Variations in Salivary Stress Biomarkers and Their Relationship with Anxiety, Self-Efficacy and Sleeping Quality in Emergency Health Care Professionals
Abstract
:1. Introduction
1.1. Stress and Anxiety
1.2. Stress, Sleep Quality and Self-Efficacy
2. Materials and Methods
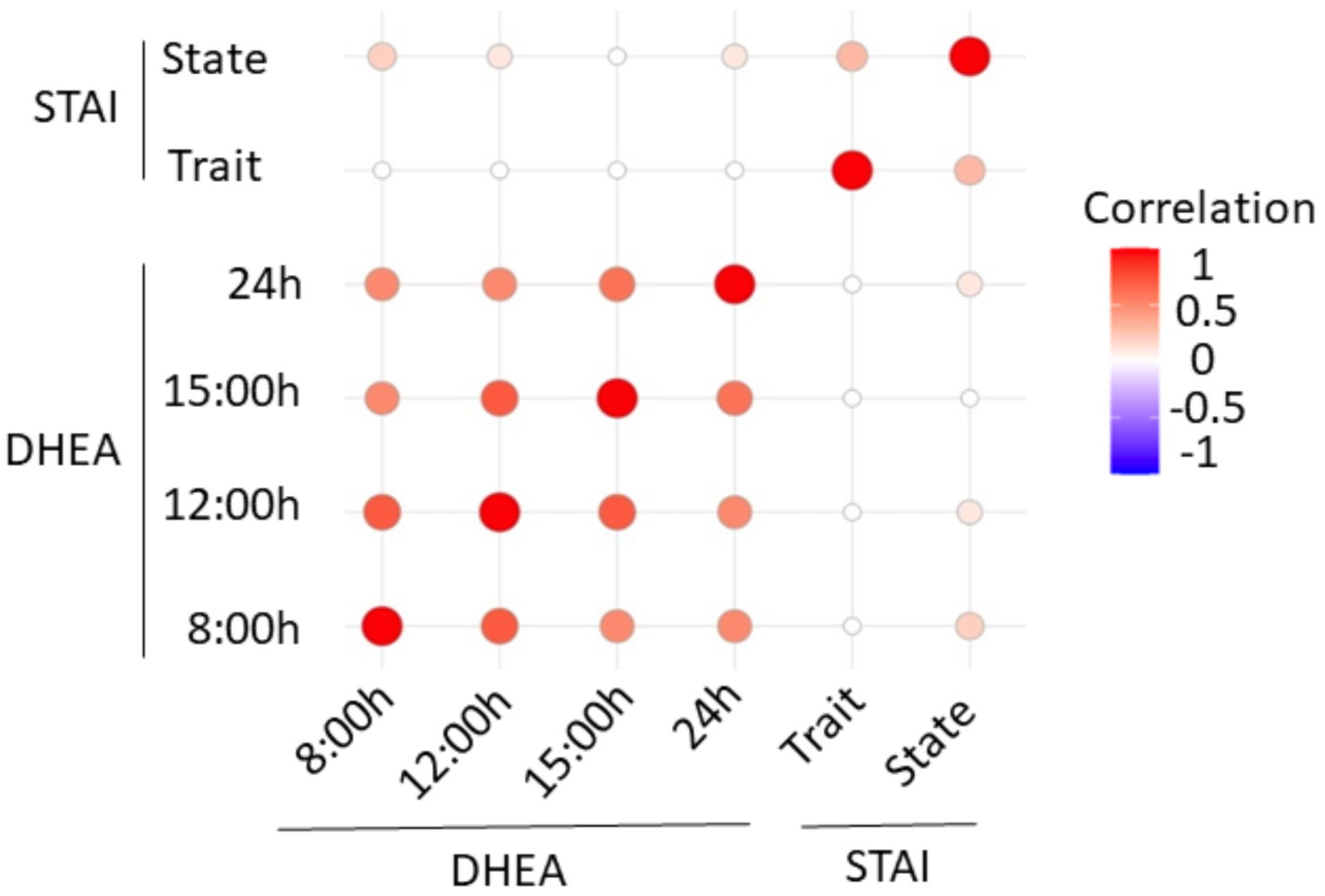
3. Results
4. Discussion
4.1. Stress and Anxiety in the Studied ED Professionals
4.2. Stress and Self-Efficacy in the Studied ED Professionals
4.3. Stress and Sleep Quality in the Studied ED Professionals
4.4. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hart, P.M.; Wearing, A.J.; Headey, B. Assessing police work experiences: Development of the police daily hassle and uplifts scales. J. Crim. Justice 1993, 21, 553–572. [Google Scholar] [CrossRef]
- Cotton, P.; Hart, P.M. Occupational well-being and performance: A review of Organisational Health Research. Aust. Psychol. 2003, 38, 118–128. [Google Scholar] [CrossRef]
- Cozma, S.; Dima-Cozma, L.C.; Ghiciuc, C.M.; Pasquali, V.; Saponaro, A.; Patacchioli, F.R. Salivary cortisol and α-amylase: Subclinical indicators of stress as cardiometabolic risk. Braz. J. Med. Biol. Res. 2017, 50, e5577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbeck, R.; Coomber, S.; Robinson, S.; Todd, C. Occupational stress in consultants in accident and emergency medicine: A national survey of levels of stress at work. Emerg. Med. J. 2002, 19, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-Lima, K.; Loureiro, S.R. Burnout, anxiety, depression, and social skills in medical residents. Psychol. Health Med. 2015, 20, 353–362. [Google Scholar] [CrossRef]
- Córdova, A. Dynamic physiology, Fisiología Dinámica; Masson-Elsevier: Barcelona, Spain, 2003. [Google Scholar]
- Dahlgren, A.; Kecklund, G.; Theorell, T.; Åkerstedt, T. Day-to-day variation in saliva cortisol—Relation with sleep, stress and self-rated health. Biol. Psychol. 2009, 82, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.T.J.; Vogelso, K.M.; Lu, Y.C.; Ellman, A.B.; Hudgens, G.A. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996, 16, 433–448. [Google Scholar] [CrossRef]
- Takaia, N.; Yamaguchib, M.; Aragakia, T.; Etoa, K.; Uchihashia, K.; Nishikawa, Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004, 4, 963–968. [Google Scholar] [CrossRef]
- Mulligan, E.M.; Hajcak, G.; Crisler, S.; Meyer, A. Increased dehydroepiandrosterone (DHEA) is associated with anxiety in adolescent girls. Psychoneuroendocrinology 2020, 119, 104751. [Google Scholar] [CrossRef]
- Chen, J.; Davis, L.S.; Davis, K.G.; Pan, W.; Daraiseh, N.M. Physiological and behavioural response patterns at work among hospital nurses. J. Nurs. Manag. 2011, 19, 57–68. [Google Scholar] [CrossRef]
- Eisenach, J.H.; Sprung, J.; Clark, M.M.; Shanafelt, T.D.; Johnson, B.D.; Kruse, T.N.; Chantigian, D.P.; Carter, J.R.; Long, T.R. The psychological and physiological effects of acute occupational stress in new anesthesiology residents: A pilot trial. Anesthesiology 2014, 121, 878–893. [Google Scholar] [CrossRef] [Green Version]
- Sierra, J.C.; Ortega, V.; Zubeidat, I. Ansiedad, angustia y estrés: Tres conceptos a diferenciar. Rev. Mal-Estar Subj. 2003, 3, 10–59. [Google Scholar]
- Caballero, L.; García, P. Ansiedad: Una Introducción para el Médico de Atención Primaria; DOYMA: Madrid, Spain, 1999. [Google Scholar]
- Ansorena, A.; Cobo, J.; Romero, I. El constructo ansiedad en Psicología. Una revisión. Estud. Psicol. 1983, 16, 31–45. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual STAI, Cuestionario de Ansiedad Estado Rasgo; TEA Ediciones: Madrid, Spain, 1982. [Google Scholar]
- Miguel-Tobal, J.J.; Cano-Vindel, A. Perfiles diferenciales de los trastornos de ansiedad. Ansiedad Estrés 1995, 1, 37–60. [Google Scholar]
- Maletic, V.; Robinson, M.; Oakes, T.; Iyengar, S.; Ball, S.G.; Russell, J. Neurobiology of depression: An integrated view of key findings. Int. J. Clin. Pract. 2007, 61, 2030–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuade, R.; Young, A.H. Future therapeutic targets in mood disorders: The glucocorticoid receptor. Br. J. Psychiatry 2000, 177, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.S. Effects of glucocorticoids on mood, memory, and the hippocampus. Ann. N. Y. Acad. Sci. 2009, 11, 41–55. [Google Scholar] [CrossRef]
- Ruotsalainen, J.H.; Verbeek, J.H.; Mariné, A.; Consol, S. Preventing occupational stress in healthcare workers. Cochrane Database Syst. Rev. 2015, 2015, CD002892. [Google Scholar] [CrossRef]
- Boudarene, M.; Legros, J.J.; Timsit-Berthier, M. Study of the stress response: Role of anxiety, cortisol and DHEAs. Encephale 2002, 28, 139–146. [Google Scholar]
- Busireddy, K.R.; Miller, J.A.; Ellison, K.; Ren, V.; Qayyum, R.; Panda, M. Efficacy of interventions to reduce resident physician burnout: A systematic review. J. Grad. Med. Educ. 2017, 9, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Weibel, L.; Gabrion, I.; Aussedat, M.; Kreutz, G. Work-related stress in an emergency medical dispatch center. Ann. Emerg. Med. 2003, 41, 500–506. [Google Scholar] [CrossRef]
- Kripke, D.F.; Garfinkel, L.; Wingard, D.; Klauber, M.R.; Marter, M.R. Mortality associated with sleep duration and insomnia. Arch. Gen. Psychiatry 2002, 59, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupfer, D.J.; Reynolds, C.F. Management of insomnia. N. Engl. J. Med. 1997, 336, 341–345. [Google Scholar] [CrossRef]
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H.; Reidel, B.W.; Bush, A.J. Epidemiology of insomnia, depression, and anxiety. Sleep 2005, 28, 1457–1464. [Google Scholar] [CrossRef]
- Fletcher, K.E.; Davis, S.Q.; Underwood, W.; Mangrulkar, R.S.; McMahon, L.F., Jr.; Saint, S. Systemic review: Effects of resident work hours on patient safety. Ann. Intern. Med. 2004, 141, 851–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miró, E.; Martínez, P.; Arriaza, R. Influencia de la cantidad y calidad subjetiva de sueño en la ansiedad y el estado de ánimo deprimido. Salud Ment. 2006, 29, 30–37. [Google Scholar]
- Víctor, M.; Ropper, A. Principles of Neurology, Principios de Neurología; McGraw-Hill: Mexico, Mexico, 2002. [Google Scholar]
- Quintero, M.A.; Pérez, E.; Correa, S. La relación entre la autoeficacia y la ansiedad ante las ciencias en estudiantes del nivel medio superior. Rev. Int. Cienc. Soc. Humanid. SOCIOTAM 2009, 19, 69–91. [Google Scholar]
- Bandura, A. Self-Efficacy: The Exercise of Control; N.H. Freeman: New York, NY, USA, 1997. [Google Scholar]
- Contreras, F.; Espinosa, J.C.; Esguerra, G.; Haikal, A.; Polanía, A.; Rodríguez, A. Autoeficacia, ansiedad y rendimiento académico. Perspectivas en Psicología. Diversitas 2005, 2, 184–194. [Google Scholar]
- Gerin, W.; Litt, M.; Deich, J.; Pickering, T. Self-efficacy as a moderator of perceived control effects on cardiovascular reactivity: Is enhanced control always beneficial? Psychosom. Med. 1995, 57, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, P.M.; Bargh, J.A. The Psychology of Action: Linking Cognition and Motivation to Behaviour; The Guilford Press: New York, NY, USA, 1996. [Google Scholar]
- Cardoso, S.; Santos, O.; Nunes, C.; Loureiro, I. Association between general self-efficacy and physical activity among adolescents. BMC Health Serv. Res. 2016, 16, 106. [Google Scholar]
- Nater, U.M.; Rohleder, N.; Schlotz, W.; Ehlert, U.; Kirschbaum, C. Determinants of the diurnal course of salivary alphaamylase. Psychoneuroendocrinology 2007, 32, 392–401. [Google Scholar] [CrossRef]
- Pérez-Valdecantos, D.; Caballero-García, A.; Del Castillo-Sanz, T.; Bello, H.J.; Roche, E.; Córdova, A. Stress salivary biomarkers variation during the work day in emergencies in health professionals. Int. J. Environ. Res. Public. Health 2021, 18, 3937. [Google Scholar] [CrossRef]
- Baessler, J.; Schwarcer, R. Evaluación de la autoeficacia: Adaptación española de la escala de Autoeficacia General. Ansiedad Estrés 1996, 2, 1–8. [Google Scholar]
- Bobes, J.; González, M.P.; Sáiz, P.A.; Bascarán, M.T.; Iglesias, C.; Fernández, J.M. Propiedades psicométricas del cuestionario Oviedo de sueño. Psicothema 2000, 12, 107–112. [Google Scholar]
- Rohleder, N.; Nater, U.M.; Wolf, J.M.; Ehlert, U.; Kirschbaum, C. Psychosocial stress induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Ann. N. Y. Acad. Sci. 2004, 1032, 258–263. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Valentin, B.; Grottke, O.; Skorning, M.; Bergrath, S.; Fischermann, H.; Rörtgen, D.; Mennig, M.-T.; Fitzner, C.; Müller, M.P.; Kirschbaum, C.; et al. Cortisol and alpha-amylase as stress response indicators during pre-hospital emergency medicine training with repetitive high-fidelity simulation and scenarios with standardized patients. Scand. J. Trauma Resusc. Emerg. Med. 2015, 8, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.P.; Hansel, M.; Fichtner, A.; Hardt, F.; Weber, S.; Kirschbaum, C.; Rüder, S.; Walcher, F.; Koch, T.; Eich, C. Excellence in performance and stress reduction during two different full scale simulator training courses: A pilot study. Resuscitation 2009, 80, 919–924. [Google Scholar] [CrossRef]
- Adam, E.K.; Till Hoyt, L.; Granger, D.A. Diurnal alpha amylase patterns in adolescents: Associations with puberty and momentary mood states. Biol. Psychol. 2011, 88, 170–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoma, V.; Kirschbaum, C.; Wolf, J.; Rohleder, N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol. Psychol. 2012, 91, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; Fisher, S., Reason, J., Eds.; Wiley & Sons: New York, NY, USA, 1988; pp. 631–651. [Google Scholar]
- Kelsey, R.M.; Blascovich, J.; Tomaka, J.; Leitten, C.L.; Schneider, T.R.; Wiens, S. Cardiovascular reactivity and adaptation to recurrent psychological stress: Effects of prior task exposure. Psychophysiology 1999, 36, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Ellershaw, J.; Fullarton, C.; Rodwell, J.; Mcwilliams, J. Conscientiousness, openness to experience and extraversion as predictors of nursing work performance: A facet-level analysis. J. Nurs. Manag. 2016, 24, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Usta, M.B.; Tuncel, O.K.; Akbas, S.; Aydin, B.; Say, G.N. Decreased dehydroepiandrosterone sulphate levels in adolescents with post-traumatic stress disorder after single sexual trauma. Nord. J. Psychiatry 2016, 70, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Bicanic, I.A.; Postma, R.M.; Sinnema, G.; De Roos, C.; Olff, M.; Van Wesel, F.; Van de Putte, E.M. Salivary cortisol and dehydroepiandrosterone sulfate in adolescent rape victims with post traumatic stress disorder. Psychoneuroendocrinology 2013, 38, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Trickett, P.K.; Noll, J.G.; Susman, E.J.; Shenk, C.E.; Putnam, F.W. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psychopathol. 2010, 22, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viljoen, M.; Benecke, R.M.; Martin, L.; Adams, R.C.M.; Seedat, S.; Smith, C. Anxiety: An overlooked confounder in the characterisation of chronic stress-related conditions? PLoS ONE 2020, 15, e0230053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2000, 30, 65–91. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.A.; Southwick, S.; Hazlett, G.; Rasmusson, A.; Hoyt, G.; Zimolo, Z.; Charney, D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch. Gen. Psychiatry 2004, 61, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, C. Positive correlation between anxiety severity and plasma levels of dehydroepiandrosterone sulfate in medication free patients experiencing a major episode of depression. Psychiatry Clin. Neurosci. 2006, 60, 746–750. [Google Scholar] [CrossRef]
- Dubrovsky, B.O. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 169–192. [Google Scholar] [CrossRef]
- Castaño, M.B.; Gelaber, M.; Terol-Cantero, M.C. La autoeficacia y su relación con la salud psicosocial ocupacional en médicos de urgencias hospitalarias. UCMalue Rev. Acad. 2017, 9, 75–91. [Google Scholar]
- Lozano, J.L.; Seva, A.M.; Díaz, J.L.; Gutiérrez, L.L.; Leal, C. Burnout, habilidades de comunicación y autoeficacia en los profesionales de urgencias y cuidados críticos. Enferm. Glob. 2020, 19, 59. [Google Scholar] [CrossRef]
- Cañadas-de la Fuente, G.A.; Albendín-García, L.; Cañadas, R.G.; San Luis-Costas, C.; Ortega-Campos, E.; de la Fuente-Solana, E.I. Nurse burnout in critical care units and emergency departments: Intensity and associated factors. Emergencias 2018, 30, 328–331. [Google Scholar]
- Mo, Y.; Deng, L.; Zhang, L.; Lang, Q.; Pang, H.; Liao, C.; Wang, N.; Tao, P.; Huang, H. Anxiety of nurses to support Wuhan in fighting against COVID-19 epidemic and its correlation with work stress and social support. J. Clin. Nurs. 2021, 30, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Yu, P.; Chang, S.Y. Longitudinal relationships between two self-efficacy types and stress in active older adults in Taichung city, Taiwan. Int. J. Ment. Health Promot. 2016, 18, 95–105. [Google Scholar] [CrossRef]
- Pérez-Fuentes, M.C.; Molero-Jurado, M.M.; Gázquez, J.J.; Barragán, A.B.; Simón, M.M.; Martos, A.; Tortosa, B.M.; González, A.; Oropesa, N.F. Engagement y autoeficacia en profesionales de medicina. Calid. Vida Salud 2019, 12, 16–28. [Google Scholar]
- Holland, B.; Gosselin, K.; Mulcahy, A. The effect of autogenic training on self-efficacy, anxiety, and performance on nursing student simulation. Nurs. Educ. Perspect. 2017, 38, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Molero-Jurado, M.; Pérez-Fuentes, M.; Oropesa-Ruiz, N.F.; Simón-Márquez, M.; Gázquez-Linares, J.J. Self-efficacy and emotional intelligence as predictors of perceived stress in nursing professionals. Medicina 2019, 55, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villamarín, F. Papel de la auto-eficacia en los trastornos de ansiedad y depresión. Análisis Modif. Conducta 1990, 16, 55–79. [Google Scholar]
- Del Río, I.Y. Estrés y sueño. Rev. Mex. Neurocienc. 2006, 7, 15–20. [Google Scholar]
- Selye, H.; Fortier, C. Adaptive reactions to stress. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1949, 29, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, V.S.; Arshavsky, V.V. REM sleep, stress and search activity. A short critical review and a new conception. Waking Sleep. 1979, 3, 235–244. [Google Scholar]
- Fried, Y.; Levi, A.; Ben-David, H.; Tiegs, R. Rater positive and negative mood predispositions as predictors of performance ratings of rates in simulates and real organizational settings: Evidence from US and Israeli samples. J. Occup. Org. Psychol. 2000, 73, 373–378. [Google Scholar] [CrossRef]
- Gruber, G.; Saletu, B.; Klösch, G.; Frey, R.; Anderer, P. Insomnia based on generalized anxiety disorder: A comparison of self-reported sleep-quality and polysomnographic data. J. Sleep Res. 1996, 5, 158. [Google Scholar]
- Karasek, R.A., Jr. Job demands, job decision latitude, and mental strain: Implications for job redesign. Adm. Sci. Q. 1979, 24, 285–308. [Google Scholar] [CrossRef]
- Johnson, J.V.; Hall, E.M. Job strain, work place social support, and cardiovascular disease: A cross-sectional study of a random sample of the Swedish working population. Am. J. Publ. Health 1988, 78, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, A.; Iwata, N.; Watanabe, N.; De Jonge, J.; Pikhart, H.; Fernández-López, J.A.; Xu, L.; Peter, P.; Knutsson, A.; Niedhammer, I.; et al. Application of item response theory to achieve cross-cultural comparability of occupational stress measurement. Int. J. Methods Psychiatr. Res. 2009, 18, 58–67. [Google Scholar] [CrossRef]
- Steiler, D.; Rosnet, E. La mesure du stress professionnel. Différentes méthodologies de recueil. La Rev. Sci. Gest. 2011, 251, 71–79. [Google Scholar] [CrossRef]

| Professionals | n | Age |
|---|---|---|
| Total | 97 | 39.5 ± 12.1 (women) 39.9 ± 15.2 (men) |
| Nurses | 59 | 39.0 ± 13.2 |
| Medical Doctors | 38 | 39.6 ± 13.5 |
| HCUV | 45 | 34.7 ± 9.7 |
| HSBS | 52 | 42.4 ± 12.5 |
| STAI State | STAI Trait | |
|---|---|---|
| Women | 25.05 ± 1.15 | 24.69 ± 1.23 |
| Men | 25.67 ± 1.77 | 24.31 ± 1.93 |
| Nurses | 25.61 ± 0.92 | 24.94 ± 1.18 |
| Medical Doctors | 24.53 ± 2.03 | 24.27 ± 1.89 |
| HCUV | 25.04 ± 1.28 | 24.89 ± 1.33 |
| HSBS | 24.66 ± 1.68 | 24.02 ± 1.98 |
| TOTAL | 25.18 ± 0.99 | 24.73 ± 1.07 |
| Self-Efficacy Scores | |
|---|---|
| Women | 29.07 ± 0.99 |
| Men | 30.02 ± 2.03 |
| Nurses | 29.12 ± 0.92 |
| Medical Doctors | 29.54 ± 1.79 |
| HCUV | 30.22 ± 0.85 |
| HSBS | 28.26 ± 1.66 |
| TOTAL | 29.30 ± 1.01 |
| Subjetive Satisfaction of Sleep | Insomnia | Hypersomnia | |
|---|---|---|---|
| Women | 4.13 ± 1.36 | 19.95 ± 7.23 | 5.68 ± 2.66 |
| Men | 4.15 ± 1.90 | 17.33 ± 8.09 | 5.74 ± 3.60 |
| Nurses | 4.16 ± 1.47 | 19.47 ± 7.56 | 5.46 ± 2.78 |
| Medical Doctors | 3.98 ± 1.65 | 18.83 ± 7.99 | 5.97 ± 3.17 |
| HCUV | 4.22 ± 1.33 | 19.27 ± 7.04 | 5.93 ± 2.55 |
| HSBS | 4.18 ± 1.60 | 19.55 ± 7.76 | 5.69 ± 3.13 |
| TOTAL | 4.10 ± 1.54 | 19.13 ± 7.69 | 5.64 ± 2.55 |
| Shift (Morning vs. Afternoon) | Hospital (HSB vs. HCUV) | Category (Phycisian vs. Nurses) | |
|---|---|---|---|
| DHEA 8 h | >0.05 | >0.05 | >0.05 |
| DHEA 12 h | >0.05 | >0.05 | >0.05 |
| DHEA 15 h | 0.04575 | 0.005375 | >0.05 |
| DHEA 24 h | 0.03665 | 0.03187 | >0.05 |
| α-Amylase 8 h | >0.05 | 0.004259 | >0.05 |
| α-Amylase 12 h | >0.05 | 0.0295 | 0.05578 |
| α-Amylase 15 h | 0.01023 | >0.05 | 0.003534 |
| α-Amylase 24 h | 0.03567 | >0.05 | >0.05 |
| STAI State | 0.02403 | >0.05 | >0.05 |
| STAI Trait | >0.05 | >0.05 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Valdecantos, D.; Caballero-García, A.; del Castillo-Sanz, T.; Bello, H.J.; Roche, E.; Roche, A.; Córdova, A. Variations in Salivary Stress Biomarkers and Their Relationship with Anxiety, Self-Efficacy and Sleeping Quality in Emergency Health Care Professionals. Int. J. Environ. Res. Public Health 2021, 18, 9277. https://doi.org/10.3390/ijerph18179277
Pérez-Valdecantos D, Caballero-García A, del Castillo-Sanz T, Bello HJ, Roche E, Roche A, Córdova A. Variations in Salivary Stress Biomarkers and Their Relationship with Anxiety, Self-Efficacy and Sleeping Quality in Emergency Health Care Professionals. International Journal of Environmental Research and Public Health. 2021; 18(17):9277. https://doi.org/10.3390/ijerph18179277
Chicago/Turabian StylePérez-Valdecantos, Daniel, Alberto Caballero-García, Teodosia del Castillo-Sanz, Hugo J. Bello, Enrique Roche, Alba Roche, and Alfredo Córdova. 2021. "Variations in Salivary Stress Biomarkers and Their Relationship with Anxiety, Self-Efficacy and Sleeping Quality in Emergency Health Care Professionals" International Journal of Environmental Research and Public Health 18, no. 17: 9277. https://doi.org/10.3390/ijerph18179277
APA StylePérez-Valdecantos, D., Caballero-García, A., del Castillo-Sanz, T., Bello, H. J., Roche, E., Roche, A., & Córdova, A. (2021). Variations in Salivary Stress Biomarkers and Their Relationship with Anxiety, Self-Efficacy and Sleeping Quality in Emergency Health Care Professionals. International Journal of Environmental Research and Public Health, 18(17), 9277. https://doi.org/10.3390/ijerph18179277









