Health Related Quality of Life Measurements for Diabetes: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
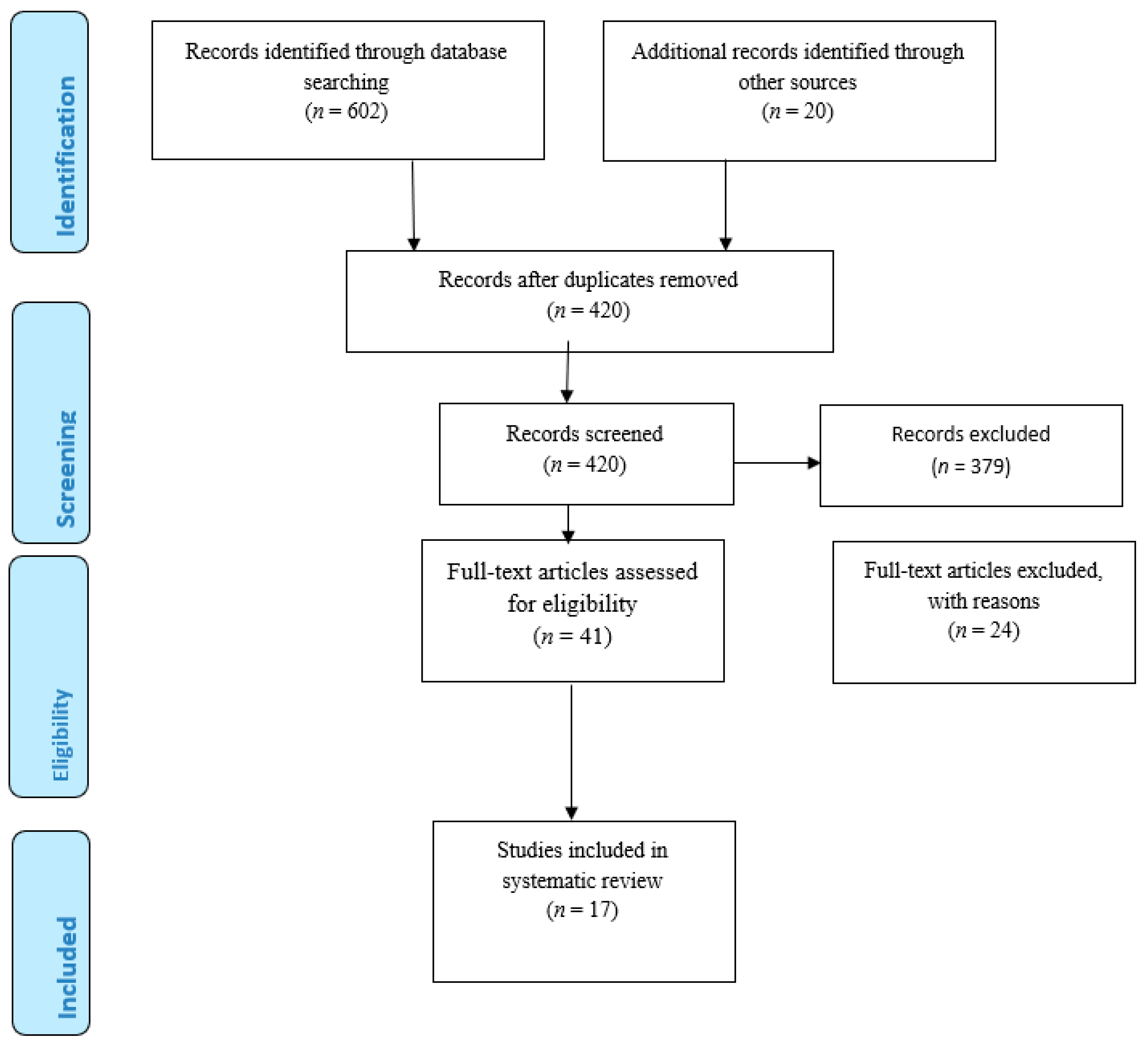
3. Eligibility Criteria
4. Results
5. Various Quality of Life Measurements for Diabetes
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moons, P.; Budts, W.; De-Geest, S. Critique on the conceptualization of quality of life: A review and evaluation of different conceptual approaches. Int. J. Nurs. Stud. 2006, 43, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.R.; Peyrot, M. Quality of life and diabetes. Diabet. Metab. Res. Rev. 1999, 5, 205–218. [Google Scholar] [CrossRef]
- Fayers, P.M.; Machin, D. Quality of Life: The Assessment, Analysis and Interpretation of Patient-Reported Outcomes, 2nd ed.; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Guyatt, G.H.; Feeny, D.H.; Patrick, D.L. Measuring health related quality of life. Ann. Intern. Med. 1993, 118, 622–629. [Google Scholar] [CrossRef]
- Holtslag, R.H.; Beeck, V.F.E.; Lichtveld, A.R.; Van Der Werken, C. Individual and population burdens of major trauma in the Netherlands. Bull. World Health Organ. 2008, 86, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Al-Aboudi, I.S.; Hassali, M.A.; Shafie, A.A.; Hassan, A.; Alrasheedy, A.A. A cross-sectional assessment of health-related quality of life among type 2 diabetes patients in Riyadh, Saudi Arabia. SAGE Open Med. 2015, 3, 2050312115610129. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.T. The outcomes movement and health status measures. J. Allied Health 1995, 24, 13–28. [Google Scholar]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Nelson, D.E.; Engelgau, M.M.; Vinicor, F.; Marks, S. Diabetes trends in the U.S. 1990–1998. Diabetes Care 2000, 23, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Dasbach, E.J.; Klein, R.; Klein, B.E.; Moss, S.E. Selfrated health and mortality in people with diabetes. Am. J. Public Health 1994, 84, 1775–1779. [Google Scholar] [CrossRef]
- Glasgow, R.E. Compliance to diabetes regimens: Conceptualization, complexity, and determinants. In Patient Compliance in Medical Practice and Clinical Trials; Cramer, J.A., Spilker, B., Eds.; Raven Press: New York, NY, USA, 1991; pp. 209–224. [Google Scholar]
- Cox, D.J.; Gonder-Frederick, L. Major developments in behavioral diabetes research. J. Consult. Clin. Psychol. 1992, 60, 628–638. [Google Scholar] [CrossRef]
- Goodall, T.A.; Halford, W.K. Self-Management of diabetes mellitus: A critical review. Health Psychol. 1991, 10, 1–8. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Bujang, M.A.; Adnan, T.H.; Hatta, N.K.B.H.; Ismail, M.; Lim, C.J. A revised version of diabetes quality of life instrument maintaining domains for satisfaction, impact, and worry. J. Diabetes Res. 2018, 2018, 5804687. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.G.K.; Rusli, B.B.; Khalid, B.A.K. Development and validation of the Asian diabetes quality of life (AsianDQOL) questionnaire. Diabetes Res. Clin. Pract. 2015, 108, 489–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bujang, M.A.; Ismail, M.; Hatta, N.K.B.H.; Othman, S.H.; Baharum, N.; Lazim, S.S.M. Validation of the Malay version of Diabetes Quality of Life (DQOL) Questionnaire for Adult Population with Type 2 Diabetes Mellitus. Malays. J. Med. Sci. 2017, 24, 86–96. [Google Scholar] [CrossRef]
- Burroughs, T.E.; Desikan, R.; Waterman, B.M.; Gilin, D.; McGill, J. Development and Validation of the Diabetes Quality of Life Brief Clinical Inventory. Diabetes Spectr. 2004, 17, 41–49. [Google Scholar] [CrossRef]
- Bradle, C.; Todd, C.; Gorton, T.; Symonds, E.; Martin, A.; Plowright, R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: The ADDQoL. Qual. Life Res. 1999, 8, 79–91. [Google Scholar] [CrossRef]
- Jacobson, A.M.; de-Groot, M.; Samson, J.A. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994, 17, 267–274. [Google Scholar] [CrossRef]
- Fitzgerald, J.T.; Davis, W.K.; Connell, C.M.; Hess, G.E.; Funnell, M.M.; Hiss, R.G. Development and Validation of the Diabetes Care Profile. Eval. Health Prof. 1996, 19, 208–230. [Google Scholar] [CrossRef]
- Hammond, G.S.; Aoki, T.T. Measurement of health status in diabetic patients: Diabetes impact measurement scales. Diabetes Care 1992, 15, 469–477. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Anderson, B.J.; Lohrer, P.A.; Welch, G.; Jacobson, A.M.; Aponte, J.E.; Schwartz, C.E. Assessment of diabetes-related distress. Diabetes Care 1995, 18, 754–760. [Google Scholar] [CrossRef]
- Carey, M.P.; Jorgensen, R.S.; Weinstock, R.S.; Sprafkin, R.P.; Lantinga, L.J.; Carnrike, C.L.M., Jr.; Baker, M.; Meisler, A.W. Reliability and validity of the Appraisal of Diabetes Scale. J. Behav. Med. 1991, 14, 43–50. [Google Scholar] [CrossRef]
- Jin, X.; Liu, G.G.; Gerstein, H.C.; Levine, M.A.H.; Steeves, K.; Guan, H.; Li, H.; Xie, F. Item reduction and validation of the Chinese version of diabetes quality-of-life measure (DQOL). Health Qual. Life Outcomes 2018, 16, 78. [Google Scholar] [CrossRef]
- Hearnshaw, H.; Wright, K.; Dale, J.; Sturt, J.; Vermeire, E.; Van Royen, O. Development and validation of the diabetes obstacles questionnaire (DOQ) to assess obstacles in living with Type 2 diabetes. Diabet. Med. 2007, 24, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Meadows, S.N.; McCol, E.; Eccles, M.; Shiels, C.; Hewison, J.; Hutchinson, A. The diabetes health profile (DHP): A new instrument for assessing the psychosocial profile of insulin requiring patients: Development and psychometric evaluation. Qual. Life Res. 1996, 5, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Alavi, N.M.; Ghofranipour, F.; Ahmadi, F.; Emami, A. Developing a culturally valid and reliable quality of life questionnaire for diabetes mellitus. East. Mediterr. Health J. 2007, 13, 177–185. [Google Scholar]
- Bott, U.; Mu-Hlhauser, I.; Overmann, H.; Berger, M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care. 1998, 21, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Izumo, Y.; Inoue, J.; Takahashi, R.; Takanashi, K.; Teshima, T.; Yatomi, N.; Shimizu, Y.; Ito, H. Development of elderly diabetes impact scales (EDIS) in elderly patients with diabetes mellitus. Nippon Ronen Igakkai Zasshi 1995, 32, 786–796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cox, W.M.; Blount, J.P.; Crowe, P.A.; Singh, S.P. Diabetic patients’ alcohol use and quality of life: Relationships with prescribed treatment compliance among older males. Alcohol. Clin. Exp. Res. 1996, 20, 327–331. [Google Scholar] [CrossRef]
- Jacobson, A.M. Quality of life in patients with diabetes mellitus. Semin. Clin. Neuropsychiatry 1997, 2, 82–93. [Google Scholar]
- Jacobson, A.; Barofsky, M.; Cleary, I.P.; Rand, L.L. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care 1988, 11, 725–732. [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. Influence of intensive diabetes treatment on quality of-life outcomes in the diabetes control and complications trial. Diabetes Care. 1996, 19, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Maskari, M.Y.; Al-Shookri, A.O.; Al-Adawi, S.H.; Lin, G.K. Assessment of quality of life in patients with type 2 diabetes mellitus in Oman. Saudi Med. J. 2011, 3, 1285–1290. [Google Scholar]
- Mohammadi, S.; Karim, N.A.; Talib, A.R.; Amani, R. Evaluation of quality of life among type 2 diabetes patients. Int. J. Community Med. Public Health 2015, 3, 51. [Google Scholar] [CrossRef][Green Version]
- Vileikyte, L.; Peyrot, M.; Bundy, C.; Rubin, R.R.; Leventhal, H.; Mora, P.; Shaw, J.E.; Baker, P.; Boulton, A.J.M. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care 2003, 26, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Hisasue, S.; Kumamoto, Y.; Sato, Y.; Masumori, N. Prevalence of female sexual dysfunction symptoms and its relationship to quality of life: A Japanese female cohort study. Urology 2005, 65, 143–148. [Google Scholar] [CrossRef]
- Fox, J. Designing research: Basics of survey construction. Minim. Invasive Surg. Nurs. 1994, 8, 77–79. [Google Scholar]
- Kotsanos, J.G.; Vignati, L.; Huster, W.; Andrejasich, C.; Boggs, M.B.; Jacobson, A.M.; Morrero, D.; Mathias, S.D.; Patrick, D.; Zalani, S.; et al. Health-Related quality-of-life results from multinational clinical trials of insulin lispro. Assessing benefits of a new diabetes therapy. Diabetes Care. 1997, 20, 948–958. [Google Scholar] [CrossRef]
- Shen, W.; Kotsanos, J.G.; Huster, W.J.; Mathias, S.D.; Andrejasich, C.M.; Patrick, D.L. Development and validation of the diabetes quality of life clinical trial questionnaire. Med. Care 1999, 37, AS45–AS66. [Google Scholar] [CrossRef]
- Welch, G.; Jacobson, A.M.; Polonsky, W.H. The Problem Areas in Diabetes (PAID) scale. An evaluation of its utility. Diabetes Care 1997, 20, 760–766. [Google Scholar] [CrossRef]
- Fitzpatrick, R.; Davey, C.; Buxton, M.J.; Jones, D.R. Evaluating patient based outcome measures for use in clinical trials. Health Technol. Assess. 1998, 2, 14. [Google Scholar] [CrossRef]
- Bott, U.; Ebrahim, S.; Hirschberger, S.; Scovlund, S.E. Effect of rapid-acting insulin analogue insulin aspart on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabet. Med. 2003, 20, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.T.; Gruppen, L.D.; Anderson, R.M.; Funnell, M.M.; Jacober, S.J.; Grunberger, G.; Aman, L.C. The influence of treatment modality and ethnicity on attitudes in Type 2 diabetes. Diabetes Care 2000, 23, 313–318. [Google Scholar] [CrossRef]
- Cunningham, V.; Mohler, M.J.; Wendel, C.S.; Hoffman, R.M.; Murata, M.G.H.; Shah, J.H.; Duckworth, W.C. Reliability and validity of the DCP among Hispanic veterans. Eval. Health Prof. 2005, 28, 447–463. [Google Scholar] [CrossRef]
- Trief, P.M.; Grant, W.; Elbert, K.; Weinstock, R.S. Family environment, glycemic control, and the psychosocial adaptation of adults with diabetes. Diabetes Care 1998, 21, 241–245. [Google Scholar] [CrossRef]
- Trief, P.M.; Aquilino, C.; Paradies, K.; Weinstock, R.S. Impact of the work environment on glycemic control and adaptation to diabetes. Diabetes Care 1999, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Ito, H. Development of elderly diabetes burden scale for elderly patients with diabetes mellitus. Geriatr. Gerontol. Int. 2003, 3, 212–222. [Google Scholar] [CrossRef]
- Garratt, A.M.; Schmidt, L.; Fitzpatrick, R. Patient-assessed health outcome measures for diabetes: A structured review. Diabet. Med. 2002, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watkins, K.; Connell, C.M. Measurement of health-related QOL in diabetes mellitus. Pharmacoeconomics 2004, 22, 1109–1126. [Google Scholar] [CrossRef] [PubMed]
- Achhab, Y.E.L.; Nejjari, C.; Chikri, M.; Lyoussi, B. Disease-Specific health-related quality of life instruments among adults diabetic: A systematic review. Diabetes Res. Clin. Pract. 2008, 80, 171–184. [Google Scholar] [CrossRef]
| Title/Author/Year of Publication | Country | Name of Instrument Used | Domains of HRQOL Used | Strength and Weakness |
|---|---|---|---|---|
| The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: The ADDQOL. Bradley et al. (1999) | United Kingdom | Audit of Diabetes-Dependent QOL measure (ADDQOL) | It has 13 Domains: Employment/Career Opportunities, Social Life, Family Relationships, Friendships, Sex, Life, Sporting, Holiday or Leisure Opportunities; The Ease with which I can Travel; Worries about my Future; Worries about the Future of my Family and Close Friends; Motivation to Achieve Things; Things I could do Physically and the Extent to which People would Fuss too much about Me | Diabetes-specific ADDQOL will be more sensitive to change and responsive to subgroup differences than a generic instrument such as the SF-36. |
| Reliability and validity of the appraisal of diabetes scale, Carey et al. (1991) | United States of America | Appraisal of diabetes scale (ADS) | Not mentioned | The ADS is easy to score and interpret and can be administered by nonprofessional support staff. The questionnaire can be completed within five minutes. The ADS could prove useful as a brief screening instrument for diabetes adjustment. The instrument can be administered to diabetic patients to identify patients that are experiencing or at risk of dysphoric reactions and noncompliance issues. |
| Development and validation of the Asian Diabetes Quality of Life Questionnaire Goh, Rusli, and Khalid (2015). | Malaysia | Asian Diabetes Quality of Life (AsianDQOL) | Not mentioned | The total score for AsianDQOL is unique to the respective language. To review the instruments used to assess the impact of PPC interventions. The AsianDQOL is more suitable for use in Malaysian population compared to DQOL, DQLCTQ-R and DSQOLS because it is disease specific and was constructed based on the Malaysian. The AsianDQOL is a valid, reliable, and stable tool for assessing QOL in multiethnic and multi-lingual NIDDM Asian populations |
| Item reduction and validation of the Chinese version of diabetes quality-of-life measure, Jin et al. (2018). | China | Chinese short versions DQOL | Four domains: Satisfaction level of “the amount of time it takes to manage your diabetes”; “the amount of time you spend getting a checkup”; “the time it takes to determine your sugar level”; “your current treatment” | Chinese DQOL was the preferred short version because it imposes a lower burden on patients without compromising the psychometric properties of the instrument. Training sample contained community-based patients, and most of them were not using insulin. This sample was relatively healthier than the diabetic population, who had more comorbidities, was inpatient, or using insulin; thus, the results cannot necessarily be generalized to the entire diabetic patient population. |
| Development and Validation of the Diabetes Care Fitzgerald et al. (1996). | United States of America | Diabetes Care Profile (DCP) | The instrument comprises of six subscales of the DCP measure diabetes-specific QOL domains comprising of Personal, Social, Emotional Functioning, and Perceptions of Control. | Using the DCP scale, results of worse QOL are associated with higher glycaemic levels, use of insulin or tablets, and if the patient is having larger number of complications due to diabetes. It takes 30 to 40 min to be complete the questionnaire. |
| The diabetes health profile (DHP): a new instrument for assessing the psychosocial profile of insulin requiring patients: development and psychometric evaluation, Meadows et al. (1996) | United Kingdom | Diabetes Health Profile (DHP) | Three subscales: The three factors were interpreted as Psychological Distress, Barriers to Activity and Disinhibited Eating. | DHP appears to be a reliable and valid instrument suitable for further development and application in a clinical and research context. |
| Measurement of Health Status in diabetic patients: Diabetes Impact Measurement Scales, Diabet. Care. Hammond and Aoki (1992) | United States of America | Diabetes Impact Measurement Scales (DIMS) | The items were grouped into four subscales: General Well-Being, Physical Symptoms, Social Functioning, and Diabetes-related Morale. | The Diabetes Impact Management Scales is an easily administered questionnaire with internal consistency and test–retest reliability. The questionnaire is simple and straightforward, comprising of items that are easily to understood; it covers a broad range of content relevant to diabetes impact. |
| Development and validation of the Diabetes Obstacles Questionnaire (DOQ) to assess obstacles in living with Type 2 diabetes Hearnshaw et al. in (2007). | United Kingdom | Diabetes Obstacles Questionnaire (DOQ) | DOQ, comprising of eight subscales covering Medication, Self-Monitoring, Knowledge and Beliefs, Diagnosis, Relationships with Health-Care Professionals, Lifestyle Changes, Coping, and Advice and Support. | DOQ covers a much wider and more detailed range of problems and obstacles than the Problem Areas in Diabetes (PAID). DOQ is a usable and valid instrument for both clinical and research settings. It helps to identify in detail the obstacles which an individual finds in living with NIDDM. |
| Development and validation of the diabetes quality of life clinical trial questionnaire Shen et al. (1999) | Multinational study: United States of America, Canada, Germany, and France | Diabetes Quality of Life Clinical Trial Questionnaire (DQLCTQ-R) | Energy/Fatigue; Health Distress; Mental Health; Satisfaction; Treatment Satisfaction; Treatment Flexibility; Frequency of Symptoms. | It is appropriate to use for IDDM and NIDDM patients. |
| Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial Jacobson, Barofsky, Cleary, and Rand, (1988). | United States of America | Diabetes Quality of Life (DQOL) | Four Domains: Life Satisfaction, Diabetes Impact, Social/Vocational Related Worries, and Diabetes-related Worries. | The Diabetes Control and Complications Trial (DQOL) questionnaire has 46 items developed for IDDM diabetes as part of the DQOL. It is particularly relevant for the worry scales, because they were developed especially for use in younger patient samples. DQOL in its full form is too lengthy to be completed as part of a provider’s routine office visit. |
| A revised version of diabetes quality of life instrument maintaining domains for satisfaction, impact, and worry. Bujang et al. (2018) | Malaysia | Diabetes Quality of Life Revised version DQOL-R | “satisfaction” domain has six items, impact domain has four items, and “worry” domain has three items. | It has lesser items, only 13 items, and, hence, less time is needed to complete the questionnaire. |
| Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes, Bott et al. (1998) | Germany | Diabetes-specific Quality of life Scale (DSQOLS) | The scale has six domains: Social Relations, Leisure Tile Flexibility, Physical Complaints, Worries Regarding the Future, Diet Restrictions and Daily Hassles. | To complete the questionnaire takes less than 20 min. |
| Development and Validation of the Diabetes Quality of Life Brief Clinical Inventory. Burroughs et al. (2004) | United States of America | DQOL Brief Clinical Inventory | Four domains: Satisfaction with Treatment, Impact of Treatment, Worry about the Future Effects of Diabetes, and Worry about Social/Vocational Issues. | The 15-item DQOL Brief Clinical Inventory provides a total health-related quality of life score that predicts self-reported diabetes care behaviors and satisfaction with diabetes control as effectively as the full version of the instrument. In addition, it provides a vehicle for quickly screening patients for readiness and specific treatment-related concerns. It takes about 10 min to administer and can be used to identify quality of life issues that might not arise during the typical patient provider encounter. |
| Development of elderly diabetes impact scales (EDIS) in elderly patients with diabetes mellitus, Araki et al. (1995) | Japan | Elderly Diabetes Burden Scale (EDBS) | The EDBS has six subscales which include Worry about Diabetes, Symptom Burden, Treatment Dissatisfaction, Burden by Tablets or Insulin, Dietary Restrictions, and Social Burden | The EDBS is useful in evaluating the quality of life in elderly patients with diabetes mellitus. |
| Developing a culturally valid and reliable quality of life questionnaire for diabetes mellitus. Alavi, Ghofranipour, Ahmadi, and Emami, (2007). | Iran | Iranian Diabetes Quality of Life (IRDQOL) | Not mentioned | The questionnaire successfully distinguished the lower QOL of patients suffering from pain in the limbs, loss of appetite, fatigue, constipation, and itching. The questionnaire could determine both general and health-related QOL for IDDM patients. |
| Validation of the Malay version of Diabetes Quality of Life (DQOL) Questionnaire for Adult Population with Type 2 Diabetes Mellitus., Bujang, et al. (2017) | Malaysia | Malay version of Diabetes Quality of Life (DQOL) | Three domains, namely Satisfaction Domain, Impact Domain, and Worry Domain. | The Malay version of diabetes quality of life (DQOL) questionnaire was found to be a valid and reliable survey instrument to be used for Malaysian adult patients with diabetes mellitus. |
| Assessment of diabetes-related distress, Polonsky et al. (1995) | United States of America | Problem Areas in Diabetes Scale (PAID) | Not mentioned | The PAID is a brief and easy to administer instrument, which may serve as a clinical tool useful in the identification of patients who are experiencing high levels of diabetes-related distress. |
| Instrument | Reliability | Validity | Responsiveness | |
|---|---|---|---|---|
| Cronbach’s α | Test– Retest | Scale Analyses | ||
| Audit of Diabetes-Dependent QOL measure (ADDQOL) | 0.85–0.92 | - | Factor analysis: All items loading >0.40 on one factor, all items loading >0.50 on one factor; item–total correlations: 0.37–0.67 | - |
| Differences between groups Better QOL associated with: non-insulin treated patients; less frequent hypoglycemia; fewer disease complications; flexible dietary regimen | ||||
| Appraisal of diabetes scale (ADS) | 0.73 | 0.85–0.89 | Principal components analysis: single factor explaining 39% variance; item–remainder correlations 0.28–0.59 | - |
| Convergent validity Diabetes Health Belief Questionnaire-Revised r = 0.31–0.42; Diabetic Daily Hassles Scale r = 0.59; Perceived Stress Scale r = 0.39–0.58 | ||||
| Asian Diabetes Quality of Life (AsianDQOL) | 0.719–0.917 | 0.60 | Confirmatory factor analysis: GFI = 0.88 | - |
| Differences between groups The component of diet and eating habits were significant in both the English language and Chinese–Mandarin versions but were not in the Malay language | ||||
| Chinese short versions DQOL | 0.884 | - | Confirmatory factor analysis Standardized root mean squared residual 0.078, Comparative fit index 0.726 | - |
| 0.822 | ||||
| Diabetes Care Profile (DCP) | 0.60–0.95 | - | Confirmatory factor analysis: GFI = 0.92 | - |
| Convergent validity Social Provisions Scale: r = −0.34 to 0.32 CES-D: r = −0.53–0.48; Happiness and Satisfaction Scale: r = −0.27 to 0.32 | ||||
| Differences between groups Not using insulin was associated with less impact on personal/social life, fewer control problems, positive outlook; number of complications (among those taking insulin) was associated with more impact on social/personal life | ||||
| Diabetes Health Profile (DHP) | - | Factor analyses: 33–35%, 32%, 40–46% of total variance explained; scale inter correlations: 0.13–0.57; item correlations: 0.47–0.75; inter-item correlations: 0.30–0.70 | - | |
| 0.77–0.86 | External validity Coefficient of congruence: sex, 0.92–0.93; age, 0.93–0.99; language, 0.98–0.99 | |||
| 0.72–0.79 | Convergent validity Hospital Anxiety and Depression Scale, r = 0.28–0.62; SF-36 r = −0.21 to −0.68, 0.07–0.65 (DHP items reverse-coded) | |||
| 0.70–0.88 | Differences between groups Younger women were more likely to be affected with psychological distress and eating disturbance than men | |||
| Diabetes Impact Measurement Scales (DIMS) | 0.60–0.94 | - | Scale intercorrelations: 0.49–0.97; principal components analysis: single factor accounting for 32% variance | - |
| Convergent validity Patient-rated diabetes control r = 0.22–0.55; Clinician-rated diabetes control r = 0.24–0.35; patient-rated general wellness r = 0.27–0.47; clinician-rated general wellness r = 0.29–0.45 | ||||
| Diabetes Obstacles Questionnaire (DOQ) | 0.766 | - | variance explained ≥ 55%, | - |
| 0.813 | ||||
| 0.866 | ||||
| 0.834 | ||||
| 0.937 | Correlation coefficient 0.86–0.271 | |||
| 0.851 | ||||
| 0.776 | ||||
| 0.880 | ||||
| Diabetes Quality of Life Clinical Trial Questionnaire (DQLCTQ-R) | 0.77–0.89 | - | Differences between groups Perceived control of diabetes is associated with better QOL, among male IDDM patients | Four domains were responsive to clinical change in metabolic control |
| Diabetes Quality of Life (DQOL) | 0.67–0.88, | 0.78–0.92 | Scale intercorrelations: r = 0.26–0.68, 0.47–0.87 Test–retest: 0.78–0.92 Convergent/discriminatory validity Symptom Checklist Global Severity Index r = 0.40–0.77; Affect Balance Scale r = −0.25 to −0.67; Psychosocial Adjustment to Illness Scale r = 0.06–0.81; SF-36 r = −0.003 to 0.59 Differences between groups Adult males reported less diabetes impact, fewer worries than adult females; number of complications associated with less satisfaction had a greater impact; taking insulin associated with less satisfaction and a greater impact; not taking insulin associated with worry | - |
| Diabetes Quality of Life Revised version DQOL-R | 0.67–0.88 | 0.78–0.92 | Scale intercorrelations: r = 0.26–0.68, 0.47–0.87 | - |
| Convergent/discriminatory validity Symptom Checklist Global Severity Index r = 0.40–0.77; Affect Balance Scale r = −0.25 to −0.67; Psychosocial Adjustment to Illness Scale | ||||
| r = 0.06–0.81; SF-36 r = −0.003 to 0.59 | ||||
| Diabetes-specific Quality of life Scale (DSQOLS) | 0.70–0.93 | - | Goodness of fit index = 0.98; scale intercorrelations r = 0.28–0.66 Convergent validity Positive well-being scale r = 0.35–0.53 | - |
| Differences between groups Age r = _0.23–0.01; social status r = _0.04–0.24; better QOL associated with greater flexibility of insulin treatment, fewer complications and use of rapid-acting insulin | ||||
| DQOL Brief Clinical Inventory | 0.61–0.94 | - | Five significant principal components that accounted for 9.23–15.35% of the total item variance each and 56.73% of the total item variance collectively. | - |
| Convergent validity Treatment satisfaction, the six-item model r = 0.254–0.562, | ||||
| Differences between groups For worry about diabetes-related events, or for females for diabetes impact, no differences between the two groups | ||||
| Elderly Diabetes Burden Scale (EDBS) | 0.55–0.89 | 0.94–0.99 | Six-factor solution explaining 69.4% of variance | - |
| Convergent validity Philadelphia geriatric center morale scale r = _0.51; Geriatric depression scale r = 0.27–0.57 | ||||
| Differences between groups It was reported that higher scores were seen among women’s dietary restrictions, worry, and less satisfaction of treatment, also more adaptive feeling to diabetes when compared to men | ||||
| Iranian Diabetes Quality of Life (IRDQOL) | 0.98 | - | Concurrent validity 0.639 | - |
| Differences between groups Quality of life has been found to be higher in males than females [22,23,24]. It seems sex can be considered | ||||
| Malay version of Diabetes Quality of Life (DQOL) | 0.846–0.941 | - | Correlation coefficients for the three domains were between 0.228 and 0.451 | - |
| Differences between groups Retinopathy group had a sizeable effect (mean score of 2.0 compared to no retinopathy group versus 2.7 from retinopathy group) | ||||
| Problem Areas in Diabetes Scale (PAID) | 0.93–0.95 | r = 0.83 | Large single factor explaining 50–52% of variance; item–total correlations: r = 0.32 to 0. 84; all >0.30 | Effect sizes range from 0.32 to 0.65 for interventions |
| Convergent validity Global Severity Index of Brief Symptom Inventory r = 0.63; ATT39 r = −0.22 to −0.81; Diabetes Coping Measure-avoidance r = 0.05–0.59; Diabetes Coping Measure-passive resignation r = −0.01 to 0.70; Diabetes Coping Measure-tackling spirit r = −0.13 to −0.82; Well- Being Questionnaire r = −0.50 to −0.53; Hypoglycaemia Fear Survey (Worry) r = 0.53–0.57; State Trait Anxiety Inventory r = 0.61 | ||||
| Differences between groups IDDM reported more diabetes-related distress than NIDDM patients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluchi, S.E.; Manaf, R.A.; Ismail, S.; Kadir Shahar, H.; Mahmud, A.; Udeani, T.K. Health Related Quality of Life Measurements for Diabetes: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9245. https://doi.org/10.3390/ijerph18179245
Oluchi SE, Manaf RA, Ismail S, Kadir Shahar H, Mahmud A, Udeani TK. Health Related Quality of Life Measurements for Diabetes: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(17):9245. https://doi.org/10.3390/ijerph18179245
Chicago/Turabian StyleOluchi, Sampson Emilia, Rosliza Abdul Manaf, Suriani Ismail, Hayati Kadir Shahar, Aidalina Mahmud, and Theophilus Kachidelu Udeani. 2021. "Health Related Quality of Life Measurements for Diabetes: A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 17: 9245. https://doi.org/10.3390/ijerph18179245
APA StyleOluchi, S. E., Manaf, R. A., Ismail, S., Kadir Shahar, H., Mahmud, A., & Udeani, T. K. (2021). Health Related Quality of Life Measurements for Diabetes: A Systematic Review. International Journal of Environmental Research and Public Health, 18(17), 9245. https://doi.org/10.3390/ijerph18179245







