Abstract
Background: Sleep is an important modulator of neuroendocrine function and glucose metabolism. Poor sleep quality is related to metabolic and endocrine alterations, including decreased glucose tolerance, decreased insulin sensitivity, and increased hunger and appetite. Objective: The aim of the present study was to determine the association between sleep quality with metabolic syndrome (MetS) markers, fitness and body fat of women with severe/morbid obesity. Methods: This cross-sectional study included 26 women with severe/morbid obesity. Fasting plasma glucose (FPG), high-density lipids (HDL-c), triglycerides (TGs), and the metabolic outcomes total cholesterol (Tc) and low-density lipids (LDL-c), systolic (SBP) and diastolic blood pressure (DBP), body composition and fitness were measured. Results: Poor sleep quality showed a positive association with body fat (%) ≥ 48.2 (OR; 8.39, 95% CI; 1.13–62.14, p = 0.037), morbid obesity (OR; 8.44, 95% CI; 1.15–66.0, p = 0.036), glucose ≥ 100 mg/dL (OR; 8.44, 95% CI; 1.15–66.0, p = 0.036) and relative handgrip strength ≤ 0.66 (OR; 12.2, 95% CI; 1.79–83.09, p = 0.011). Conclusion: sleep quality is associated with health markers in women with severe/morbid obesity.
1. Introduction
Sleep is an important modulator of neuroendocrine function and glucose metabolism, and poor sleep quality is related to metabolic and endocrine alterations, including decreased glucose tolerance, decreased insulin sensitivity, and increased hunger and appetite [1]. Poor sleep quality is related to several other metabolic syndrome (MetS) risk factors, such as higher waist circumference (WC), hypertension, elevated serum triglycerides (TGs), low serum high-density lipoprotein cholesterol (HDL-C), and hyperglycaemia [2]. Furthermore, today, a short sleeping time is very common and sleep duration has been associated with a great number of adverse health effects, including all-cause mortality, weight gain, and incident cardiovascular disease [3]. Therefore, reduced sleep is a risk factor for obesity and cardiovascular disease, and consequently, may contribute to worsening of common obesity complications such as metabolic and cardiovascular diseases and reduce cardiorespiratory fitness (CRF) [4].
In addition, evidence showed a strong relationship between sleep quality and hormonal status, with sleep restriction seeming to impair the hormonal system that regulates energy balance. This involves several hormones, including cortisol, insulin, ghrelin, leptin and melatonin, increasing the risk of diabetes [5] and mortality [6].
Moreover, morbid obesity is associated with an increase in sleep disorders such as obstructive sleep apnoea, affecting sleep quality [7]. In this sense, poor sleep quality is strongly associated with mood disturbance and poor health related to quality of life (HRQoL) among patients with morbid obesity [8]. Furthermore, women with morbid obesity have poorer sleep quality [9]. In this sense, poor sleep quality is associated with an adverse metabolic profile and low fitness [10,11], indicating that sleep may play an important role in health disparities and may represent a risk factor for MetS [12,13].
The integration of the concepts of sleep quality and duration and the examination of their combined and independent impacts on health outcomes is a sensible and necessary step in understanding the contribution of sleep as a whole to public health [14]. Accordingly, patients with severe/morbid obesity (i.e., ≥obesity class II) present more adverse MetS factors and higher levels of inflammation [5,6,8], especially women suffering from more severe obstructive sleep apnoea syndrome and worse sleep quality [8]. However, how MetS risk factors are related to sleep quality in women with morbid obesity must be studied deeply. Therefore, the present study aimed to determine the association between sleep quality with MetS markers, such as WC, hypertension, elevated TG, low HDL-C, hyperglycaemia, fitness and body fat in women with morbid obesity.
2. Materials and Methods
2.1. Study Design
This cross-sectional study included 26 female volunteers selected by convenience, who provided signed written consent for participation. Ten participants had obesity (38.5%) and 16 had morbid obesity (61.5%). The study was carried out in accordance with the Declaration of Helsinki (2013) and was approved by the Ethical Committee of the Universidad de La Frontera, Temuco, Chile (Act 080-21).
The inclusion criteria were (i) 18–60 years of age, (ii) women, (iii) medical authorization for physical testing, and (iv) body mass index (BMI) ≥ 35 kg/m2 (i.e., ≥obesity class II). The exclusion criteria were (i) physical limitations preventing the performance of the physical test (e.g., restrictive injuries of the musculoskeletal system), (ii) exercise-related dyspnoea or respiratory alterations, and (iii) chronic heart disease with any worsening in the last month.
2.2. Measurements
2.2.1. Sleep Quality Measurements
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) [15]. The PSQI is a self-report questionnaire that includes seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. In the PSQI, subjects rate perceived sleep quality as very good, fairly good, fairly bad, or very bad. These subjective scales are weighted to obtain a global PSQI score that differentiates between good and poor SQ. Their sum builds the global PSQI report, which provides an inverse score, where a score <5 denotes good sleep quality (GSQ) and a score >5 denotes poor sleep quality (PSQ). This scale has been used in previous studies [16] and publications that examined bariatric patients [17]. Conditions associated with PSQ include the use of sleep medications, difficulties in daily living and enthusiasm, and low sleep efficiency.
2.2.2. MetS Markers
The MetS markers were screened using standard criteria [2,18]. After overnight fasting for 10 ± 2 h, all patients underwent a baseline assessment (pre-test) between 08:00 and 9:00 in the morning. All participants arrived at the lab for the extraction of a 5 mL blood sample, in order to determine the MetS outcomes: fasting plasma glucose (FPG), HDL-c, and TG. The systolic (SBP) and diastolic blood pressures (DBP) were measured according to the standard criteria [19]. Blood pressure was measured in the sitting position after 5 min rest. Two recordings were taken, and the mean of the measurements was used for statistical analysis using an OMRONTM digital electronic BP monitor (model HEM 7114, Chicago, IL, United States). Caffeine, exercise, and smoking were avoided for at least 30 min prior to the measurement [20]. WC was assessed using a tape measure graduated in centimetres (Adult SECATM) at the upper hipbone and the top of the right iliac crest, with a non-elastic measuring tape in a horizontal plane around the abdomen at the level of the iliac crest. The tape was snug, but not skin compressing, and it was parallel to the floor. The measurement was performed at the end of a normal expiration [21].
2.2.3. Body Composition and Anthropometric Parameters
Body mass (kg) and body fat (%) were measured using a digital bioimpedance scale (TANITATM, model 331, Tokyo, Japan). Height (m) was measured with a SECATM stadiometer (model 214, Hamburg, Germany), with subjects wearing light clothing and without shoes. The BMI was calculated as the body mass divided by height squared (kg/m2), and then used to estimate the degree of obesity using the standard criteria for obesity class [22].
2.2.4. Six-Minutes Walking Test
The day after the metabolic measurements, the physical condition of participants in both groups was measured by endurance and muscle strength testing. First, a six-minute walking test (6Mwt) was used to estimate CRF. The test was performed in a closed space on a flat surface (30 m long), with two reflective cones placed at the ends to indicate the distance. During the test, participants were assisted with instructions from an exercise physiologist [23].
2.2.5. Handgrip Strength
Which has been used in previous studies [24]. Two attempts were performed, measuring each hand, and the best result from each was selected. As previously, the mean value obtained was taken as the total score [24]. Using these data, we calculated other outcomes such as the HGS relative to BMI (HGS/BMI).
2.3. Data Analysis
The statistical analysis of the data was carried out using the statistics programs STATA v.15.0 (StataCorp, College Station, TX, USA). The absolute frequencies were determined for the qualitative variables. The comparison between groups was evaluated using Student’s t test. In order to determine the linear relation between the sleep quality score and anthropometric, metabolic and fitness parameters, Pearson’s correlation coefficients were calculated, as well as multiple logistic regression models, determining the odds ratios. Values of p < 0.05 were considered statistically significant.
3. Results
Table 1 shows anthropometric, clinical-metabolic and fitness parameters according to sleep quality. Fourteen women presented good sleep quality (GSQ; 38.42 ± 12.64 years) and twelve poor sleep quality (PSQ; 41.66 ± 11.38 years). The poor sleep quality group reported a higher BMI (GSQ; 39.62 ± 5.72 vs. PSQ; 45.99 ± 7.61 kg/m2, p = 0.023) and body fat percentage (GSQ; 46.45 ± 4.28 vs. PSQ; 50.21 ± 4.74%, p = 0.044) than the good sleep quality group. In addition, they reported lower CRF (GSQ; 560 ± 71.14 vs. PSQ; 458.33 ± 105.12 m, p = 0.007).

Table 1.
Anthropometric, clinical-metabolic and fitness characteristics of the study population according to sleep quality.
Figure 1 shows the relationship between a poor sleep quality score and anthropometric, metabolic and fitness parameters. BMI (rho = 0.40, p = 0.03, Panel (A)) and triglyceride levels (rho= 0.44, p = 0.02, Panel (B)) were linked positively with poor sleep quality, whereas CRF showed an inverse relationship with poor sleep quality (rho = −0.66, p < 0.001, Panel (C)). There was not a significant relationship with another sociodemographic and physical variables (Table 2).
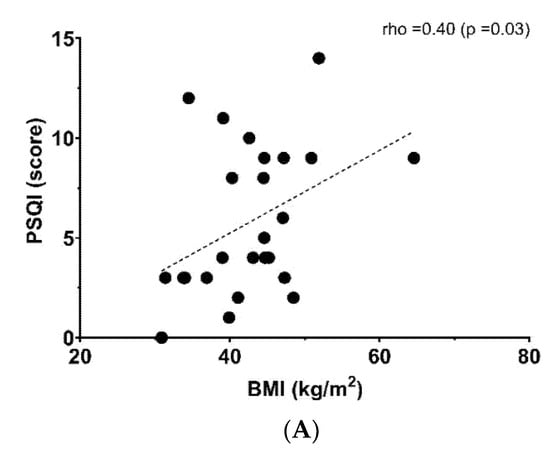
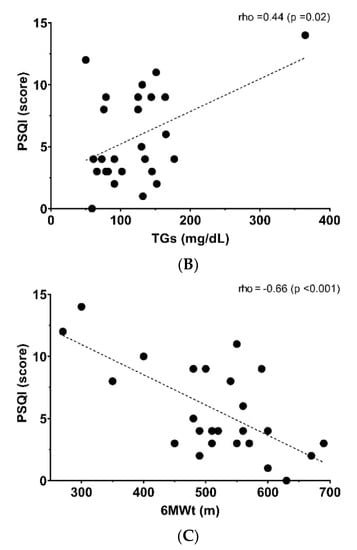
Figure 1.
Relationship between poor sleep quality score and (A) body mass index (BMI), (B) triglycerides (TGs) and (C) six-minute walking test (6MWt).

Table 2.
Relationship between sleep quality score and anthropometric, metabolic and fitness parameters.
In total, 61.5% of patients presented with morbid obesity, and 34.62% presented ≥4 MetS parameters. There were no significant differences between groups (Table 3).

Table 3.
Frequency of parameters related to MetS markers according to sleep quality.
Table 4 shows the association between poor sleep quality with MetS markers and physical status. Poor sleep quality reported a positive association with body fat (%) ≥ 48.2 (OR; 8.39, 95%CI; 1.13–62.14, p = 0.037), morbid obesity condition (OR; 8.44, 95%CI; 1.15–66.0, p = 0.036), glucose ≥ 100 mg/dL (OR; 8.44, 95%CI; 1.15–66.0, p = 0.036) and relative handgrip strength ≤ 0.66 (OR; 12.2, 95%CI; 1.79–83.09, p = 0.011).

Table 4.
Association between poor sleep quality with MetS markers and physical status.
4. Discussion
The aim of the present study was to determine the association between sleep quality with MetS markers (i.e., WC, hypertension, elevated TG, low HDL-C, and hyperglycaemia), fitness and body fat in women with severe/morbid obesity. In the present study, poor sleep quality showed an association with body fat and morbid obesity. Previous evidence showed that lack of sleep is a risk factor for obesity, insulin resistance, and type 2 diabetes [23]; therefore, a short sleep duration and other dimensions of poor sleep quality are associated with body fat [16]. In this context, it was demonstrated in adult subjects that poorer sleep efficiency is related to higher fat mass [24]. In addition, a recent study reported that BMI negatively predicted sleep duration and sleep efficiency in Chinese young adults [25]. Sweatt et al. [26] showed that lower sleep quality is associated with elevated visceral adipose tissue, and poor sleep quality was positively associated with fat mass percentage in Spanish subjects [27]. Similarly, another study reported that fewer hours of sleep may be linked to fat mass index and obesity increase in Korean adults [28]. Another study showed that body composition of subjects with obesity is related to sleep habit changes (i.e., sleep quality and quantity), and the results of this study also indicated that sleep disorders such as obstructive sleep apnoea are associated with sarcopenic obesity, and nocturnal hypoxia is linked to obesity [29].
Another important result is that poor sleep quality showed an association with glucose alteration (i.e., glucose ≥ 100 mg/dL) and reported a positive relationship with triglyceride levels. An study showed that the anti-inflammatory cytokine (IL-10) serum levels were significantly reduced in subjects with morbid obesity and obstructive sleep apnoea, and reported a strong correlation with a systemic state of hyperinsulinemia and insulin resistance [30]. Likewise, another study reported that fasting glucose is directly linked to sleep duration; nevertheless, sleep quality was not associated with fasting glucose or 2-h glucose in adults who are overweight/obese [31]. Knutson et al. [32] indicated that sleep quality and duration were predictors of haemoglobin A1c (HbA1c) level, an important marker of glucose alteration/control, in American volunteers with diabetes. Moreover, evidence showed that sleep disturbances may be linked to impaired glucose metabolism [33]. In this context, another study conducted in subjects with type 2 diabetes mellitus showed that poor sleep quality was related to worse control of glycaemia [34]. Another study conducted in women who were overweight or obese reported that the participants who had worse sleep quality obtained significantly higher HOMA2-IR; therefore, the authors concluded that more studies were needed to establish whether enhancing sleep quality improves insulin resistance [35]. The evidence also showed that women have lower sleep quality than men [8]. Chirwa et al. [36] reported that worse sleep quality was linked to higher HbA1c and attendant health complications in pregnant women. In addition, Morselli et al. [37] indicated that sleep loss affects insulin sensitivity, leading to increased diabetes risk. Although there is no complete mechanistic explanation for these observations, they point to a mismatch between circadian rhythm and energy homeostasis that may trigger or increase symptoms of obesity and diabetes [38].
Fitness levels were linked to poor sleep quality, and sleep quality is positively associated with greater physical fitness and especially with high levels of upper body strength [39]. Hence, excess fat reduces the total compliance of the respiratory system, increases pulmonary resistance, and reduces respiratory muscle strength [40], which can affect the CRF. In this context, other findings indicated that sleep patterns (i.e., quality and duration) were factors that had a significant influence on physical fitness results in Taiwanese adults. Likewise, a cross-sectional study showed that sleep quality was strongly linked to physical fitness (i.e., grip strength, one-leg standing test, back scratch test and vital capacity) in Chinese adults [41]. Moreover, it was reported that worse sleep quality is related to lower levels of physical fitness and less physical activity in young adults [42]. In this context, another study showed that poor sleep quality was associated with worse CRF in adolescent women [43]. On the other hand, evidence showed that a good CRF is fundamental to decrease sleep problems [44]. Likewise, Lee and Lin [45] reported that young adults with worse sleep quality had lower levels of CRF and muscular endurance. Another study showed that sleep problems were associated with poorer physical fitness in women [4]. Therefore, sleep quality is an important marker related to health in different dimensions.
Limitations
The main limitation of this study was that the participants’ lifestyle such as physical activity levels and eating habits were not measured. Moreover, menopausal status and transition were not considered, which can have an impact on sleep quality and duration.
5. Conclusions
In the present study, poor sleep quality in women with severe/morbid obesity showed a negative association with body fat, metabolic outcomes and fitness. For this reason, poor sleep quality represents an important factor that can further aggravate the health of women with morbid obesity. It is necessary to integrate the concepts of sleep quality and duration to examine their combined and independent impacts on health outcomes in order to understand the contribution of sleep as a whole to public health.
Author Contributions
Conceptualization, P.D.-F. and C.A.V.; methodology, C.A.V., P.D.-F. and I.P.G.-G.; software, D.J.-M.; validation, L.J.C.-R., C.A.V. and P.D.-F.; formal analysis, I.P.G.-G.; investigation, P.D.-F.; resources, P.D.-F.; data curation, P.D.-F.; writing—original draft preparation, P.D.-F. and C.A.V.; writing—review and editing, P.D.-F. and L.J.C.-R.; visualization, F.C.-N.; supervision, F.C.-N.; project administration, C.A.V.; funding acquisition, P.D.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de La Frontera, Temuco, Chile Project DI21-0030 and Project FRO 1895.
Institutional Review Board Statement
The study was carried out in accordance with the Declaration of Helsinki (2013) and was approved by the Ethical Committee of the Universidad de La Frontera, Temuco, Chile (Act 080-21).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors thank the team from the research and treatment programme for severe/morbid obesity, Department of Physical Education, Sports, and Recreation of the La Frontera University, for their support in the development of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beccuti, G.; Pannain, S. Sleep and obesity. Current opinion in clinical nutrition and metabolic care. PMC 2011, 14, 402–412. [Google Scholar]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Badran, M.; Yassin, B.A.; Fox, N.; Laher, I.; Ayas, N. Epidemiology of sleep disturbances and cardiovascular consequences. Can. J. Cardiol. 2015, 31, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Di Somma, C.; Laudisio, D.; Colao, A.; Savastano, S. Obesity and sleep disturbance: The chicken or the egg? Crit. Rev. Food Sci. Nutr. 2019, 59, 2158–2165. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E. Associations between sleep loss and increased risk of obesity and diabetes. Ann. N. Y. Acad. Sci. 2008, 1129, 287–304. [Google Scholar] [CrossRef]
- Grandner, M.A.; Hale, L.; Moore, M.; Patel, N.P. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med. Rev. 2010, 14, 191–203. [Google Scholar] [CrossRef]
- Raveendran, R.; Wong, J.; Chung, F. Morbid obesity, sleep apnea, obesity hypoventilation syndrome: Are we sleepwalking into disaster? Perioper. Care Oper. Room Manag. 2017, 9, 24–32. [Google Scholar] [CrossRef]
- Araghi, M.H.; Jagielski, A.; Neira, I.; Brown, A.; Higgs, S.; Thomas, G.N.; Taheri, S. The complex associations among sleep quality, anxiety-depression, and quality of life in patients with extreme obesity. Sleep 2013, 36, 1859–1865. [Google Scholar] [CrossRef]
- André, S.; Andreozzi, F.; Van Overstraeten, C.; Youssef, S.B.; Bold, I.; Carlier, S.; Gruwez, A.; Bruyneel, A.-V.; Bruyneel, M. Cardiometabolic comorbidities in obstructive sleep apnea patients are related to disease severity, nocturnal hypoxemia, and decreased sleep quality. Respir. Res. 2020, 21, 35. [Google Scholar] [CrossRef]
- Garfield, V. The association between body mass index (BMI) and sleep duration: Where are we after nearly two decades of epidemiological research? Int. J. Environ. Res. Public Health 2019, 16, 4327. [Google Scholar] [CrossRef]
- Vanhecke, T.E.; Franklin, B.A.; Zalesin, K.C.; Sangal, R.B.; deJong, A.T.; Agrawal, V.; McCullough, P.A. Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest 2008, 134, 539–545. [Google Scholar] [CrossRef]
- Rangaraj, V.R.; Knutson, K.L. Association between sleep deficiency and cardiometabolic disease: Implications for health disparities. Sleep Med. 2016, 18, 19–35. [Google Scholar]
- McHill, A.W.; Wright, K.P., Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 2017, 18 (Suppl. 1), 15–24. [Google Scholar] [CrossRef]
- Bin, Y.S. Is sleep quality more important than sleep duration for public health? Sleep 2016, 39, 1629–1630. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Rahe, C.; Czira, M.E.; Teismann, H.; Berger, K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015, 16, 1225–1228. [Google Scholar] [CrossRef]
- Toor, P.; Kim, K.; Buffington, C.K. Sleep quality and duration before and after bariatric surgery. Obes. Surg. 2012, 22, 890–895. [Google Scholar]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A. 2013 ESH/ESC practice guidelines for the management of arterial hypertension: ESH-ESC the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press. 2014, 23, 3–16. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef]
- National Institutes of Health; National Heart, Lung, and Blood Institute. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; North American Association for the Study of Obesity: Long Beach, CA, USA, 2000.
- Johnson Stoklossa, C.A.; Sharma, A.M.; Forhan, M.; Siervo, M.; Padwal, R.S.; Prado, C.M. Prevalence of sarcopenic obesity in adults with class II/III obesity using different diagnostic criteria. J. Nutr. Metab. 2017, 2017, 7307618. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef]
- Kahlhöfer, J.; Karschin, J.; Breusing, N.; Bosy-Westphal, A. Relationship between actigraphy-assessed sleep quality and fat mass in college students. Obesity 2016, 24, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, Q.; Zeng, N.; McDonough, D.J.; Tao, K.; Peng, Q.; Gao, Z. Relationships between college students’ sedentary behavior, sleep quality, and body mass index. Int. J. Environ. Res. Public Health 2021, 18, 3946. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, S.K.; Gower, B.A.; Chieh, A.Y.; Liu, Y.; Li, L. Sleep quality is differentially related to adiposity in adults. Psychoneuroendocrinology 2018, 98, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la-O, A.; Dote-Montero, M.; Gutiérrez, Á.; Castillo, M.J. Association between sleep quality and body composition in sedentary middle-aged adults. Medicina 2018, 54, 91. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shin, D.; Jung, G.U.; Lee, D.; Park, S.M. Association between sleep duration, fat mass, lean mass and obesity in Korean adults: The fourth and fifth Korea National Health and Nutrition Examination Surveys. J. Sleep Res. 2017, 26, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, R.D.; Hirotsu, C.; Moizinho, R.; de Sá Souza, H.; D’Almeida, V.; Tufik, S.; Poyares, D. Associations between sleep conditions and body composition states: Results of the EPISONO study. J. Cachexia Sarcopenia Muscle 2019, 10, 962–973. [Google Scholar] [CrossRef]
- Leon-Cabrera, S.; Arana-Lechuga, Y.; Esqueda-Leon, E.; Teran-Perez, G.; Gonzalez-Chavez, A.; Escobedo, G.; Velazquez Moctezuma, J. Reduced systemic levels of IL-10 are associated with the severity of obstructive sleep apnea and insulin resistance in morbidly obese humans. Mediat. Inflamm. 2015, 2015, 493409. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Temple, K.A.; Tjaden, A.H.; Edelstein, S.L.; Utzschneider, K.M.; Nadeau, K.J.; Hannon, T.S.; Sam, S.; Barengolts, E.; Manchanda, S.; et al. Association of self-reported sleep and circadian measures with glycemia in adults with prediabetes or recently diagnosed untreated type 2 diabetes. Diabetes Care 2019, 42, 1326–1332. [Google Scholar] [CrossRef]
- Knutson, K.L.; Ryden, A.M.; Mander, B.A.; Van Cauter, E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 2006, 166, 1768–1774. [Google Scholar] [CrossRef]
- Trento, M.; Broglio, F.; Riganti, F.; Basile, M.; Borgo, E.; Kucich, C.; Passera, P.; Tibaldi, P.; Tomelini, M.; Cavallo, F.; et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008, 45, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.W.; Kann, N.H.; Tung, T.H.; Chao, Y.J.; Lin, C.J.; Chang, K.C.; Chang, S.S.; Chen, J.Y. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Fam. Pract. 2012, 29, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.E.; Hall, M.H.; Buysse, D.J.; Earnest, C.P.; Church, T.S. Poor sleep quality is associated with insulin resistance in postmenopausal women with and without metabolic syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 183–189. [Google Scholar] [CrossRef]
- Chirwa, S.; Nwabuisi, C.R.; Ladson, G.M.; Korley, L.; Whitty, J.E.; Atkinson, R.; Clark, J.T. Poor sleep quality is associated with higher hemoglobin A1c in pregnant women: A pilot observational study. Int. J. Environ. Res. Public Health 2018, 15, 2287. [Google Scholar] [CrossRef] [PubMed]
- Morselli, L.; Leproult, R.; Balbo, M.; Spiegel, K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract. Research. Clin. Endocrinol. Metab. 2010, 24, 687–702. [Google Scholar] [CrossRef]
- Jha, P.K.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 2015, 418, 74–88. [Google Scholar]
- Moreno-Vecino, B.; Arija-Blazquez, A.; Pedrero-Chamizo, R.; Gomez-Cabello, A.; Alegre, L.M.; Perez-Lopez, F.R.; Gonzalez-Gross, M.; Casajus, J.A.; Ara, I.; on behalf of the EXERNET Group. Sleep disturbance, obesity, physical fitness and quality of life in older women: EXERNET study group. Climacteric 2017, 20, 72–79. [Google Scholar] [CrossRef]
- Mafort, T.T.; Rufino, R.; Costa, C.H.; Lopes, A.J. Obesity: Systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip. Respir. Med. 2016, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, N.; Zhang, X.; Bao, X.; Xie, Y.; Huang, J.; Wang, P.; Du, Q. Associations between objectively assessed physical fitness levels and sleep quality in community-dwelling elderly people in South China. Sleep Breath. 2019, 23, 679–685. [Google Scholar] [CrossRef]
- Štefan, L.; Krističević, T.; Sporiš, G. The associations of self-reported physical fitness and physical activity with sleep quality in young adults: A population-based study. Ment. Health Phys. Act. 2018, 14, 131–135. [Google Scholar] [CrossRef]
- Mota, J.; Vale, S. Associations between sleep quality with cardiorespiratory fitness and BMI among adolescent girls. Am. J. Hum. Biol. 2010, 22, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; Sui, X.; Church, T.S.; Kline, C.E.; Youngstedt, S.D.; Blair, S.N. Decline in cardiorespiratory fitness and odds of incident sleep complaints. Med. Sci. Sports Exerc. 2015, 47, 960–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, A.; Lin, W. Association between sleep quality and physical fitness in female young adults. J. Sports Med. Phys. Fit. 2007, 47, 462. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).