Risk Prediction of Second Primary Endometrial Cancer in Obese Women: A Hospital-Based Cancer Registry Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Njoku, K.; Abiola, J.; Russell, J.; Crosbie, E.J. Endometrial cancer prevention in high-risk women. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 65, 66–78. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Zwahlen, M.; Kitchener, H.C.; Egger, M.; Renehan, A.G. Body mass index, hormone replacement therapy and endometrial cancer risk: A meta-analysis. Cancer Epidemiol. Prev. Biomark. 2010, 19, 3119–3130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine learning in medicine: A practical introduction. BMC Med. Res. Methodol. 2019, 19, 64. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 1998. [Google Scholar]
- Pham, T.-H.; Vicnesh, J.; Wei, J.K.E.; Oh, S.L.; Arunkumar, N.; Abdulhay, E.W.; Ciaccio, E.J.; Acharya, U.R. Autism spectrum disorder diagnostic system using HOS bispectrum with EEG signals. Int. J. Environ. Res. Public Health 2020, 17, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinbaum, D.G.; Klein, M. Introduction to logistic regression. In Logistic Regression: A Self-Learning Text; Kleinbaum, D.G., Klein, M., Eds.; Springer: New York, NY, USA, 2010; pp. 1–39. [Google Scholar]
- Levy, J.J.; O’Malley, A.J. Don’t dismiss logistic regression: The case for sensible extraction of interactions in the era of machine learning. BMC Med. Res. Methodol. 2020, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, J.R.C. Programs for Machine Learning; Morgan Kaufmann Publishers: Burlington, MA, USA, 1993; Volume 4. [Google Scholar]
- Dima, S.; Wang, K.-J.; Chen, K.-H.; Huang, Y.-K.; Chang, W.-J.; Lee, S.-Y.; Teng, N.-C. Decision tree approach to the impact of parents’ oral health on dental caries experience in children: A cross-sectional study. Int. J. Environ. Res. Public Health 2018, 15, 692. [Google Scholar] [CrossRef] [Green Version]
- Esmaily, H.; Tayefi, M.; Doosti, H.; Ghayour-Mobarhan, M.; Nezami, H.; Amirabadizadeh, A. A comparison between decision tree and random forest in determining the risk factors associated with Type 2 diabetes. J. Res. Health Sci. 2018, 18, e00412. [Google Scholar] [PubMed]
- Peng, J.; Chen, C.; Zhou, M.; Xie, X.; Zhou, Y.; Luo, C.H. A machine-learning approach to forecast aggravation risk in patients with acute exacerbation of chronic obstructive pulmonary disease with clinical indicators. Sci. Rep. 2020, 10, 3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth Publishing: Belmont, CA, USA, 1984. [Google Scholar]
- Sun, Z.; Wang, J.; Chen, Y.; Lu, H. Influence factors on injury severity of traffic accidents and differences in urban functional zones: The empirical analysis of Beijing. Int. J. Environ. Res. Public Health 2018, 15, 2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, C.-C.; Lu, C.-J.; Chen, G.-D.; Chang, C.-C. Risk Prediction for Early Chronic Kidney Disease: Results from an Adult Health Examination Program of 19,270 Individuals. Int. J. Environ. Res. Public Health 2020, 17, 4973. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.J.; Chang, C.C.; Lu, C.J.; Chen, G.D. Application of machine learning to predict the recurrence proneness for cervical cancer. Neural Comput. Appl. 2014, 24, 1311–1316. [Google Scholar] [CrossRef]
- Tseng, C.J.; Lu, C.J.; Chang, C.C.; Chen, G.D.; Cheewakriangkrai, C. Integration of data mining classification techniques and ensemble learning to identify risk factors and diagnose ovarian cancer recurrence. Artif. Intell. Med. 2017, 78, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraga, J.; Nakamura, K.; Haruma, T.; Nyuya, A.; Nagasaka, T.; Masuyama, H. Molecular characterization of second primary endometrial cancer. Anticancer Res. 2020, 40, 3811–3818. [Google Scholar] [CrossRef] [PubMed]
- Gerber, B.; Krause, A.; Muller, H.; Reimer, T.; Külz, T.; Kundt, G.; Friese, K. Ultrasonographic detection of asymptomatic endometrial cancer in postmenopausal patients offers no prognostic advantage over symptomatic disease discovered by uterine bleeding. Eur. J. Cancer 2001, 37, 64–71. [Google Scholar] [CrossRef]
- Potischman, N.; Hoover, R.N.; Brinton, L.A.; Siiteri, P.; Dorgan, J.F.; Swanson, C.A.; Berman, M.L.; Mortel, R.; Twiggs, L.B.; Barrett, R.J.; et al. Case-control study of endogenous steroid hormones and endometrial cancer. J. Natl. Cancer Inst. 1996, 88, 1127–1135. [Google Scholar] [CrossRef]
- Suvanto-Luukkonen, E.; Sundstrom, H.; Penttinen, J.; Kauppila, A.; Rutanen, E.M. Insulin-like growth factor-binding protein-1: A biochemical marker of endometrial response to progestin during hormone replacement therapy. Maturitas 1995, 22, 255–262. [Google Scholar] [CrossRef]
- Ayabe, T.; Tsutsumi, O.; Sakai, H.; Yoshikawa, H.; Yano, T.; Kurimoto, F.; Taketani, Y. Increased circulating levels of insulin-like growth factor-I and decreased circulating levels of insulin-like growth factor binding protein-1 in postmenopausal women with endometrial cancer. Endocr. J. 1997, 44, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Adesanya, O.O.; Zhou, J.; Samathanam, C.; Powell-Braxton, L.; Bondy, C.A. Insulin-like growth factor 1 is required for G2 progression in the estradiol-induced mitotic cycle. Proc. Natl. Acad. Sci. USA 1999, 96, 3287–3291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, J.V., Jr.; Potischman, N.; Madigan, M.P.; Berman, M.L.; Mortel, R.; Twiggs, L.B.; Barrett, R.J.; Wilbanks, G.D.; Lurain, J.R.; Fillmore, C.-M.; et al. Insulin-like growth factors, insulin-like growth factor-binding proteins, and endometrial cancer in postmenopausal women: Results from a US case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 607–612. [Google Scholar]
- Ogawa, C.; Nakamura, K.; Matsuoka, H.; Matsubara, Y.; Haraga, J.; Masuyama, H. Risk of gynecologic cancer as second versus first primary cancer in Japan. Acta Med. Okayama 2020, 74, 109–114. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.; Keeney, G.; Haddock, M.G.; Calori, G.; Podratz, K.C. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am. J. Obstet. Gynecol. 2000, 182, 1506–1519. [Google Scholar]
| Rank | Variable Name |
|---|---|
| 1 | Clinical stage group |
| 2 | Tumor size |
| 2 | Pathologic stage group |
| 2 | Date of first surgical procedure |
| 5 | BMI |
| 6 | Age at diagnosis |
| 7 | Sequence of locoregional therapy and systemic therapy |
| 8 | Grade/differentiation |
| 8 | Surgical margins of the primary site |
| 10 | Sequence of RT and surgery |
| Characteristics | Endometrial Cancer (N = 1560) | ||
|---|---|---|---|
| Without SPEC | With SPEC | p-Value | |
| N (%) | 1040 (66.7%) | 520 (33.3%) | |
| Age at Diagnosis (years) | <0.001 ** | ||
| <50 | 372 (35.7%) | 140 (26.9%) | |
| ≥50 | 668 (64.3%) | 380 (73.1%) | |
| Grade/Differentiation | 0.014 * | ||
| 1, 2 | 705 (67.8%) | 320 (61.5%) | |
| Others | 335 (32.2%) | 200 (38.5%) | |
| Tumor Size (cm) | <0.001 ** | ||
| <2 | 262 (25.2%) | 220 (42.3%) | |
| ≥2 c | 778 (74.8%) | 300 (57.7%) | |
| Clinical Stage | <0.001 ** | ||
| <II | 838 (80.6%) | 280 (53.8%) | |
| ≥II | 202 (19.4%) | 240 (46.2%) | |
| Pathologic Stage | <0.001 ** | ||
| <II | 834 (80.2%) | 480 (92.3%) | |
| ≥II | 206 (19.8%) | 40 (7.7%) | |
| Surgical Margin Involvement | 0.405 | ||
| No | 947 (91.1%) | 480 (92.3%) | |
| Yes | 93 (8.9%) | 40 (7.7%) | |
| Surgical Procedure | <0.001 ** | ||
| No | 40 (3.8%) | 0 (0.0%) | |
| Yes | 1000 (96.2%) | 520 (100.0%) | |
| Sequence of Radiotherapy/Surgery | 0.689 | ||
| No | 611 (58.8%) | 300 (42.9%) | |
| Yes | 429 (41.2%) | 220 (57.1%) | |
| Sequence of Locoregional/Systemic Therapy | <0.001 ** | ||
| No | 740 (71.2%) | 320 (57.7%) | |
| Yes | 300 (28.8%) | 200 (42.3%) | |
| BMI (kg/m2) | 0.001 * | ||
| ≤25 | 464 (44.6%) | 280 (53.8%) | |
| >25 | 576 (55.4%) | 240 (46.2%) | |
| Variable | Name | Definition of Normal Test Data |
|---|---|---|
| X1 | Age at diagnosis (years) | <50/≥50 |
| X2 | Grade/differentiation | ≤2/>2 |
| X3 | Tumor size (cm) | <2/≥2 |
| X4 | Clinical stage group | <II/≥II |
| X5 | Pathologic stage group | <II/≥II |
| X6 | Surgical margins of the primary site | No/Yes |
| X7 | Date of first surgical procedure | No/Yes |
| X8 | Sequence of RT and surgery | No/Yes |
| X9 | Sequence of locoregional therapy and systemic therapy (chemotherapy) | No/Yes |
| X10 | BMI (kg/m2) | ≤25/>25 |
| Y | SPEC | No/Yes |
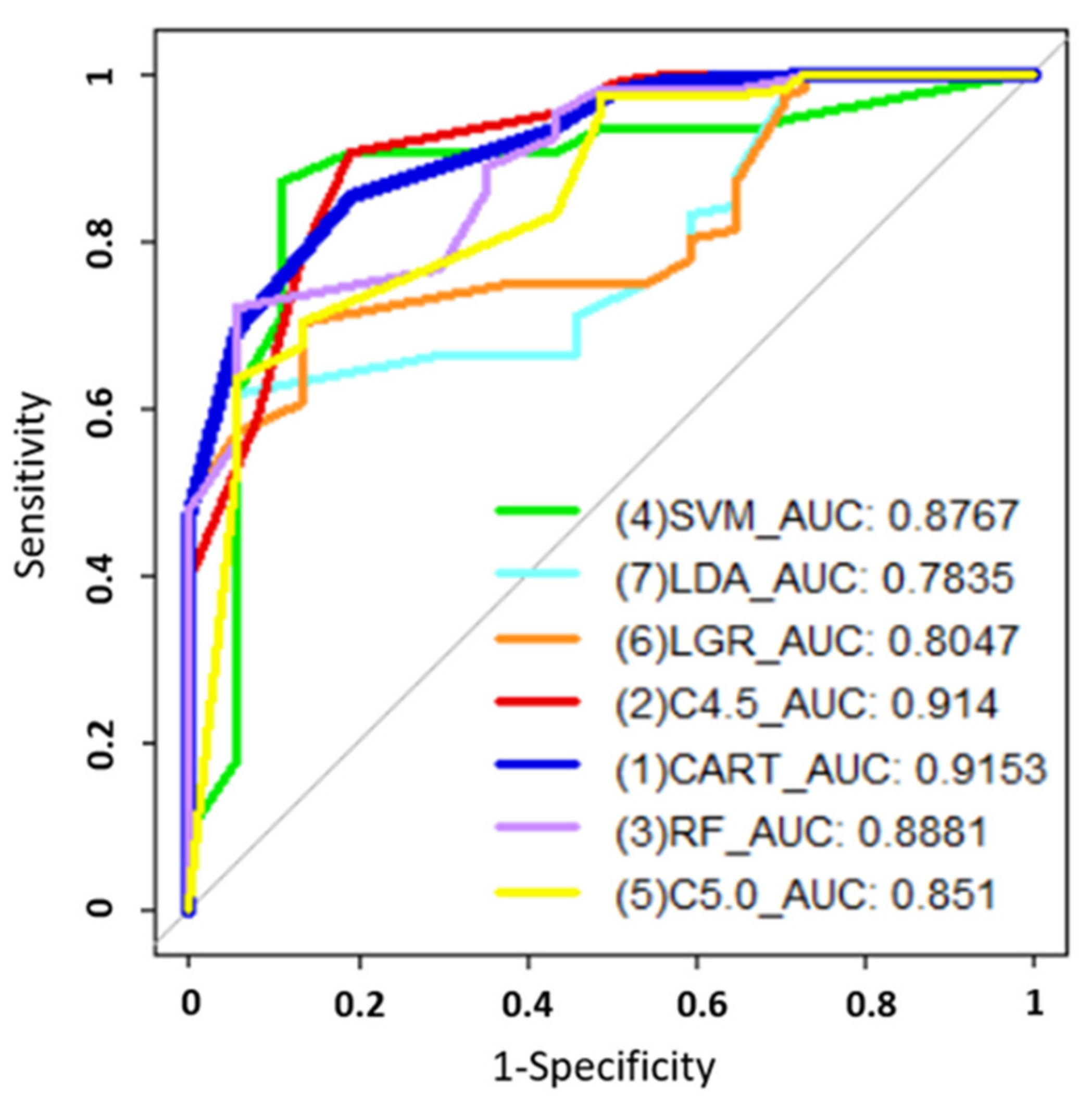
| Method | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| SVM | 0.875 | 0.8919 | 0.8692 | 0.8767 |
| LDA | 0.7014 | 0.9459 | 0.6168 | 0.7835 |
| LGR | 0.7431 | 0.8649 | 0.7009 | 0.8047 |
| C4.5 | 0.8819 | 0.9065 | 0.8108 | 0.914 |
| CART | 0.8403 | 0.8108 | 0.8505 | 0.9153 |
| RF | 0.7778 | 0.9459 | 0.7196 | 0.8881 |
| C5.0 | 0.7153 | 0.9459 | 0.6355 | 0.851 |
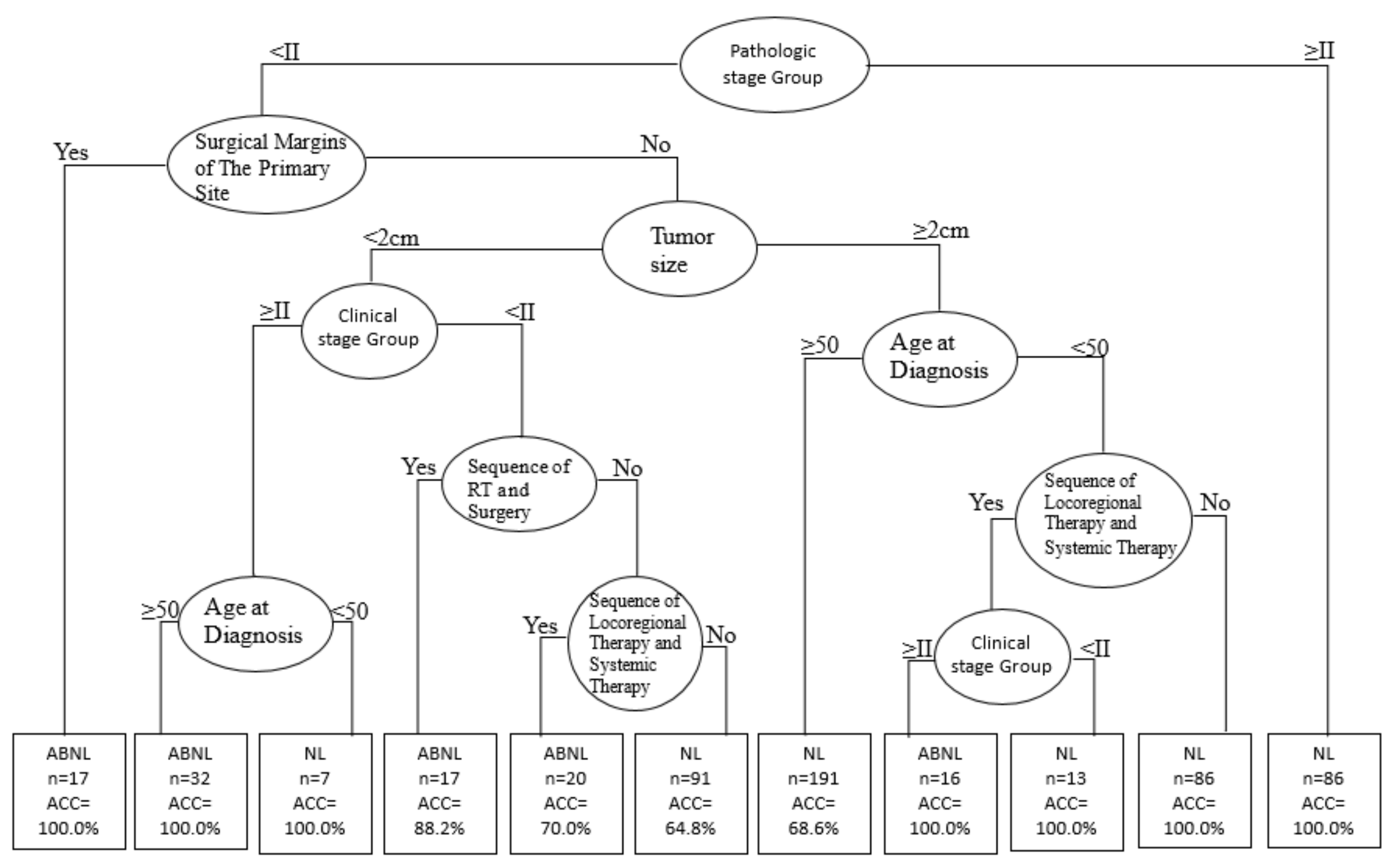
| Rules No. | Combinations of Condition Variables | SPEC/Observed (n) | Accuracy |
|---|---|---|---|
| 1 | Pathologic stage (<II) + Surgical Margins involvement (Yes) | 17/20 | 100.0% |
| 2 | Pathologic stage (<II) + Surgical Margins involvement (No) + Tumor size (<2 cm) + Clinical stage (≥II) + Age at Diagnosis (≥50) | 32/40 | 100.0% |
| 4 | Pathologic stage (<II) + Surgical Margins involvement (No) + Tumor size (<2 cm) + Clinical stage (<II)+ Sequence of Radiotherapy (Yes) | 17/24 | 88.2% |
| 5 | Pathologic stage (<II) + Surgical Margins involvement (No) + Tumor size (<2 cm) + Clinical stage (<II) + Sequence of Radiotherapy (No) + Sequence of Locoregional/Systemic Therapy (Yes) | 20/28 | 70.0% |
| 8 | Pathologic stage (<II) + Surgical Margins involvement (No) + Tumor size (≥2 cm) + Age at Diagnosis (<50) + Sequence of Locoregional/Systemic Therapy (Yes) + Clinical stage (≥II) | 16/20 | 100.0% |
| Characteristics | BMI ≤ 25 kg/m2 | BMI > 25 kg/m2 | ||||
|---|---|---|---|---|---|---|
| Without SPEC | With SPEC | p-Value | Without SPEC | With SPEC | p-Value | |
| N (%) | 560 (66.7%) | 280 (33.3%) | 480 (66.7%) | 240 (33.3%) | ||
| Age at Diagnosis | 0.117 | 0.019 * | ||||
| <50 years | 190 (33.9%) | 200 (71.4%) | 161 (33.5%) | 60 (25.0%) | ||
| ≥50 years | 370 (66.1%) | 80 (28.6%) | 319 (66.5%) | 180 (75.0%) | ||
| Grade/Differentiation | 0.004 | 0.698 | ||||
| 1, 2 | 377 (67.3%) | 160 (57.1%) | 313 (65.2%) | 160 (66.7%) | ||
| Others | 183 (32.7%) | 120 (42.9%) | 167 (34.8%) | 80 (33.3%) | ||
| Tumor Size | 0.144 | <0.001 ** | ||||
| <2 cm | 172 (30.7%) | 100 (35.7%) | 102 (21.3%) | 120 (50.0%) | ||
| ≥2 cm | 388 (69.3%) | 180 (64.3%) | 378 (78.7%) | 120 (50.0%) | ||
| Clinical Stage | <0.001 | <0.001** | ||||
| <II | 463 (82.7%) | 120 (42.9%) | 385 (80.2%) | 160 (66.7%) | ||
| ≥II | 97 (17.3%) | 160 (57.1%) | 95 (19.8%) | 80 (33.3%) | ||
| Pathologic Stage | 0.032 | <0.001 ** | ||||
| <II | 446 (79.6%) | 240 (85.7%) | 384 (80.0%) | 240 (100.0%) | ||
| ≥II | 114 (20.4%) | 40 (14.3%) | 96 (20.0%) | 0 (0.0%) | ||
| Surgical Margins Involved | 0.648 | 0.170 | ||||
| No | 515 (92.0%) | 260 (92.9%) | 424 (88.3%) | 220 (91.7%) | ||
| Yes | 45 (8.0%) | 20 (7.1%) | 56 (11.7%) | 20 (8.3%) | ||
| Surgical Procedures | 0.002 | 0.014 * | ||||
| No | 19 (3.4%) | 0 (0.0%) | 12 (2.5%) | 0(0.0%) | ||
| Yes | 541 (96.6%) | 280 (100.0%) | 468 (97.5%) | 240(100.0%) | ||
| Radiotherapy/Surgery | <0.001 | <0.001 ** | ||||
| No | 332 (3.4%) | 120 (42.9%) | 249 (51.9%) | 180 (75.0%) | ||
| Yes | 228 (96.6%) | 160 (57.1%) | 231 (48.1%) | 60 (25.0%) | ||
| Locoregional/Systemic Therapy | <0.001 | 0.867 | ||||
| No | 402 (71.8%) | 160 (57.1%) | 317 (66.0%) | 160 (66.7%) | ||
| Yes | 158 (28.2%) | 120 (42.9%) | 163 (34.0%) | 80 (33.3%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Chen, C.-C.; Cheewakriangkrai, C.; Chen, Y.C.; Yang, S.-F. Risk Prediction of Second Primary Endometrial Cancer in Obese Women: A Hospital-Based Cancer Registry Study. Int. J. Environ. Res. Public Health 2021, 18, 8997. https://doi.org/10.3390/ijerph18178997
Chang C-C, Chen C-C, Cheewakriangkrai C, Chen YC, Yang S-F. Risk Prediction of Second Primary Endometrial Cancer in Obese Women: A Hospital-Based Cancer Registry Study. International Journal of Environmental Research and Public Health. 2021; 18(17):8997. https://doi.org/10.3390/ijerph18178997
Chicago/Turabian StyleChang, Chi-Chang, Chun-Chia Chen, Chalong Cheewakriangkrai, Ying Chen Chen, and Shun-Fa Yang. 2021. "Risk Prediction of Second Primary Endometrial Cancer in Obese Women: A Hospital-Based Cancer Registry Study" International Journal of Environmental Research and Public Health 18, no. 17: 8997. https://doi.org/10.3390/ijerph18178997
APA StyleChang, C.-C., Chen, C.-C., Cheewakriangkrai, C., Chen, Y. C., & Yang, S.-F. (2021). Risk Prediction of Second Primary Endometrial Cancer in Obese Women: A Hospital-Based Cancer Registry Study. International Journal of Environmental Research and Public Health, 18(17), 8997. https://doi.org/10.3390/ijerph18178997










