Analysis of Risk Factors for Major Complications of 1500 Transvenous Lead Extraction Procedures with Especial Attention to Tricuspid Valve Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lead Extraction Procedure
2.2. TEE Monitoring during TLE
2.3. Statistical Analysis
2.4. Approval of the Bioethics Committee
3. Results
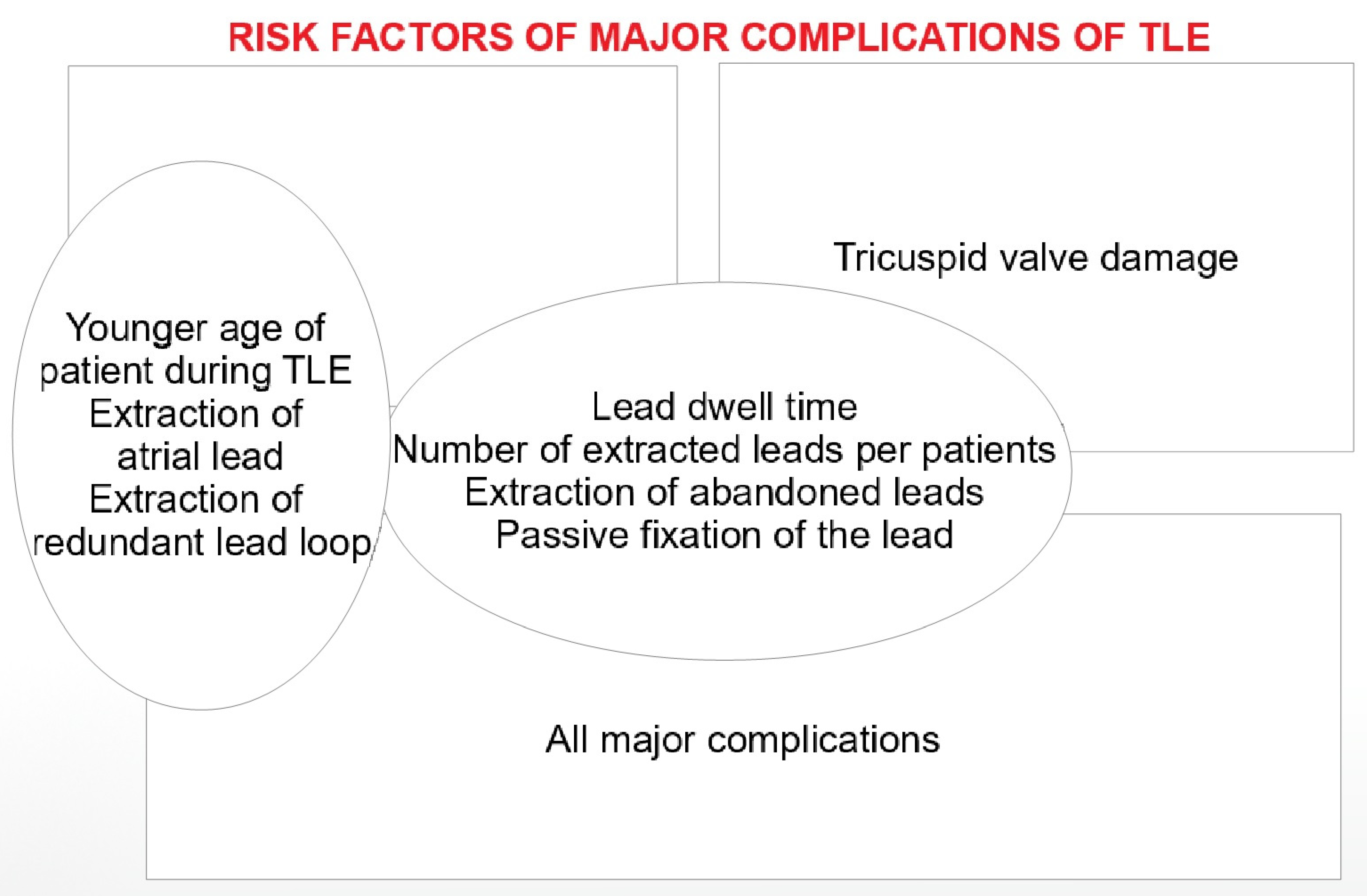
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Love, C.J.; Wilkoff, B.L.; Byrd, C.L.; Belott, P.H.; Brinker, J.A.; Fearnot, N.E.; Friedman, R.A.; Furman, S.; Goode, L.B.; Hayes, D.L.; et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: Indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin. Electrophysiol. 2000, 23, 544–551. [Google Scholar]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H., 3rd; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009, 6, 1085–1104. [Google Scholar] [CrossRef]
- Deharo, J.C.; Bongiorni, M.G.; Rozkovec, A.; Bracke, F.; Defaye, P.; Fernandez-Lozano, I.; Golzio, P.G.; Hansky, B.; Kennergren, C.; Manolis, A.S.; et al. European Heart Rhythm Association. Pathways for training and accreditation for transvenous lead extraction: A European Heart Rhythm Association position paper. Europace 2012, 14, 124–134. [Google Scholar] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [Green Version]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2012, 14, 994–1001. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Tułecki, Ł.; Kleinrok, A.; Kutarski, A. Echocardiographic findings in patients with cardiac implantable electronic devices-analysis of factors predisposing to lead-associated changes. Clin. Physiol. Funct. Imaging 2021, 41, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.P.; Cronin, E.M.; Wazni, O.; Baranowski, B.; Saliba, W.I.; Sabik, J.F.; Lindsay, B.D.; Wilkoff, B.L.; Tarakji, K.G. Outcomes of patients requiring emergent surgical or endovascular intervention for catastrophic complications during transvenous lead extraction. Heart Rhythm 2014, 11, 419–425. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Modry, D.; Wang, S. Cardiopulmonary bypass standby avoids fatality due to vascular laceration in laser-assisted lead extraction. J. Card. Surg. 2014, 29, 274–278. [Google Scholar] [CrossRef]
- Bashir, J.; Fedoruk, L.M.; Ofiesh, J.; Karim, S.S.; Tyers, G.F.O. Classification and surgical repair of injuries sustained during transvenous lead extraction. Circ. Arrhythmia Electrophysiol. 2016, 9, e003741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.M.; Rozen, G.; Kaadan, M.I.; Galvin, J.; Ruskin, J.N. Safety and In-Hospital Outcomes of Transvenous Lead Extraction for Cardiac Implantable Device-Related Infections: Analysis of 13 Years of Inpatient Data in the United States. JACC Clin. Electrophysiol. 2019, 5, 1450–1458. [Google Scholar] [CrossRef]
- Hauser, R.G.; Katsiyiannis, W.T.; Gornick, C.C.; Almquist, A.K.; Kallinen, L.M. Deaths and cardiovascular injuries due to device assisted implantable cardioverter–defibrillator and pacemaker lead extraction. Europace 2010, 12, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Gentry, J.L., 3rd; Varma, N.; Wazni, O.; Tarakji, K.G.; Mehta, A.; Mick, S.; Grimm, R.; Wilkoff, B.L. Transvenous Extraction of Pacemaker and Defibrillator Leads and the Risk of Tricuspid Valve Regurgitation. JACC Clin. Electrophysiol. 2018, 4, 1421–1428. [Google Scholar] [CrossRef]
- Pecha, S.; Castro, L.; Gosau, N.; Linder, M.; Vogler, J.; Willems, S.; Reichenspurner, H.; Hakmi, S. Evaluation of tricuspid valve regurgitation following laser lead extraction†. Eur. J. Cardiothorac. Surg. 2017, 51, 1108–1111. [Google Scholar] [CrossRef]
- Givon, A.; Vedernikova, N.; Luria, D.; Vatury, O.; Kuperstein, R.; Feinberg, M.S.; Eldar, M.; Glikson, M.; Nof, E. Tricuspid Regurgitation following Lead Extraction: Risk Factors and Clinical Course. Isr. Med. Assoc. J. 2016, 18, 18–22. [Google Scholar] [PubMed]
- Regoli, F.; Caputo, M.; Conte, G.; Faletra, F.F.; Moccetti, T.; Pasotti, E.; Cassina, T.; Casso, G.; Schlotterbeck, H.; Engeler, A.; et al. Clinical utility of routine use of continuous transesophageal echocardiography monitoring during transvenous lead extraction procedure. Heart Rhythm 2015, 12, 313–320. [Google Scholar] [CrossRef]
- Coffey, J.O.; Sager, S.J.; Gangireddy, S.; Levine, A.; Viles-Gonzalez, J.F.; Fischer, A. The impact of transvenous lead extraction on tricuspid valve function. Pacing Clin. Electrophysiol. 2014, 37, 19–24. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Mesa, J.; Arguelles, E.; Carrillo, R.G. Tricuspid insufficiency after laser lead extraction. Pacing Clin. Electrophysiol. 2013, 36, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, F.; Thuny, F.; Giorgi, R.; Sanaa, I.; Peyrouse, E.; Assouan, X.; Prévôt, S.; Bastard, E.; Habib, G.; Deharo, J.C. Incidence, risk factors and outcome of traumatic tricuspid regurgitation after percutaneous ventricular lead removal. J. Am. Coll. Cardiol. 2009, 53, 2168–2174. [Google Scholar] [CrossRef] [Green Version]
- Zucchelli, G.; Di Cori, A.; Segreti, L.; Laroche, C.; Blomstrom-Lundqvist, C.; Kutarski, A.; Regoli, F.; Butter, C.; Defaye, P.; Pasquié, J.L.; et al. ELECTRa Investigators. Major cardiac and vascular complications after transvenous lead extraction: Acute outcome and predictive factors from the ESC-EHRA ELECTRa (European Lead Extraction ConTRolled) registry. Europace 2019, 21, 771–780. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Janion, M.; Kutarski, A. Risk Factors Predicting Complications of Transvenous Lead Extraction. Biomed Res. Int. 2018, 2018, 8796704. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.P.; Cronin, E.M.; Duarte, V.E.; Yu, C.; Tarakji, K.G.; Martin, D.O.; Callahan, T.; Cantillon, D.J.; Niebauer, M.J.; Saliba, W.I.; et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014, 11, 799–805. [Google Scholar] [CrossRef]
- Wazni, O.; Epstein, L.M.; Carrillo, R.G.; Love, C.; Adler, S.W.; Riggio, D.W.; Karim, S.S.; Bashir, J.; Greenspon, A.J.; DiMarco, J.P.; et al. Lead extraction in the contemporary setting: The LExICon study: An observational retrospective study of consecutive laser lead extractions. J. Am. Coll. Cardiol. 2010, 55, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Kutarski, A. Transvenous Lead Extraction, S.A.FeTY Score for Risk Stratification and Proper Patient Selection for Removal Procedures Using Mechanical Tools. J. Clin. Med. 2020, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, B.S.; Ayis, S.; Gould, J.; Elliott, M.K.; Mehta, V.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Maggioni, A.P.; et al. ELECTRa Investigators Group. Risk stratification of patients undergoing transvenous lead extraction with the ELECTRa Registry Outcome Score (EROS): An ESC EHRA EORP European lead extraction ConTRolled, E.L.ECTRa registry analysis. Europace 2021, euab037, online ahead of print. [Google Scholar] [CrossRef]
- Kancharla, K.; Acker, N.G.; Li, Z.; Samineni, S.; Cai, C.; Espinosa, R.E.; Osborn, M.; Mulpuru, S.K.; Asirvatham, S.J.; Friedman, P.A.; et al. Efficacy and safety of transvenous lead extraction in the device laboratory and operating room guided by a novel risk stratification scheme. JACC Clin. Electrophysiol. 2019, 5, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, L.; Vassanelli, F.; Cerini, M.; D’Aloia, A.; Vizzardi, E.; Gargaro, A.; Chiusso, F.; Mamedouv, R.; Lipari, A.; Curnis, A. Predicting the difficulty of a lead extraction procedure: The LED index. J. Cardiovasc. Med. 2014, 15, 668–673. [Google Scholar] [CrossRef]
- Fu, H.X.; Huang, X.M.; Zhong, L.I.; Osborn, M.J.; Asirvatham, S.J.; Espinosa, R.E.; Brady, P.A.; Lee, H.C.; Greason, K.L.; Baddour, L.M.; et al. Outcomes and complications of lead removal: Can we establish a risk stratification schema for a collaborative and effective approach? Pacing Clin. Electrophysiol. 2015, 38, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Tomaszewski, A.; Brzozowski, W.; Szcześniak-Stańczyk, D.; Kleinrok, A.; et al. Transesophageal Echocardiography as a Monitoring Tool During Transvenous Lead Extraction-Does It Improve Procedure Effectiveness? J. Clin. Med. 2020, 9, 1382. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Kleinrok, A.; Kutarski, A. A new approach to the continuous monitoring of transvenous lead extraction using transesophageal echocardiography—Analysis of 936 procedures. Echocardiography 2020, 37, 601–611. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Kleinrok, A.; Tułecki, Ł.; Kutarski, A. Transesophageal echocardiography for the monitoring of transvenous lead extraction. Kardiol. Pol. 2020, 78, 1206–1214. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Kleinrok, A.; Tułecki, Ł.; Kutarski, A. The prognostic value of transesophageal echocardiography after transvenous lead extraction: Landscape after battle. Cardiovasc. Diagn. Ther. 2021, 11, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Morita, J.; Yamaji, K.; Nagashima, M.; Kondo, Y.; Sadohara, Y.; Hirokami, J.; Kuji, R.; Korai, K.; Fukunaga, M.; Hiroshima, K.; et al. Predictors of lead break during transvenous lead extraction. J. Arrhythmia 2021, 37, 645–652. [Google Scholar] [CrossRef]
- Pecha, S.; Ziegelhoeffer, T.; Yildirim, Y.; Choi, Y.H.; Willems, S.; Reichenspurner, H.; Burger, H.; Hakmi, S. Safety and efficacy of transvenous lead extraction of very old leads. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 402–407. [Google Scholar] [CrossRef]
- Mehrotra, D.; Kejriwal, N.K. Tricuspid valve repair for torrential tricuspid regurgitation after permanent pacemaker lead extraction. Tex. Heart Inst. J. 2011, 38, 305–307. [Google Scholar] [PubMed]
- Schaller, R.D.; Sadek, M.M. Intracardiac echocardiography during transvenous lead extraction. Card. Electrophysiol. Clin. 2021, 13, 409–418. [Google Scholar] [CrossRef] [PubMed]
| Hemorrhagic Complication (Cardiac/Vascular Wall Tear) | Tricuspid Valve Damage | All Major Complications (Mixed Damages 1 Case) | Control Group (No Major Complications) | |
|---|---|---|---|---|
| Groups of patients | A N = 22 (1.5%) Mean ± SD n (%) | B N = 12 (0.8%) Mean ± SD n (%) | C N = 33 (2.2%) Mean ± S n (%) | D N = 1467 Mean ± SD n (%) |
| Patients | ||||
| Patient age during TLE [years] | 63.14 ± 13.91 p = 0.009 | 68.75 ± 21.98 p = 0.116 | 65.82 ± 16.84 p = 0.005 | 68.16 ± 13.96 |
| Patient age at first implantation [years] | 45.32 ± 16.98 p < 0.001 | 50.58 ± 26.16 p = 0.007 | 47.97 ± 20.23 p < 0.001 | 59.06 ± 15.58 |
| Sex (% of female patients) | 17 (77.30) p = 0.004 | 5 (41.70) p = 0.979 | 21 (63.60) p = 0.005 | 555 (37.80) |
| NYHA class III & IV (%) | 1 (4.50) p = 0.192 | 0 (0.00) p = 0.227 | 1 (3.00) p = 0.052 | 256 (17.50) |
| LVEF < 40% | 1 (4.50) p = 0.003 | 2 (16.70) p < 0.001 | 3 (9.10) p < 0.001 | 555 (37.80) |
| Renal failure (any) | 3 (13.60) p = 0.316 | 1 (8.30) p = 0.127 | 4 (12.10) p = 0.127 | 371 (25.30) |
| Charlson comorbidity index [points] | 2.55 ± 2.41 p < 0.001 | 3.67 ± 3.53 p = 0.013 | 3.03 ± 2.85 p < 0.001 | 5.14 ± 3.76 |
| TLE Indications | ||||
| CIED-related infection (any) | 4 (18.20) p = 0.917 | 2 (16.70) p = 0.964 | 6 (18.20) p = 0.817 | 314 (21.40) |
| Non-infectious indications | 18 (81.80) p = 0.917 | 10 (83.30) p = 0.964 | 27 (81.80) p = 0.817 | 1153 (78.60) |
| System | ||||
| Pacemaker-with RA lead | 18 (81.80) p = 0.028 | 8 (66.70) p = 0.621 | 26 (78.80) p = 0.012 | 812 (55.40) |
| Pacemaker-without RA lead and only abandoned PM lead | 2 (9.10) p = 0.974 | 3 (25.00) p = 0.294 | 4 (12.10) p = 0.663 | 164 (11.20) |
| ICD-with RA lead | 0 (0.00) p = 0.170 | 0 (0.00) p = 0.424 | 1 (3.00) p = 0.210 | 170 (11.60) |
| ICD-without RA lead and only HV lead | 1 (4.50) p = 0.409 | 1 (8.30) p = 0.982 | 1 (3.00) p = 0.379 | 187 (12.70) |
| ICD-CRT-D pacing system | 1 (4.50) p = 0.726 | 0 (0.00) p = 0.562 | 1 (3.00) p = 0.377 | 132 (9.90) |
| Number of leads in the heart before TLE | 2.14 ± 0.94 p = 0.690 | 2.08 ± 0.67 p = 0.684 | 2.15 ± 0.83 p = 0.365 | 1.92 ± 0.69 |
| Abandoned leads before TLE | 5 (22.70) p = 0.019 | 4 (33.30) p = 0.004 | 9 (27.30) p < 0.001 | 106 (7.20) |
| Large lead loop on X-rays before TLE | 3 (13.60) p = 0.015 | 1 (8.30) p = 0.754 | 4 (12.10) p < 0.001 | 39 (2.70) |
| Small lead loop on X-rays before TLE | 5 (22.70) p = 0.250 | 1 (8.30) p = 0.978 | 6 (18.20) p = 0.452 | 180 (12.30) |
| Number of procedures before lead extraction | 3.00 ± 2.00 p < 0.001 | 2.83 ± 1.34 p = 0.003 | 2.90 ± 1.77 p < 0.001 | 1.79 ± 0.91 |
| Dwell time of the oldest lead per patient before TLE [months] | 214.9 ± 91.86 p < 0.001 | 217.9 ± 106.2 p < 0.001 | 215.0 ± 96.87 p < 0.001 | 109.8 ± 76.15 |
| Mean implant duration (per patient) before TLE [months] | 201.25 ± 81.14 p < 0.001 | 178.0 ± 62.89 p < 0.001 | 191.4 ± 75.59 p < 0.001 | 103.3 ± 68.79 |
| Global implant duration (sum of lead dwell times) [years] | 36.96 ± 23.12 p < 0.001 | 30.56 ± 13.85 p < 0.001 | 35.12 ± 20.50 p < 0.001 | 16.60 ± 13.29 |
| Hemorrhagic Complication (Cardiac/Vascular Wall Tear) | Tricuspid Valve Damage | All Major Complications (Mixed Damages 1 Case) | Control Group (No Major Complications) | |
|---|---|---|---|---|
| A N = 22 Mean ± SD n (%) | B N = 12 Mean ± SD n (%) | C N = 33 Mean ± SD n (%) | D N = 1467 Mean ± SD n (%) | |
| TLE Procedure Potential Risk Factors of Major TLE Complications and Procedure Complicity | ||||
| Number of leads extracted per patient | 2.30 ± 1.58 p = 0.079 | 2.39 ± 1.81 p = 0.189 | 2.21 ± 1.34 p = 0.008 | 1.63 ± 0.71 |
| Three or more leads extracted | 5 (22.79) p = 0.091 | 2 (16.70) p = 0.739 | 7 (21.20) p = 0.056 | 141 (9.60) |
| Extraction of leads with redundant loop (large) | 3 (13.60) p = 0.083 | 1 (8.30) p = 0.696 | 4 (12.10) p = 0.004 | 35 (2.40) |
| Extraction of abandoned lead(s) (any) | 4 (18.20) p = 0.094 | 4 (33.30) p = 0.002 | 8 (24.20) p < 0.001 | 99 (6.70) |
| HV therapy (ICD) lead extracted | 2 (9.10) p = 0.043 | 1 (8.30) p = 0.158 | 3 (9.10) p = 0.010 | 462 (31.50) |
| Atrial lead extracted (any) | 19 (86.40) p = 0.018 | 6 (50.00) p = 0.080 | 25 (75.80) p = 0.044 | 867 (59.10) |
| CS (LV pacing) lead extracted | 1 (4.50) p = 0.964 | 0 (0.00) p = 0.737 | 1 (3.00) p = 0.640 | 97 (6.60) |
| Dwell time of the oldest lead extracted | 214.9 (91.86) p < 0.001 | 217.9 ± 106.2 p = 0.001 | 215.0 ± 96.87 p = 0 < 001 | 108.8 ± 75.74 |
| Average (per patient) dwell time of lead extracted | 201.3 (81.14) p < 0.001 | 176.9 ± 63.75 p = 0.001 | 191.0 ± 75.91 p < 0.001 | 103.8 ± 69.47 |
| Cumulative dwell time of lead extracted (in years) | 36.34 (23.81) p < 0.001 | 28.72 ± 14.88 p = 0.001 | 34.05 ± 21.37 p < 0.001 | 14.96 ± 13.23 |
| SAFeTY TLE calculator of risk of MC of TLE—[number of points] | 13.03 (4.73) p < 0.001 | 11.42 ± 4.60 p = 0.001 | 12.31 ± 4.69 p < 0.001 | 6.11 ± 4.32 |
| Risk of MC calculated by SAFeTY TLE calculator (%) | 9.40 (12.70) p < 0.001 | 6.17 ± 6.06 p < 0.001 | 8.06 ± 10.89 p < 0.001 | 1.79 ± 2.58 |
| Analysis of Extracted Leads: Lead Model, Tip Location and Mechanism of Tip Fixation. | ||||
| Tip Location | ||||
| RAA | 22 (47.73) p = 0.139 | 7 (30.43) p = 0.355 | 29 (42.64) p = 0.355 | 901 (37.04) |
| BB | 1 (2.27) p = 0.693 | 0 (0.00) p = 0.911 | 1 (1.47) p = 0.911 | 15 (0.62) |
| CS | 1 (2.27) p = 0.967 | 0 (0.00) p = 0.811 | 1 (1.47) p = 0.811 | 25 (1.03) |
| CSO | 1 (2.27) p = 0.772 | 0 (.00) p = 0.783 | 1 (1.47) p = 0.783 | 44 (1.82) |
| RVA | 17 (38.64) p = 0.505 | 10 (43.48) p = 480 | 27 (39.71) p = 0.483 | 1069 (43.69) |
| Outside RVA | 2 (4.55) p = 0.231 | 6 (26.09) p = 0.985 | 8 (11.76) p = 0.985 | 274 (11.31) |
| LV vein | 1 (2.27 p = 0.726 | 0 (0.00) p = 0.390 | 1 (1.47) p = 0.390 | 108 (4.46) |
| Lead Type | ||||
| BP pacemaker leads | 39 (86.67) p = 0.106 | 18 (78.26) p = 0.146 | 57 (83.82) p = 0.146 | 1828 (75.04) |
| VDD pacemaker leads | 0 (0.00) p = 0.952 | 0 (0.00) p = 0.730 | 0 (0.00) p = 0.730 | 30 (1.19) |
| UP pacemaker leads | 4 (8.89) p = 0.256 | 4 (17.39) p = 0.007 | 8 (11.76) p = 0.007 | 104 (4.19) |
| ICD leads single coil | 2 (4.44) p = 0.231 | 0 (0.00) p = 0.053 | 2 (2.94) p = 0.053 | 274 (11.25) |
| ICD leads dual coil | 0 (0.00) p = 0.084 | 1 (4.35) p = 0.077 | 1 (1.47) p = 0.077 | 200 (8.21) |
| All | 45 (100) | 23 (100.0) | 68 (100.0) | 2436 (100.0) |
| Tip Fixation Mode | ||||
| Active fixation lead | 9 (20.00) p < 0.001 | 11 (47.83) p < 0.001 | 20 (29.41) p < 0.001 | 1408 (57.73) |
| Passive fixation lead | 36 (80.00) p ≤ 0.001 | 12 (52.17) p < 0.001 | 48 (70.59) p < 0.001 | 1028 (42.18) |
| Hemorrhagic Complication (Cardiac/Vascular Wall Tear) | Tricuspid Valve Damage | All Major Complications (Mixed Damages 1 Case) | Control Group (No Major Complications) | |
|---|---|---|---|---|
| Groups of patients | A N = 22 Mean ± SD n (%) | B N = 12 Mean ± SD n (%) | C N = 33 Mean ± SD n (%) | D N = 1467 Mean ± SD n (%) |
| TTE before TLE | ||||
| LVEF average [%] | 59.43 ± 10.85 p = 0.002 | 56.00 ± 11.78 p = 0.049 | 58.06 ± 11.29 p < 0.001 | 49.07 ± 15.96 |
| TVR-mild (0,1) | 15 (68.20) p = 0.214 | 8 (66.70) p = 0.214 | 22 (66.70) p = 0.153 | 771 (52.60) |
| TVR-intermediate/mid (2,3) | 6 (27.30) p = 0.417 | 4 (33.30) p = 0.469 | 10 (30.30) 0.469 | 558 (38.00) |
| TVR-severe (4) | 0 (0.00) p = 0.382 | 0 (0.00) p = 0.215 | 0 (0.00) p = 0.215 | 104 (7.10) |
| Lack of examination | 1 (4.50) p = 0.610 | 0 (0.00) p = 0.997 | 1 (3.00) p = 0.997 | 67 (4.60) |
| RVSP [mm Hg] | 27.24 ± 8.57 p = 0.075 | 26.08 (7.12) p = 0.010 | 27.06 ± 7.98 p = 0.010 | 32.07 (11.82) |
| TEE Findings before TLE | ||||
| Oscillating tissue scar on the lead | 7 (38.80) p = 0.080 | 3 (25.00) p = 0.044 | 10 (30.30) p < 0.044 | 231 (15.70) |
| Lead thickening (encapsulation) | 14(63.60) p < 0.001 | 9 (75.00) p < 0.001 | 22 (66.70) p < 0.001 | 398 (27.10) |
| Lead-to-lead binding | 10 (45.50) p < 0.001 | 5 (41.70) p < 0.001 | 15 (45.50) p < 0.001 | 208 (14.20) |
| Lead adhering to any heart structure | 11 (50.00) p < 0.001 | 10 (83.30) p < 0.001 | 20 (60.60) p < 0.001 | 242 (16.50) |
| Lead adhering to tricuspid valve | 5 (22.70) p = 0.031 | 6 (50.00) p < 0.001 | 11 (33.30) p < 0.001 | 115 (7.80) |
| Lead adhering to superior vena cava | 5 (22.70) p = 0.004 | 4 (33.30) p < 0.001 | 9 (27.30 p < 0.001) | 83 (5.70) |
| Lead adhering to RA wall | 9 (40.90) p < 0.001 | 1 (8.30) p < 0.001 | 10 (30.30) p < 0.001 | 92 (6.30) |
| Lead adhering to RV wall | 7 (31.80) p = 0.002 | 8 (66.70) p < 0.001 | 14 (42.40) p < 0.001 | 140 (9.50) |
| Tissue scar occurrence (any form) (possible multiple options) | 3.272 ± 1.725 p < 0.001 | 3.917 ± 1.647 p < 0.001 | 3.515 ± 1.587 p < 0.001 | 1.188 ± 1.225 |
| Occurrence of any form of tissue scar | 14 (63.60) p = 0.026 | 10 (83.30) p < 0.001 | 23 (69.70) p < 0.001 | 558 (38.00) |
| Perforation of RV wall/ECHO finding | 1 (4.50) p = 0.578 | 1 (8.30) p = 0.591 | 2 (6.10) p = 0.591 | 154 (10.50) |
| Hemorrhagic Complication (Cardiac/Vascular Wall Tear) | Tricuspid Valve Damage | All Major Complications (Mixed Damages 1 Case) | Control Group (No Major Complications) | |
|---|---|---|---|---|
| Groups of patients | A N = 22 Mean ± SD n (%) | B N = 12 Mean ± SD n (%) | C N = 33 Mean ± SD n (%) | D N = 1467 Mean ± SD n (%) |
| TLE Procedure Complexity and Efficacy | ||||
| Procedure duration (skin-to-skin) | 104.4 ± 52.24 p < 0.001 | 81.33 ± 31.76 p = 0.004 | 94.61 ± 46.62 p < 0.001 | 60.93 ± 25.93 |
| Procedure duration (sheath-to-sheath) | 55.53 ± 55.93 p < 0.001 | 29.92 ± 21.16 p < 0.001 | 46.67 ± 48.69 p < 0.001 | 13.91 ± 21.32 |
| Average time of single lead extr. (sheath-to-sheath/number of extracted leads) | 25.62 ± 21.63 p < 0.001 | 16.69 ± 13.00 p < 0.001 | 21.79 ± 19.19 p < 0.001 | 8.40 ± 13.26 |
| Technical problem during TLE (any) | 14 (63.60) p < 0.001 | 8 (66.70) p < 0.001 | 21 (63.60) p < 0.001 | 321 (21.90) |
| Lead-to-lead binding (intraoperative diagnosis) | 11 (50.00) p < 0.001 | 5 (41.70) p < 0.001 | 16 (48.50) p < 0.001 | 106 (7.20) |
| Block at venous entry site | 4 (18.20 p = 0.497 | 3 (25.00) p = 0.497 | 7 (21.20) p = 0.134 | 165 (11.20) |
| Fracture of extracted lead | 7 (31.80) p < 0.001 | 3 (25.00) p < 0.001 | 10 (30.30) p < 0.001 | 65 (4.40) |
| Byrd dilator torsion/collapse | 2 (9.10) p = 0.544 | 4 (33.30) p = 0.544 | 6 (18.20) p = 0.544 | 61 (4.20) |
| Three or more technical problems | 3 (13.60) p < 0.001 | 1 (8.30) p < 0.001 | 4 (12.10) p < 0.001 | 25 (1.70) |
| Use of Evolution (old and new) or TightRail | 3 (13.60) p = 0.007 | 3 (25.00) p = 0.007 | 5 (15.20) p = 0.003 | 34 (2.30) |
| Use of lasso catheters/snares | 5 (22.70) p < 0.001 | 3 (25.00) p < 0.001 | 8 (24.20) p < 0.001 | 47 (3.20) |
| Temporary pacing during procedure | 3 (13.60) 0.348 | 5 (41.70) p = 0.348 | 8 (24.20) p = 0.3876 | 361 (24.60) |
| TEE and Blood Pressure Monitoring | ||||
| RAA pulling/drawing | 15 (5) p < 0.001 | 3 (25.00) p < 0.001 | 18 (54.50) p = 0.012 | 472 (32.20) |
| TV pulling/drawing | 6 (27.30) p < 0.001 | 11 (91.70) p < 0.001 | 16 (48.50) p < 0.001 | 100 (6.80) |
| RV wall pulling | 10 (45.50) p = 0.015 | 8 (66.70) p = 0.015 | 17 (51.50) p < 0.001 | 317 (21.60) |
| Other lead pulling | 10 (45.50) | 5 (41.70) p < 0.001 | 15 (45.50) p < 0.001 | 116 (7.90) |
| Pulling/drawing of heart structures or other lead (possible multiple options) | 1.864 ± 1.46 p < 0.001 | 2.250 ± 1.224 p < 0.001 | 2.00 ± 1.350 p < 0.001 | 0.670 ± 0.928 |
| Max blood pressure drop during TLE [mm Hg] | 54.43 ± 23.42 p < 0.001 | 38.89 ± 21.03 p < 0.001 | 48.38 ± 22.64 p < 0.001 | 20.79 ± 14.53 |
| Significant blood pressure drop during TLE (different reasons) | 13 (59.10) p < 0.001 | 3 (25.00) p < 0.001 | 15 (45.50) p < 0.001 | 137 (9.30) |
| TLE Efficacy and Complications | ||||
| Worsening TR for 1 degree | 2 (9.10) p = 0.956 | 0 (0.00) p = 0.956 | 2 (6.10) p = 0.908 | 104 (7.10) |
| Worsening TR for 2 degrees | 0 (0.00) p = 0.95 | 4 (33.30) p = 0.95 | 4 (12.10) p = 0.002 | 31 (2.10) |
| Worsening TR for 3 degrees | 1 (4.50) p < 0.001 | 8 (66.70) p < 0.001 | 8 (24.20) p < 0.001 | 0 (0.00) |
| Tricuspid valve damage during TLE (severe) | 0 (0.00) N | 12 (100.0) p < 0.001 | 12 (36.40) p < 0.001 | 0 (0.00) |
| Procedure-related death (intra-, post-procedural) | 0 (0.00) N | 0 (0.00) N | 0 (0.00) N | 0 (0.00) |
| Clinical success | 21 (95.50) p = 0.114 | 0 (0.00) p = 0.114 | 21 (63.60) p < 0.001 | 1463 (99.70) |
| Complete procedural success | 20 (90.90) p = 0.322 | 0 (0.00) p = 0.322 | 20 (60.60) p < 0.001 | 1422 (96.90) |
| Short-, Mid-and Long-Term Mortality after TLE (Any Reason) | ||||
| First day (first 48 h) | 0 (0.00) p = 0862 | 0 (0.00) p = 0.862 | 0 (0.00) p = 0.832 | 2 (0.14) |
| Mortality at 1 month after TLE (2–30 days) | 0 (0.00) p = 0.78 | 0 (0.00) p = 0.780 | 0 (0.00) p = 0.993 | 23 (1.57) |
| Mortality at 1 year after TLE (31–365 days) | 1 (4.55) 0.985 | 1 (8.33) 0.985 | 2 (6.06) 0.845 | 99 (6.75) |
| Mortality at 3 years TLE (366–1095 days) | 1 (4.55) 0.855 | 0 (0.00) p = 0.855 | 1 (3.03) p = 0.841 | 116 (7.91) |
| Mortality > 3 years after TLE (> 1095 days) | 0 (0.00) 0.673 | 1 (8.33) p = 0.673 | 1 (3.03) p = 0.888 | 60 (4.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tułecki, Ł.; Polewczyk, A.; Jacheć, W.; Nowosielecka, D.; Tomków, K.; Stefańczyk, P.; Kosior, J.; Duda, K.; Polewczyk, M.; Kutarski, A. Analysis of Risk Factors for Major Complications of 1500 Transvenous Lead Extraction Procedures with Especial Attention to Tricuspid Valve Damage. Int. J. Environ. Res. Public Health 2021, 18, 9100. https://doi.org/10.3390/ijerph18179100
Tułecki Ł, Polewczyk A, Jacheć W, Nowosielecka D, Tomków K, Stefańczyk P, Kosior J, Duda K, Polewczyk M, Kutarski A. Analysis of Risk Factors for Major Complications of 1500 Transvenous Lead Extraction Procedures with Especial Attention to Tricuspid Valve Damage. International Journal of Environmental Research and Public Health. 2021; 18(17):9100. https://doi.org/10.3390/ijerph18179100
Chicago/Turabian StyleTułecki, Łukasz, Anna Polewczyk, Wojciech Jacheć, Dorota Nowosielecka, Konrad Tomków, Paweł Stefańczyk, Jarosław Kosior, Krzysztof Duda, Maciej Polewczyk, and Andrzej Kutarski. 2021. "Analysis of Risk Factors for Major Complications of 1500 Transvenous Lead Extraction Procedures with Especial Attention to Tricuspid Valve Damage" International Journal of Environmental Research and Public Health 18, no. 17: 9100. https://doi.org/10.3390/ijerph18179100
APA StyleTułecki, Ł., Polewczyk, A., Jacheć, W., Nowosielecka, D., Tomków, K., Stefańczyk, P., Kosior, J., Duda, K., Polewczyk, M., & Kutarski, A. (2021). Analysis of Risk Factors for Major Complications of 1500 Transvenous Lead Extraction Procedures with Especial Attention to Tricuspid Valve Damage. International Journal of Environmental Research and Public Health, 18(17), 9100. https://doi.org/10.3390/ijerph18179100






