Fatal Clostridium Infection in a Leg-Amputated Patient after Unsuccessful Knee Arthroplasty
Abstract
:1. Introduction
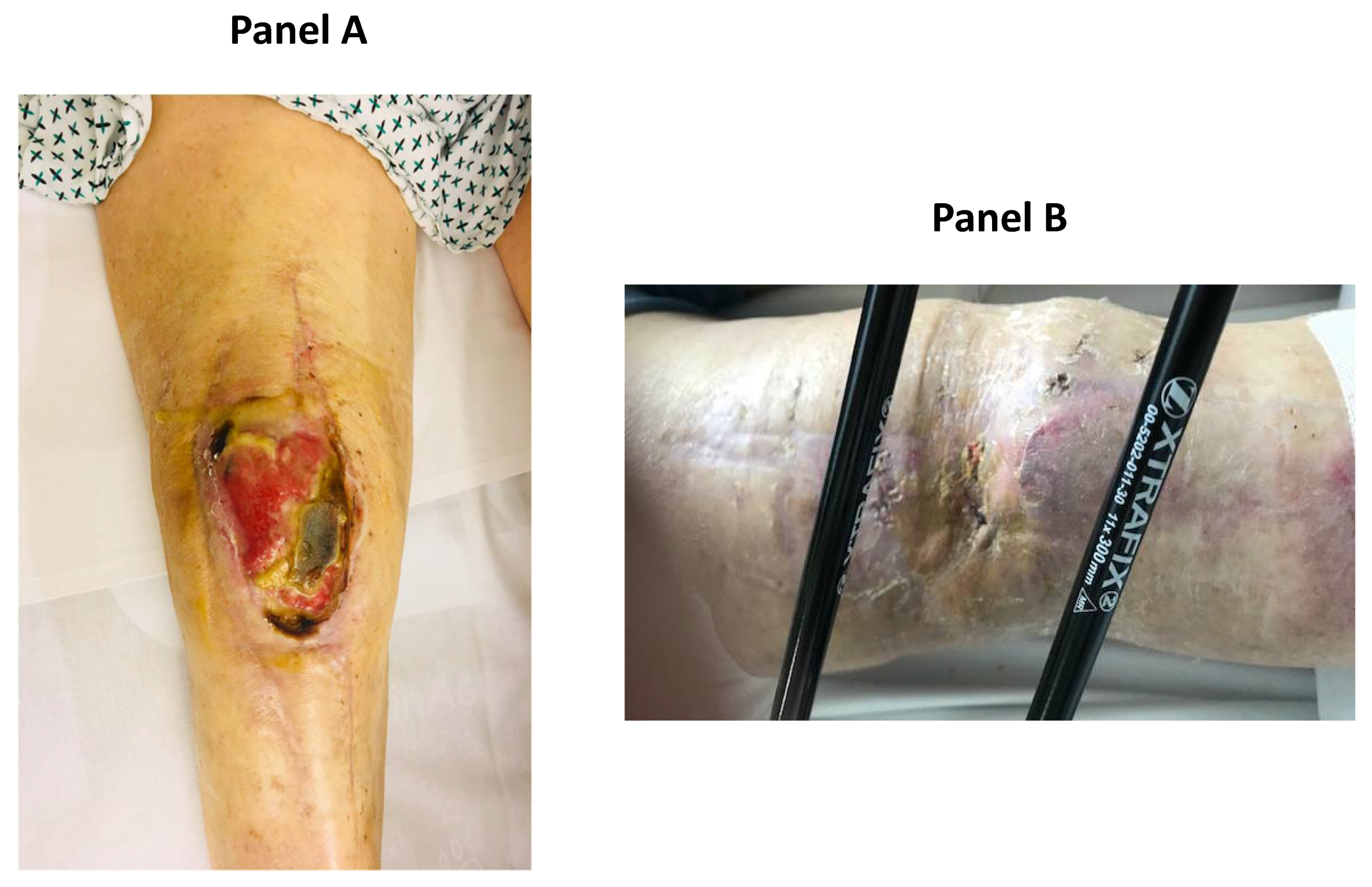
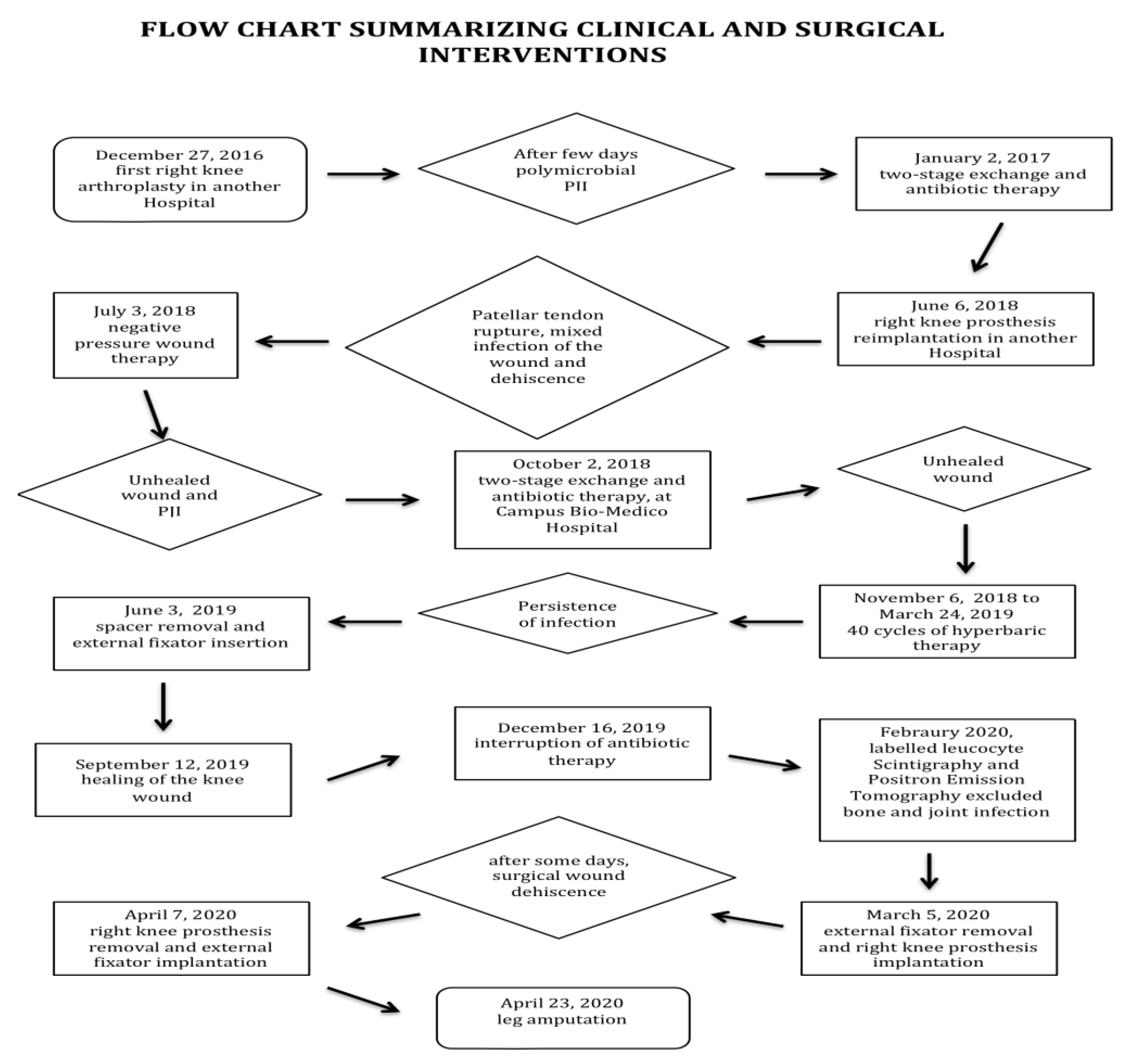
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRP | C reactive protein |
| DAIR | Debridement: antibiotics, irrigation, and implant retention |
| ESBL | Extended spectrum β-lactamases |
| ESR | Erythrocyte sedimentation rate |
| MDR | Multidrug resistant |
| PJI | Prosthetic joint infection |
References
- Marang-van de Mheen, P.J.; Bragan Turner, E.; Liew, S.; Mutalima, N.; Tran, T.; Rasmussen, S.; Nelissen, R.G.H.H.; Gordon, A. Variation in Prosthetic Joint Infection and treatment strategies during 4.5 years of follow-up after primary joint arthroplasty using administrative data of 41,397 patients across Australian, European and United States hospitals. BMC. Musculoskelet. Disord. 2017, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Tsukayama, D.T.; Estrada, R.; Gustilo, R.B. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Jt. Surg. Am. 1996, 78, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Marculescu, C.E.; Cantey, J.R. Polymicrobial prosthetic joint infection. Clin. Orthop. Relat. Res. 2008, 466, 1397–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, E.; Masters, S.; Berendt, A.R.; McLardy-Smith, P.; Byren, I.; Atkins, B.L. Guiding empirical antibiotic therapy in orthopedics: The microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J. Infect. 2007, 55, 1–7. [Google Scholar] [CrossRef]
- Zardi, E.M.; Franceschi, F. Prosthetic joint infection. A relevant public health issue. J. Infect. Public Health 2020, 13, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Tatarelli, P.; Romani, T.; Santoro, V.; Spezia, M.; Gallo, A.; Ripamonti, G.; Carducci, M.; Trotti, C.; Parisini, A.; Nicolini, L.A.; et al. Debridement, antibiotics and implant retention (DAIR): An effective treatment option for early prosthetic joint infections. J. Infect. Chemother. 2021, 27, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Stasch, P.; Preiss, S.; Lucke, K.; Vogt, M. Characteristics and treatment outcomes of 69 cases with early prosthetic joint infections of the hip and knee. Infection 2014, 42, 511–519. [Google Scholar] [CrossRef] [PubMed]
- George, D.A.; Drago, L.; Scarponi, S.; Gallazzi, E.; Haddad, F.S.; Romano, C.L. Predicting lower limb periprosthetic joint infections: A review of risk factors and their classification. World J. Orthop. 2017, 8, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, N.A.; Hutt, J.R.; Kendoff, D.O.; Mitchell, P.A.; Citak, M.; Granger, L. Prolonged suppressive antibiotic therapy is successful in the management of prosthetic joint infection. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Hung, J.F.; Chang, C.H.; Lee, S.H.; Shih, H.N.; Chang, Y.H. Periprosthetic knee infection reconstruction with a hinged prosthesis: Implant survival and risk factors for treatment failure. Knee 2020, 27, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.D.; Friedrich, M.J.; Randau, T.M.; Ploeger, M.M.; Schmolders, J.; Strauss, A.A.; Hischebeth, G.T.; Pennekamp, P.H.; Vavken, P.; Gravius, S. Polymicrobial infections reduce the cure rate in prosthetic joint infections: Outcome analysis with two-stage exchange and follow-up ≥two years. Int. Orthop. 2016, 40, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Zeller, V.; Kerroumi, Y.; Meyssonnier, V.; Heym, B.; Metten, M.A.; Desplaces, N.; Marmor, S. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J. Infect. 2018, 76, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Coll, P.; Gálvez, M.L.; Jordán, M.; López-Contreras, J.; Pomar, V.; Monllau, J.C.; Mirelis, B.; Gurguí, M. Etiology of surgical site infections after primary total joint arthroplasties. J. Orthop. Res. 2014, 32, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Tornero, E.; Morata, L.; Martínez-Pastor, J.C.; Angulo, S.; Combalia, A.; Bori, G.; García-Ramiro, S.; Bosch, J.; Mensa, J.; Soriano, A. Importance of selection and duration of antibiotic regimen in prosthetic joint infections treated with debridement and implant retention. J. Antimicrob. Chemother. 2016, 71, 1395–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasticci, M.B.; Di Filippo, P.; Pasqualini, L.; Mencacci, A.; Pallotto, C.; Malincarne, L.; Baldelli, F. Tolerability and efficacy of long-term treatment with daptomycin, ceftazidime and colistin in a patient with a polymicrobial, multidrug-resistant prosthetic joint reinfection: A case report. J. Med. Case Rep. 2014, 8, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, B.J.H.; Aichmair, A.; Simon, S.; Schwarz, G.M.; Dominkus, M.; Hofstaetter, J.G. Analysis of Culture Positive First and Second Stage Procedures in Periprosthetic Knee and Hip Joint Infections. J. Arthroplast. 2021, 36, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.W.; Wilson, R.B. Clostridium difficile colitis and zoonotic origins-a narrative review. Gastroenterol. Rep. 2018, 6, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheir, M.M.; Tan, T.L.; Gomez, M.M.; Chen, A.F.; Parvizi, J. Patients with failed prior two-stage exchange have poor outcomes after further surgical intervention. J. Arthroplast. 2017, 32, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Löwik, C.A.M.; Zijlstra, W.P.; Knobben, B.A.S.; Ploegmakers, J.J.W.; Dijkstra, B.; de Vries, A.J.; Kampinga, G.A.; Mithoe, G.; Al Moujahid, A.; Jutte, P.C.; et al. Northern Infection Network Joint Arthroplasty (NINJA). Obese patients have higher rates of polymicrobial and Gram-negative early periprosthetic joint infections of the hip than non-obese patients. PLoS ONE 2019, 14, e0215035. [Google Scholar] [CrossRef] [PubMed]
- Seugendo, M.; Janssen, I.; Lang, V.; Hasibuan, I.; Bohne, W.; Cooper, P.; Daniel, R.; Gunka, K.; Kusumawati, R.L.; Mshana, S.E.; et al. Prevalence and Strain Characterization of Clostridioides (Clostridium) difficile in Representative Regions of Germany, Ghana, Tanzania and Indonesia—A Comparative Multi-Center Cross-Sectional Study. Front. Microbiol. 2018, 9, 1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zardi, E.M.; Persichetti, P.; Palumbo, A.; Franceschetti, E.; Franceschi, F. Fatal Clostridium Infection in a Leg-Amputated Patient after Unsuccessful Knee Arthroplasty. Int. J. Environ. Res. Public Health 2021, 18, 9186. https://doi.org/10.3390/ijerph18179186
Zardi EM, Persichetti P, Palumbo A, Franceschetti E, Franceschi F. Fatal Clostridium Infection in a Leg-Amputated Patient after Unsuccessful Knee Arthroplasty. International Journal of Environmental Research and Public Health. 2021; 18(17):9186. https://doi.org/10.3390/ijerph18179186
Chicago/Turabian StyleZardi, Enrico Maria, Paolo Persichetti, Alessio Palumbo, Edoardo Franceschetti, and Francesco Franceschi. 2021. "Fatal Clostridium Infection in a Leg-Amputated Patient after Unsuccessful Knee Arthroplasty" International Journal of Environmental Research and Public Health 18, no. 17: 9186. https://doi.org/10.3390/ijerph18179186
APA StyleZardi, E. M., Persichetti, P., Palumbo, A., Franceschetti, E., & Franceschi, F. (2021). Fatal Clostridium Infection in a Leg-Amputated Patient after Unsuccessful Knee Arthroplasty. International Journal of Environmental Research and Public Health, 18(17), 9186. https://doi.org/10.3390/ijerph18179186





