcutaneous electrical nerve stimulation on balance and gait function in elderly people with dementia: A pilot study. Phys. Ther. Rehabil. Sci. 2017, 6, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Okamura, K.; Kanai, S.; Hasegawa, M.; Otsuka, A.; Oki, S. The effect of additional activation of the plantar intrinsic foot muscles on foot dynamics during gait. Foot 2018, 34, 1–5. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Takahashi, T.; Kawade, S.; Maeda, N.; Maruyama, H.; Hyngstrom, A. The Effect of Electrical Muscle Stimulation on Muscle Mass and Balance in Older Adults with Dementia. Brain Sci. 2021, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur. J. Appl. Physiol. 2010, 110, 223–234. [Google Scholar] [CrossRef]
- Piva, S.R.; Khoja, S.S.; Toledo, F.G.S.; Chester-Wasko, M.; Fitzgerald, G.K.; Goodpaster, B.H.; Smith, C.N.; Delitto, A. Neuromuscular Electrical Stimulation Compared to Volitional Exercise for Improving Muscle Function in Rheumatoid Arthritis: A Randomized Pilot Study. Arthritis Care Res. 2019, 71, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Halback, J.; Straus, D. Comparison of Electro-Myo Stimulation to lsokinetic Training in Increasing -Power of the Knee Extensor Mechanism. J. Orthop. Sports Phys. Ther. 1980, 2, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.K.; Nam, B.R.; Lee, Y.S.; Cheon, S.H. Relationship between the Application of TENS to the Lower Limbs and Balance of Healthy Subjects. J. Phys. Ther. Sci. 2013, 25, 1079–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi-Demneh, E.; Tyson, S.F.; Nester, C.J.; Cooper, G. The Effect of Transcutaneous Electrical Nerve Stimulation (TENS) Applied to the Foot and Ankle on Strength, Proprioception and Balance: A Preliminary Study. Clin. Res. Foot Ankle 2015, 3. [Google Scholar] [CrossRef]
- Quinlan, S.; Fong Yan, A.; Sinclair, P.; Hunt, A. The evidence for improving balance by strengthening the toe flexor muscles: A systematic review. Gait Posture 2020, 81, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ferlinc, A.; Fabiani, E.; Velnar, T.; Gradisnik, L. The Importance and Role of Proprioception in the Elderly: A Short Review. Mater. Sociomed. 2019, 31, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Mignardot, J.B.; Deschamps, T.; Le Goff, C.G.; Roumier, F.X.; Duclay, J.; Martin, A.; Sixt, M.; Pousson, M.; Cornu, C. Neuromuscular electrical stimulation leads to physiological gains enhancing postural balance in the pre-frail elderly. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Latey, P.J.; Burns, J.; Nightingale, E.J.; Clarke, J.L.; Hiller, C.E. Reliability and correlates of cross-sectional area of abductor hallucis and the medial belly of the flexor hallucis brevis measured by ultrasound. J. Foot Ankle Res. 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Sakuraba, K. Strength training for the intrinsic flexor muscles of the foot: Effects on muscle strength, the foot arch, and dynamic parameters before and after the training. J. Phys. Ther. Sci. 2014, 26, 373–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, H.A.; Vilaça, K.H.C.; Carvalho, G.A. Assessment of muscle mass, risk of falls and fear of falling in elderly people with diabetic neuropathy. Fisioter Mov. 2015, 28, 677–683. [Google Scholar] [CrossRef]
| Observe Parameter | Mean ± SD | p-Value, ES | |||||
|---|---|---|---|---|---|---|---|
| Control | TENS | Within Group Effect | Between Group Effect | Inter-Action Effect | |||
| Pre-Test | Post-Test | Pre-Test | Post-Test | ||||
| NDT (mm) | 6.60 ± 2.24 | 5.95 ± 2.21 | 6.21 ± 2.48 | 4.53 ± 2.33 | p < 0.001 * ES = 0.48 | p = 0.167 ES = 0.04 | p = 0.006 * ES = 0.16 |
| Peak weight: front (kg) | 55.46 ± 17.20 | 61.73 ± 13.81 | 53.00 ± 18.40 | 66.28 ± 14.39 | p < 0.001 * ES = 0.36 | p = 0.806 ES < 0.01 | p = 0.077 ES = 0.07 |
| Peak weight: back (kg) | 51.57 ± 17.67 | 59.13 ± 11.35 | 53.28 ± 16.26 | 64.82 ± 8.82 | p < 0.001 * ES = 0.357 | p = 0.305 ES = 0.02 | p = 0.298 ES = 0.02 |
| Peak weight: Rt. (kg) | 62.42 ± 13.96 | 66.48 ± 12.65 | 53.23 ± 16.43 | 68.82 ± 10.85 | p < 0.001 * ES = 0.34 | p = 0.315 ES = 0.02 | p = 0.007 * ES = 0.15 |
| Peak weight: Lt. (kg) | 61.59 ± 15.70 | 69.65 ± 13.74 | 57.14 ± 15.51 | 73.61 ± 14.02 | p < 0.001 * ES = 0.41 | p = 0.946 ES < 0.01 | p = 0.06 ES = 0.08 |
| FES-I | 25.96 ± 6.42 | 24.63 ± 4.41 | 26.63 ± 6.99 | 21.79 ± 4.82 | p < 0.001 * ES = 0.36 | p = 0.488 ES = 0.01 | p = 0.006 * ES = 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namsawang, J.; Muanjai, P.; Luangpon, N.; Kiatkulanusorn, S. The Effects of Electrical Stimulation Program on Navicular Height, Balance, and Fear of Falling in Community-Dwelling Elderly. Int. J. Environ. Res. Public Health 2021, 18, 9351. https://doi.org/10.3390/ijerph18179351
Namsawang J, Muanjai P, Luangpon N, Kiatkulanusorn S. The Effects of Electrical Stimulation Program on Navicular Height, Balance, and Fear of Falling in Community-Dwelling Elderly. International Journal of Environmental Research and Public Health. 2021; 18(17):9351. https://doi.org/10.3390/ijerph18179351
Chicago/Turabian StyleNamsawang, Juntip, Pornpimol Muanjai, Nongnuch Luangpon, and Sirirat Kiatkulanusorn. 2021. "The Effects of Electrical Stimulation Program on Navicular Height, Balance, and Fear of Falling in Community-Dwelling Elderly" International Journal of Environmental Research and Public Health 18, no. 17: 9351. https://doi.org/10.3390/ijerph18179351
APA StyleNamsawang, J., Muanjai, P., Luangpon, N., & Kiatkulanusorn, S. (2021). The Effects of Electrical Stimulation Program on Navicular Height, Balance, and Fear of Falling in Community-Dwelling Elderly. International Journal of Environmental Research and Public Health, 18(17), 9351. https://doi.org/10.3390/ijerph18179351






