How Much Does HIV Positivity Affect the Presence of Oral HPV? A Molecular Epidemiology Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Samples Collection
2.3. DNA Extraction
2.4. Detection of HPV-DNA
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
6. Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Khalil, A.I.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2020, 9, e161–e169. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Rohner, E.; Bütikofer, L.; Schmidlin, K.; Sengayi, M.; Maskew, M.; Giddy, J.; Taghavi, K.; Moore, R.D.; Goedert, J.J.; Gill, M.J.; et al. Cervical cancer risk in women living with HIV across four continents: A multicohort study. Int. J. Cancer 2019, 146, 601–609. [Google Scholar] [CrossRef]
- Amini Lari, M.; Faramarzi, H.; Shams, M.; Marzban, M.; Joulaei, H. Sexual dysfunction.; depression and quality of life in patients with HIV infection. Iran J. Psychiatry Behav. Sci. 2013, 7, 61–68. [Google Scholar]
- Dominiak-Felden, G.; Cohet, C.; Atrux-Tallau, S.; Gilet, H.; Tristram, A.; Fiander, A. Impact of human papillomavirus-related genital diseases on quality of life and psychosocial wellbeing: Results of an observational, health-related quality of life study in the UK. BMC Public Health 2013, 13, 1065. [Google Scholar] [CrossRef]
- Rooney, A.S.; Moore, R.C.; Paolillo, E.W.; Gouaux, B.; Umlauf, A.; Letendre, S.L.; Jeste, D.V.; Moore, D.J. HIV Neurobehavioral Research Program. Depression and aging with HIV: Associations with health-related quality of life and positive psychological factors. J. Affect. Disord. 2019, 251, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, A.; Franzetti, M.; Bonfati, P. HIV and HPV coinfections: A dangerous liaison. JHA 2017, 2, 102–108. [Google Scholar] [CrossRef]
- Bogale, A.L.; Belay, N.B.; Medhin, G.; Ali, J.H. Molecular epidemiology of human papillomavirus among HIV infected women in developing countries: Systematic review and meta-analysis. Virol. J. 2020, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Riso, R.; Facciolà, A.; Mondello, P.; Caruso, C.; Picerno, I.; Di Pietro, A.; Spataro, P.; Bertuccio, M.P. Higher levels of oxidative DNA damage in cervical cells are correlated with the grade of dysplasia and HPV infection. J. Med. Virol. 2015, 88, 336–344. [Google Scholar] [CrossRef]
- Visalli, G.; Currò, M.; Facciolà, A.; Riso, R.; Mondello, P.; Laganà, P.; Di Pietro, A.; Picerno, I.; Spataro, P. Prevalence of human papillomavirus in saliva of women with HPV genital lesions. Infect. Agents Cancer 2016, 11, 48. [Google Scholar] [CrossRef]
- Méndez-Martínez, R.; Maldonado-Frías, S.; Vázquez-Vega, S.; Caro-Vega, Y.; Rendón-Maldonado, J.G.; Guido-Jiménez, M.; Crabtree-Ramírez, B.; Sierra-Madero, J.G.; García-Carrancá, A. High prevalent human papillomavirus infections of the oral cavity of asymptomatic HIV-positive men. BMC Infect. Dis. 2020, 20, 27–29. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C. Human Papillomavirus-Related Cancers. Adv. Exp. Med. Biol. 2017, 1018, 23–34. [Google Scholar] [CrossRef]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2019, 8, e180–e190. [Google Scholar] [CrossRef]
- Dunne, E.F.; Nielson, C.M.; Stone, K.M.; Markowitz, L.E.; Giuliano, A.R. Prevalence of HPV Infection among Men: A Systematic Review of the Literature. J. Infect. Dis. 2006, 194, 1044–1057. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Nielson, C.M.; Flores, R.; Dunne, E.F.; Abrahamsen, M.; Papenfuss, M.R.; Markowitz, L.E.; Smith, D.; Harris, R.B. The Optimal Anatomic Sites for Sampling Heterosexual Men for Human Papillomavirus (HPV) Detection: The HPV Detection in Men Study. J. Infect. Dis. 2007, 196, 1146–1152. [Google Scholar] [CrossRef]
- Pathak, N.; Dodds, J.; Zamora, J.; Khan, K.S. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: Systematic review and meta-analysis. Br. Med. J. 2014, 349, g5264. [Google Scholar] [CrossRef]
- Leeman, A.; Del Pino, M.; Molijn, A.; Rodriguez, A.; Torné, A.; De Koning, M.; Ordi, J.; Van Kemenade, F.; Jenkins, D.; Quint, W. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: Cross-sectional data from a triage population. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1356–1363. [Google Scholar] [CrossRef]
- Youshya, S.; Purdie, K.; Breuer, J.; Proby, C.; Sheaf, M.T.; Oliver, R.T.D.; Baithun, S. Does human papillomavirus play a role in the development of bladder transitional cell carcinoma? A comparison of PCR and immunohistochemical analysis. J. Clin. Pathol. 2005, 58, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Jimenez, A.; de Dios Luna, J.; Soto, M.J.; Sorlozano, A. Meta-Analysis of Studies Analyzing the Relationship Between Bladder Cancer and Infection by Human Papillomavirus. J. Urol. 2006, 176, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Facciolà, A.; d’Aleo, F.; Pinzone, M.R.; Condorelli, F.; Picerno, I.; Nunnari, G.; Pellicanò, G.F.; Ceccarelli, M.; Venanzi Rullo, E. HPV and Urinary Bladder Carcinoma: A Review of the Literature. WCRJ 2018, 5, e1038. [Google Scholar] [CrossRef]
- Silverman, S., Jr. Epidemiology. In Oral Cancer Hamilton, 4th ed.; Silverman, S., Jr., Ed.; Decker Inc.: Ontario, BC, Canada, 1998; pp. 1–6. [Google Scholar]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Pytynia, K.B.; Dahlstrom, K.; Sturgis, E.M. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; Kotsantis, I.; Psyrri, A. Special Issue about Head and Neck Cancers: HPV Positive Cancers. Int. J. Mol. Sci. 2020, 21, 3388. [Google Scholar] [CrossRef]
- Donà, M.G.; Spriano, G.; Pichi, B.; Rollo, F.; Laquintana, V.; Covello, R.; Pellini, R.; Giuliani, M.; Pescarmona, E.; Benevolo, M. Human papillomavirus infection and p16 overexpression in oropharyngeal squamous cell carcinoma: A case series from 2010 to 2014. Future Microbiol. 2015, 10, 1283–1291. [Google Scholar] [CrossRef]
- Saulle, R.; Semyonov, L.; Mannocci, A.; Careri, A.; Saburri, F.; Ottolenghi, L.; Guerra, F.; La Torre, G. Human papillomavirus and cancerous diseases of the head and neck: A systematic review and meta-analysis. Oral Dis. 2014, 21, 417–431. [Google Scholar] [CrossRef]
- Golusinski, P. Risk Factors for Oral Infection with Human Papillomavirus. Recent Results Cancer Res. 2016, 206, 73–85. [Google Scholar] [CrossRef]
- Heck, J.; Berthiller, J.; Vaccarella, S.; Winn, D.M.; Smith, E.M.; Shan’Gina, O.; Schwartz, S.M.; Purdue, M.; Pilarska, A.; Eluf-Neto, J.; et al. Sexual behaviours and the risk of head and neck cancers: A pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int. J. Epidemiol. 2010, 39, 166–181. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Klussmann, J.P.; Giorgi, M.R.S.; Wuerdemann, N.; Dikkers, F.G. Oral and laryngeal HPV infection: Incidence, prevalence and risk factors, with special regard to concurrent infection in head, neck and genitals. Vaccine 2021, 39, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Drake, V.E.; Fakhry, C.; Windon, M.J.; Stewart, C.M.; Akst, L.; Hillel, A.; Chien, W.; Ha, P.; Miles, B.; Gourin, C.G.; et al. Timing, number, and type of sexual partners associated with risk of oropharyngeal cancer. Cancer 2021, 127, 1029–1038. [Google Scholar] [CrossRef]
- Wong, I.K.J.; Poynten, I.M.; Cornall, A.; Templeton, D.J.; Molano, M.; Garland, S.M.; Fairley, C.K.; Law, C.; Hillman, R.J.; Polizzotto, M.N.; et al. Sexual behaviours associated with incident high-risk anal human papillomavirus among gay and bisexual men. Sex. Transm. Infect. 2020. [Google Scholar] [CrossRef]
- Farahmand, M.; Monavari, S.H.; Tavakoli, A. Prevalence and genotype distribution of human papillomavirus infection in different anatomical sites among men who have sex with men: A systematic review and meta-analysis. Rev. Med. Virol. 2021. [Google Scholar] [CrossRef]
- Cranston, R.D.; Carballo-Diéguez, A.; Gundacker, H.; Richardson, B.A.; Giguere, R.; Dolezal, C.; Siegel, A.; KunjaraNaAyudhya, R.P.; Gomez, K.; Piper, J.M.; et al. Prevalence and determinants of anal human papillomavirus infection in men who have sex with men and transgender women. Int. J. STD AIDS 2018, 30, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Rullo, E.V.; Ceccarelli, M.; D’Andrea, F.; Coco, M.; Micali, C.; Cacopardo, B.; Marino, A.; Cannavò, S.P.; Di Rosa, M.; et al. Malignant melanoma in HIV: Epidemiology, pathogenesis, and management. Dermatol. Ther. 2019, 33, e13180. [Google Scholar] [CrossRef]
- D’Andrea, F.; Ceccarelli, M.; Facciolà, A.; Nunnari, G.; Pellicanò, G.F.; Venanzi Rullo, E. Breast cancer in women living with HIV. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1158–1164. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Rullo, E.V.; Vaccaro, M.; Facciolà, A.; D’Aleo, F.; Paolucci, I.A.; Cannavò, S.P.; Cacopardo, B.; Pinzone, M.R.; Pellicano’, G.F.; et al. HIV-associated psoriasis: Epidemiology, pathogenesis, and management. Dermatol. Ther. 2018, 32, e12806. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Rullo, E.V.; Facciolà, A.; Madeddu, G.; Cacopardo, B.; Taibi, R.; D’Aleo, F.; Pinzone, M.R.; Picerno, I.; Di Rosa, M.; et al. Head and neck squamous cell carcinoma and its correlation with human papillomavirus in people living with HIV: A systematic review. Oncotarget 2018, 9, 17171–17180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Facciolà, A.; Venanzi Rullo, E.; Ceccarelli, M.; D’Aleo, F.; Di Rosa, M.; Pinzone, M.R.; Condorelli, F.; Visalli, G.; Picerno, I.; Fisichella, R.; et al. Kaposi’s sarcoma in HIV-infected patients in the era of new antiretrovirals. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5868–5869. [Google Scholar] [CrossRef]
- D’Aleo, F.; Ceccarelli, M.; Venanzi Rullo, E.; Facciolà, A.; Di Rosa, M.; Pinzone, M.R.; Condorelli, F.; Visalli, G.; Picerno, I.; Berretta, M.; et al. Hepatitis C-related hepatocellular carcinoma: Diagnostic and therapeutic management in HIV-patients. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5859–5867. [Google Scholar] [CrossRef] [PubMed]
- United Nations Programme on HIV/AIDS (UNAIDS). Global HIV and AIDS Statistics-2020 Factsheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 13 June 2021).
- Borges, H. Combination antiretroviral therapy and cancer risk. Curr. Opin. HIV AIDS 2017, 12, 12–19. [Google Scholar] [CrossRef]
- Trickey, A.; May, M.T.; Vehreschild, J.; Obel, N.; Gill, M.J.; Crane, H.; Boesecke, C.; Samji, H.; Grabar, S.; Cazanave, C.; et al. Cause-Specific Mortality in HIV-Positive Patients Who Survived Ten Years after Starting Antiretroviral Therapy. PLoS ONE 2016, 11, e0160460. [Google Scholar] [CrossRef] [PubMed]
- Trickey, A.; May, M.T.; Gill, M.J.; Grabar, S.; Vehreschild, J.; Wit, F.W.; Bonnet, F.; Cavassini, M.; Abgrall, S.; Berenguer, J.; et al. Cause-specific mortality after diagnosis of cancer among HIV-positive patients: A collaborative analysis of cohort studies. Int. J. Cancer 2020, 146, 3134–3146. [Google Scholar] [CrossRef]
- Beachler, D.C.; Abraham, A.G.; Silverberg, M.J.; Jing, Y.; Fakhry, C.; Gill, M.J.; Dubrow, R.; Kitahata, M.M.; Klein, M.B.; Burchell, A.N.; et al. Incidence and risk factors of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma in HIV-infected individuals. Oral Oncol. 2014, 50, 1169–1176. [Google Scholar] [CrossRef]
- Chaitanya, N.C.S.K.; Allam, N.S.J.; Babu, D.B.G.; Waghray, S.; Badam, R.K.; Lavanya, R. Systematic meta-analysis on association of human papilloma virus and oral cancer. J. Cancer Res. Ther. 2016, 12, 969–974. [Google Scholar] [CrossRef]
- Schache, A.G.; Powell, N.G.; Cuschieri, K.S.; Robinson, M.; Leary, S.; Mehanna, H.; Rapozo, D.; Long, A.; Cubie, H.; Junor, E.; et al. HPV-Related Oropharynx Cancer in the United Kingdom: An Evolution in the Understanding of Disease Etiology. Cancer Res. 2016, 76, 6598–6606. [Google Scholar] [CrossRef]
- Parisi, S.G.; Cruciani, M.; Scaggiante, R.; Boldrin, C.; Andreis, S.; Bello, F.D.; Pagni, S.; Barelli, A.; Sattin, A.; Mengoli, C.; et al. Anal and oral human papillomavirus (HPV) infection in HIV-infected subjects in northern Italy: A longitudinal cohort study among men who have sex with men. BMC Infect. Dis. 2011, 11, 150. [Google Scholar] [CrossRef]
- Read, T.R.H.; Hocking, J.; Vodstrcil, L.; Tabrizi, S.N.; McCullough, M.; Grulich, A.E.; Garland, S.M.; Bradshaw, C.; Chen, M.Y.; Fairley, C.K. Oral Human Papillomavirus in Men Having Sex with Men: Risk-Factors and Sampling. PLoS ONE 2012, 7, e49324. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Tamburro, M.; Falasca, K.; Sammarco, M.L.; Ripabelli, G.; Vecchiet, J. Prevalence of anal, oral, penile and urethral Human Papillomavirus in HIV infected and HIV uninfected men who have sex with men. J. Med. Virol. 2017, 90, 358–366. [Google Scholar] [CrossRef]
- Beachler, D.C.; Sugar, E.A.; Margolick, J.B.; Weber, K.M.; Strickler, H.D.; Wiley, D.J.; Cranston, R.D.; Burk, R.D.; Minkoff, H.; Reddy, S.; et al. Risk Factors for Acquisition and Clearance of Oral Human Papillomavirus Infection Among HIV-Infected and HIV-Uninfected Adults. Am. J. Epidemiol. 2014, 181, 40–53. [Google Scholar] [CrossRef] [PubMed]
- King, E.M.; Oomeer, S.; Gilson, R.; Copas, A.; Beddows, S.; Soldan, K.; Jit, M.; Edmunds, W.J.; Sonnenberg, P. Oral Human Papillomavirus Infection in Men Who Have Sex with Men: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0157976. [Google Scholar] [CrossRef] [PubMed]
- Echelman, D.; Feldman, S. Management of cervical pre cancers: A global perspective. Hematol. Oncol. Clin. N. Am. 2012, 26, 31–44. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Hirschhorn, J.; Zhao, Z.; Davis, M.R.; Feldman, S. Human Papillomavirus and Its Testing As-says, Cervical Cancer Screening, and Vaccination, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 81. [Google Scholar]
- Steben, M.; Duarte-Franco, E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol. Oncol. 2007, 107, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Trottier, H.; Franco, E.L. The epidemiology of genital human papillomavirus infection. Vaccine 2006, 24, S4–S15. [Google Scholar] [CrossRef] [PubMed]
- Gheit, T.; Simões, R.T.; Tommasino, M.; Donadi, E.; Gonçalves, M.A.G. Brief report HPV16 Variants in Squamous Intraepithelial Lesions in Human Immunodeficiency Virus–Negative and –Positive Brazilian Women. Viral Immunol. 2006, 19, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G. Clinical implications of the interaction between HPV and HIV infections. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 47, 95–106. [Google Scholar] [CrossRef]
- Remis, R.S.; Liu, J.; Loutfy, M.; Tharao, W.; Rebbapragada, A.; Perusini, S.J.; Chieza, L.; Saunders, M.; Green-Walker, L.; Kaul, R. The epidemiology of sexually transmitted co-infections in HIV-positive and HIV-negative African-Caribbean women in Toronto. BMC Infect. Dis. 2013, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M.; Herrera, R.; Chin-Hong, P.; Veluppillai, P.; Greenspan, D.; Berry, J.M.; Pilcher, C.D.; Shiboski, C.H.; Jay, N.; Rubin, M.; et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 2013, 446, 378–388. [Google Scholar] [CrossRef]
- Silverberg, M.J.; Ahdieh, L.; Munoz, A.; Anastos, K.; Burk, R.D.; Cu-Uvin, S.; Duerr, A.; Greenblatt, R.M.; Klein, R.S.; Massad, S.; et al. The Impact of HIV Infection and Immunodeficiency on Human Papillomavirus Type 6 or 11 Infection and on Genital Warts. Sex. Transm. Dis. 2002, 29, 427–435. [Google Scholar] [CrossRef]
- Hawes, S.E.; Critchlow, C.W.; Sow, P.S.; Touré, P.; N’Doye, I.; Diop, A.; Kuypers, J.M.; Kasse, A.A.; Kiviat, N.B. Incident High-Grade Squamous Intraepithelial Lesions in Senegalese Women with and Without Human Immunodeficiency Virus Type 1 (HIV-1) and HIV-2. J. Natl. Cancer Inst. 2006, 98, 100–109. [Google Scholar] [CrossRef]
- Massad, L.S.; Xie, X.; D’Souza, G.; Darragh, T.M.; Minkoff, H.; Wright, R.; Colie, C.; Sanchez-Keeland, L.; Strickler, H.D. Incidence of cervical precancers among HIV-seropositive women. Am. J. Obstet. Gynecol. 2014, 212, 606-e1. [Google Scholar] [CrossRef]
- Ahdieh, L.; Klein, R.S.; Burk, R.; Cu-Uvin, S.; Schuman, P.; Duerr, A.; Safaeian, M.; Astemborski, J.; Daniel, R.; Shah, K. Prevalence, Incidence, and Type-Specific Persistence of Human Papillomavirus in Human Immunodeficiency Virus (HIV)-Positive and HIV-Negative Women. J. Infect. Dis. 2001, 184, 682–690. [Google Scholar] [CrossRef]
- Clifford, G.M.; de Vuyst, H.; Tenet, V.; Plummer, M.; Tully, S.; Franceschi, S. Effect of HIV Infection on Human Papillomavirus Types Causing Invasive Cervical Cancer in Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 73, 332–339. [Google Scholar] [CrossRef]
- Strickler, H.D.; Palefsky, J.M.; Shah, K.V.; Anastos, K.; Klein, R.S.; Minkoff, H.; Duerr, A.; Massad, L.S.; Celentano, D.D.; Hall, C.; et al. Human Papillomavirus Type 16 and Immune Status in Human Immunodeficiency Virus-Seropositive Women. J. Natl. Cancer Inst. 2003, 95, 1062–1071. [Google Scholar] [CrossRef]
- Massad, L.S.; Xie, X.; Burk, R.D.; D’Souza, G.; Darragh, T.M.; Minkoff, H.; Colie, C.; Burian, P.; Palefsky, J.; Atrio, J.; et al. Association of cervical precancer with human papillomavirus types other than 16 among HIV co-infected women. Am. J. Obstet. Gynecol. 2015, 214, 354-e1. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.; Webster-Cyriaque, J.; Syrianen, S.; Chattopadyay, A.; Sroussi, H.; Zhang, L.; Kaushal, A. Mechanisms of Viral Infections Associated with HIV. Adv. Dent. Res. 2011, 23, 130–136. [Google Scholar] [CrossRef]
- Cameron, J.; Mercante, D.; O’Brien, M.; Gaffga, A.M.; Leigh, J.E.; Fidel, P.L.; Hagensee, M.E. The Impact of Highly Active Antiretroviral Therapy and Immunodeficiency on Human Papillomavirus Infection of the Oral Cavity of Human Immunodeficiency Virus–Seropositive Adults. Sex. Transm. Dis. 2005, 32, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, Z.; Zhang, H.; Li, X.; Yu, Q.; Lin, H.; Yang, W. HIV-1 Tat protein alter the tight junction integrity and function of retinal pigment epithelium: An in vitro study. BMC Infect. Dis. 2008, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Smart, E.J.; Weksler, B.B.; Couraud, P.-O.; Hennig, B.; Toborek, M. Caveolin-1 Regulates Human Immunodeficiency Virus-1 Tat-Induced Alterations of Tight Junction Protein Expression via Modulation of the Ras Signaling. J. Neurosci. 2008, 28, 7788–7796. [Google Scholar] [CrossRef]
- Kanmogne, G.D.; Schall, K.; Leibhart, J.; Knipe, B.; Gendelman, H.E.; Persidsky, Y. HIV-1 gp120 Compromises Blood–Brain Barrier Integrity and Enhance Monocyte Migration across Blood–Brain Barrier: Implication for Viral Neuropathogenesis. J. Cereb. Blood Flow Metab. 2006, 27, 123–134. [Google Scholar] [CrossRef]
- Nakamuta, S.; Endo, H.; Higashi, Y.; Kousaka, A.; Yamada, H.; Yano, M.; Kido, H. Human immunodeficiency virus type 1 gp120–mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J. Neurovirol. 2008, 14, 186–195. [Google Scholar] [CrossRef]
- Kim, R.H.; Yochim, J.M.; Kang, M.; Shin, K.H.; Christensen, R.; Park, N.H. Parkash HIV-1 Tat enhances replicative potential of human oral keratinocytes harboring HPV-16 genome. Int. J. Oncol. 1992, 33, 777–782. [Google Scholar] [CrossRef]
- András, I.E.; Pu, H.; Tian, J.; Deli, M.; Nath, A.; Hennig, B.; Toborek, M. Signaling Mechanisms of HIV-1 Tat-Induced Alterations of Claudin-5 Expression in Brain Endothelial Cells. J. Cereb. Blood Flow Metab. 2005, 25, 1159–1170. [Google Scholar] [CrossRef]
- Pu, H.; Tian, J.; Andras, I.E.; Hayashi, K.; Flora, G.; Hennig, B.; Toborek, M. HIV-1 Tat Protein-Induced Alterations of ZO-1 Expression are Mediated by Redox-Regulated ERK1/2 Activation. J. Cereb. Blood Flow Metab. 2005, 25, 1325–1335. [Google Scholar] [CrossRef]
- Minkoff, H.; Zhong, Y.; Burk, R.D.; Palefsky, J.M.; Xue, X.; Watts, D.H.; Levine, A.M.; Wright, R.L.; Colie, C.; D’Souza, G.; et al. Influence of Adherent and Effective Antiretroviral Therapy Use on Human Papillomavirus Infection and Squamous Intraepithelial Lesions in Human Immunodeficiency Virus–Positive Women. J. Infect. Dis. 2010, 201, 681–690. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, V.; Lima, I.; Ziegelmann, P.; Paranhos, L.R.; Matos, F. Impact of highly active antiretroviral therapy on the prevalence of oral lesions in HIV-positive patients: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 1497–1504. [Google Scholar] [CrossRef]
- Toljić, B.; Trbovich, A.M.; Petrović, S.M.; Kannosh, I.Y.; Dragović, G.; Jevtović, D.; De Luka, S.R.; Ristić-Djurović, J.L.; Milašin, J. Ageing with HIV—A periodontal perspective. New Microbiol. 2018, 41, 61–66. [Google Scholar]
- Clifford, G.M.; Franceschi, S.; Keiser, O.; Schöni-Affolter, F.; Lise, M.; Dehler, S.; Levi, F.; Mousavi, M.; Bouchardy, C.; Wolfensberger, A.; et al. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: A nested case-control study in the Swiss HIV cohort study. Int. J. Cancer 2015, 138, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Zeier, M.D.; Botha, M.H.; Van Der Merwe, F.H.; Eshun-Wilson, I.; Van Schalkwyk, M.; La Grange, M.; Mason, D.; Louw, M.; Nachega, J. Progression and Persistence of Low-Grade Cervical Squamous Intraepithelial Lesions in Women Living with Human Immunodeficiency Virus. J. Low. Genit. Tract Dis. 2012, 16, 243–250. [Google Scholar] [CrossRef]
- Dries, L.V.D.; Claassen, M.A.; Groothuismink, Z.M.; van Gorp, E.; Boonstra, A. Immune activation in prolonged cART-suppressed HIV patients is comparable to that of healthy controls. Virology 2017, 509, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Palefsky, J. Human papillomavirus-related disease in people with HIV. Curr. Opin. HIV AIDS 2009, 4, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Palefsky, J.M.; Holly, E.A.; Efirdc, J.T.; Da Costa, M.; Jay, N.; Berry, J.M.; Darragh, T.M. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS 2005, 19, 1407–1414. [Google Scholar] [CrossRef]
- Hessol, N.A.; Holly, E.A.; Efird, J.T.; Minkoff, H.; Schowalter, K.; Darragh, T.M.; Burk, R.D.; Strickler, H.D.; Greenblatt, R.M.; Palefsky, J.M. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS 2009, 23, 59–70. [Google Scholar] [CrossRef] [PubMed]
- De Pokomandy, A.; Rouleau, D.; Ghattas, G.; Vézina, S.; Coté, P.; Macleod, J.; Allaire, G.; Franco, E.; Coutlée, F. Prevalence, Clearance, and Incidence of Anal Human Papillomavirus Infection in HIV-Infected Men: The HIPVIRG Cohort Study. J. Infect. Dis. 2009, 199, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Saavedra, G.; Flores-Moreno, B.; Carranca, A.M.G.; Irigoyen-Camacho, E.; Guido-Jiménez, M.; Ramírez-Amador, V. HPV oral lesions in HIV-infected patients: The impact of long-term HAART. J. Oral Pathol. Med. 2012, 42, 443–449. [Google Scholar] [CrossRef] [PubMed]

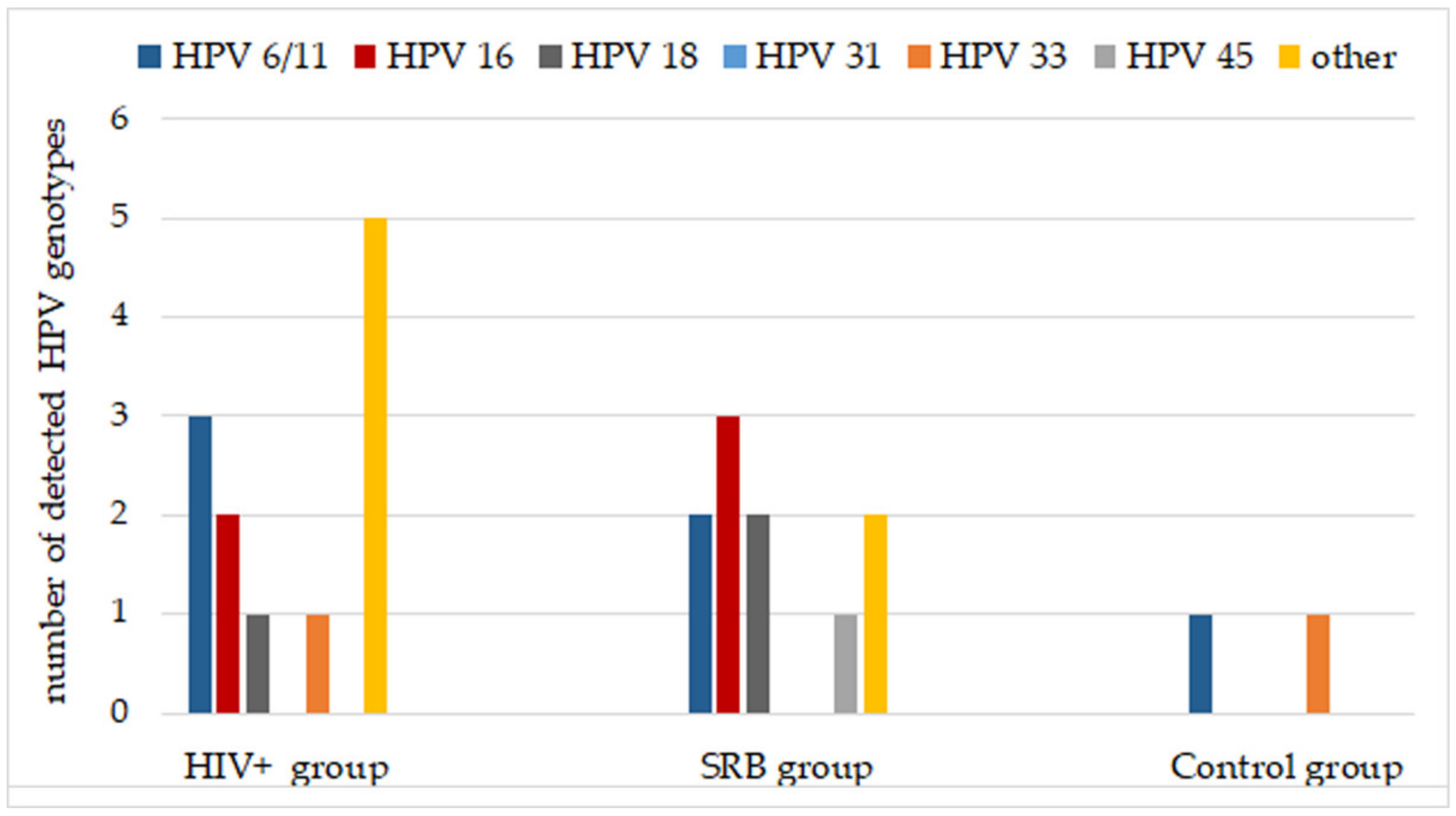
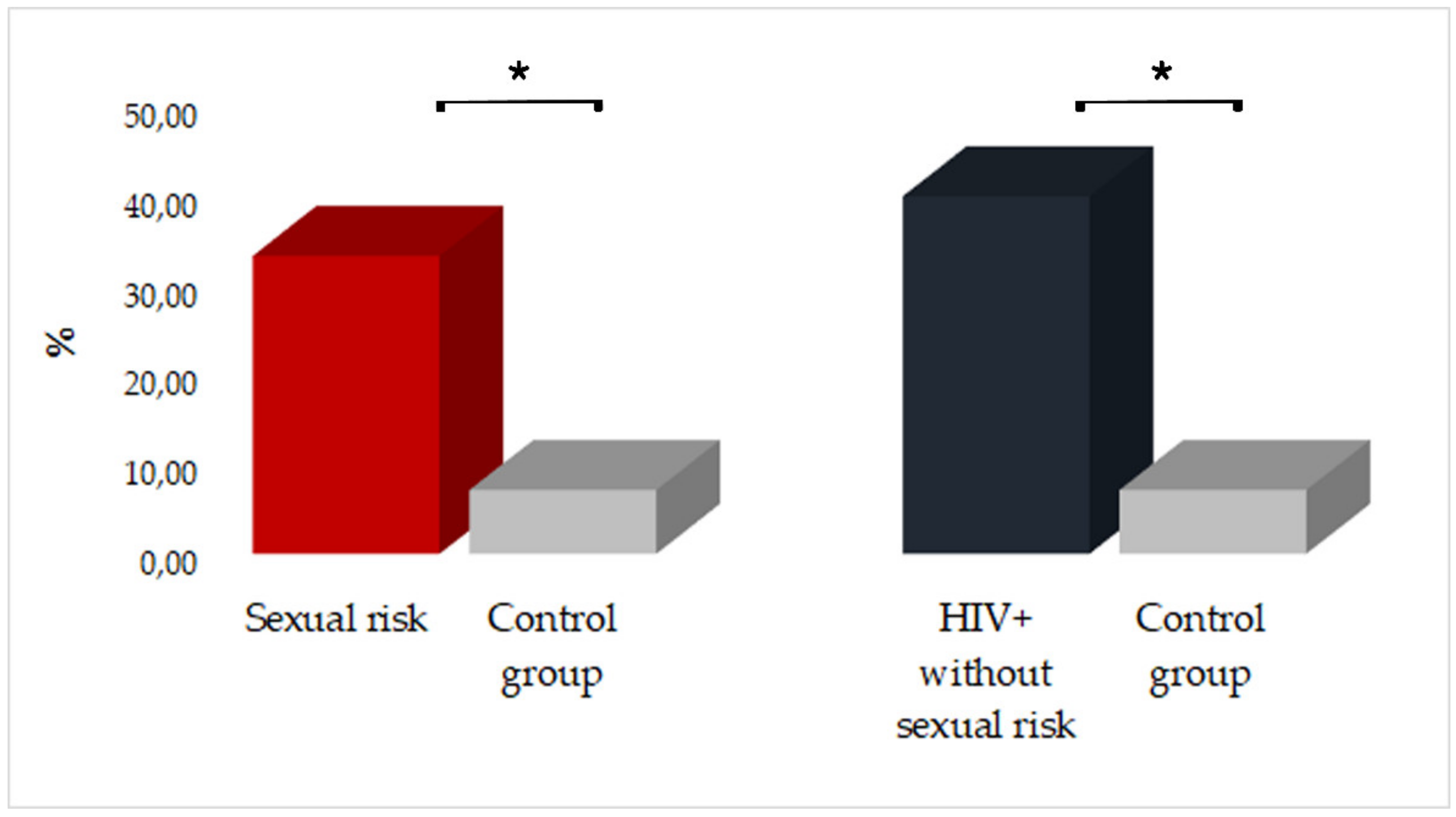
| HIV+ Group | SRB Group | Control Group | ||
|---|---|---|---|---|
| Without Sexual Risk | With Sexual Risk | |||
| N° SAMPLES | 15 | 28 | 21 | 28 |
| GENDER | ||||
| Men | 78.57% | 85.71% | 66.67% | 65.32% |
| Women | 21.43% | 14.29% | 33.33% | 34.68% |
| MEAN AGE (DS) | 41.36 (11.73) | 38.25 (10.18) | 28.17 (8.33) | 34.33 (10.29) |
| min–max | 24–59 | 22–59 | 19–51 | 25–54 |
| NATIONALITY | ||||
| Italian | 71.43% | 95.24% | 95.83% | 96.43% |
| Foreigners | 28.57% | 4.76% | 4.17% | 3.57% |
| EDUCATIONAL LEVEL | ||||
| Elementary school | 7.14% | 0.00% | 0.00% | 0.00% |
| Middle school | 28.57% | 23.81% | 4.17% | 0.00% |
| High school | 50.00% | 42.86% | 62.50% | 21.43% |
| University | 14.29% | 33.33% | 33.34% | 78.57% |
| SEXUAL HABITS | ||||
| Heterosexual | 64.29% | 33.33% | 54.17% | 100% |
| Homosexual | 21.43% | 57.14% | 33.33% | |
| Bisexual | 14.28% | 9.52% | 12.50% | |
| β Value | Standard Error of β Value | p Level | |
|---|---|---|---|
| Gender | 0.101 | 0.182 | 0.584 |
| Age | −0.349 | 0.189 | 0.078 |
| Nationality | −0.282 | 0.170 | 0.111 |
| Education level | −0.042 | 0.163 | 0.797 |
| Sexual orientation | −0.053 | 0.204 | 0.798 |
| SRB | −0.132 | 0.174 | 0.454 |
| CD4+ count | 0.203 | 0.170 | 0.245 |
| HIV viral load | 0.470 | 0.202 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visalli, G.; Di Pietro, A.; Currò, M.; Pruiti Ciarello, M.; D’Andrea, F.; Nunnari, G.; Pellicanò, G.F.; Facciolà, A. How Much Does HIV Positivity Affect the Presence of Oral HPV? A Molecular Epidemiology Survey. Int. J. Environ. Res. Public Health 2021, 18, 8999. https://doi.org/10.3390/ijerph18178999
Visalli G, Di Pietro A, Currò M, Pruiti Ciarello M, D’Andrea F, Nunnari G, Pellicanò GF, Facciolà A. How Much Does HIV Positivity Affect the Presence of Oral HPV? A Molecular Epidemiology Survey. International Journal of Environmental Research and Public Health. 2021; 18(17):8999. https://doi.org/10.3390/ijerph18178999
Chicago/Turabian StyleVisalli, Giuseppa, Angela Di Pietro, Monica Currò, Marianna Pruiti Ciarello, Flavia D’Andrea, Giuseppe Nunnari, Giovanni Francesco Pellicanò, and Alessio Facciolà. 2021. "How Much Does HIV Positivity Affect the Presence of Oral HPV? A Molecular Epidemiology Survey" International Journal of Environmental Research and Public Health 18, no. 17: 8999. https://doi.org/10.3390/ijerph18178999
APA StyleVisalli, G., Di Pietro, A., Currò, M., Pruiti Ciarello, M., D’Andrea, F., Nunnari, G., Pellicanò, G. F., & Facciolà, A. (2021). How Much Does HIV Positivity Affect the Presence of Oral HPV? A Molecular Epidemiology Survey. International Journal of Environmental Research and Public Health, 18(17), 8999. https://doi.org/10.3390/ijerph18178999








