Open Issues in the Transition from NAFLD to MAFLD: The Experience of the Plinio Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. MAFLD Diagnosis
2.2. Statistical Analysis
3. Results
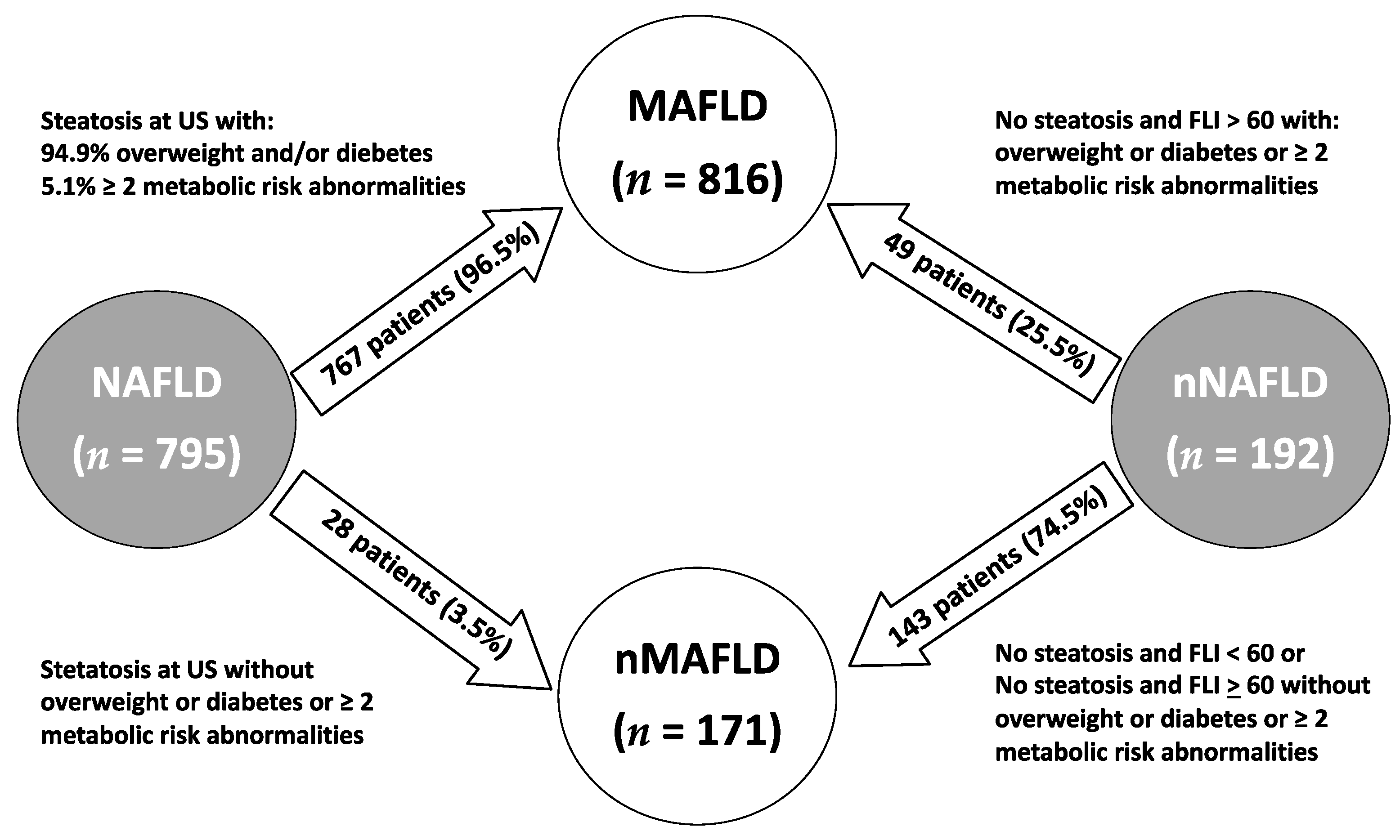
Reclassification Groups
4. Discussion
Study Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [PubMed]
- Cortez-Pinto, H.; Camilo, M.E.; Baptista, A.; De Oliveira, A.G.; De Moura, M.C. Non-alcoholic fatty liver: Another feature of the metabolic syndrome? Clin. Nutr. 1999, 18, 353–358. [Google Scholar] [CrossRef]
- Schaffner, F.; Thaler, H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986, 8, 283–298. [Google Scholar] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- European Association for the Study of The Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Polimeni, L.; Del Ben, M.; Baratta, F.; Perri, L.; Albanese, F.; Pastori, D.; Violi, F.; Angelico, F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J. Hepatol. 2015, 7, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Bartimoccia, S.; Cammisotto, V.; Cocomello, N.; Colantoni, A.; Nocella, C.; Carnevale, R.; Ferro, D.; Angelico, F.; et al. Poor Adherence to Mediterranean Diet and Serum Lipopolysaccharide are Associated with Oxidative Stress in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased liver localization of lipopolysaccharides in human and experimental non-alcoholic fatty liver disease. Hepatology 2020, 72, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Brancorsini, M.; Di Costanzo, A.; D’Erasmo, L.; Baratta, F.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Violi, F.; et al. Non-alcoholic fatty liver disease, metabolic syndrome and patatin-like phospholipase domain-containing protein3 gene variants. Eur. J. Intern. Med. 2014, 25, 566–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Costanzo, A.; Belardinilli, F.; Bailetti, D.; Sponziello, M.; D’Erasmo, L.; Polimeni, L.; Baratta, F.; Pastori, D.; Ceci, F.; Montali, A.; et al. Evaluation of Polygenic Determinants of Non-Alcoholic Fatty Liver Disease (NAFLD) By a Candidate Genes Resequencing Strategy. Sci. Rep. 2018, 8, 3702. [Google Scholar] [CrossRef]
- Carpino, G.; Pastori, D.; Baratta, F.; Overi, D.; Labbadia, G.; Polimeni, L.; Di Costanzo, A.; Pannitteri, G.; Carnevale, R.; Del Ben, M.; et al. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: A possible role for oxidative stress. Sci. Rep. 2017, 7, 15756. [Google Scholar] [CrossRef]
- Di Costanzo, A.; D’Erasmo, L.; Polimeni, L.; Baratta, F.; Coletta, P.; Di Martino, M.; Loffredo, L.; Perri, L.; Ceci, F.; Montali, A.; et al. Non-alcoholic fatty liver disease and subclinical atherosclerosis: A comparison of metabolically- versus genetically-driven excess fat hepatic storage. Atherosclerosis 2017, 257, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Panel, I.C. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Rinella, M.E.; Sanyal, A.; Harrison, S.A.; Brunt, E.; Goodman, Z.; Cohen, D.E.; Loomba, R. From NAFLD to MAFLD: Implications of a premature change in terminology. Hepatology 2021, 73, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Jeong, W.K.; Baik, S.K. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J. Gastroenterol. 2014, 20, 4300–4315. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Bourliere, M.; Penaranda, G.; Renou, C.; Botta-Fridlund, D.; Tran, A.; Portal, I.; Lecomte, L.; Castellani, P.; Rosenthal-Allieri, M.A.; Gerolami, R.; et al. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: Proposal for a pragmatic approach classification without liver biopsies. J. Viral Hepat. 2006, 13, 659–670. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chrysavgis, L.; Ztriva, E.; Protopapas, A.; Tziomalos, K.; Cholongitas, E. Nonalcoholic fatty liver disease in lean subjects: Prognosis, outcomes and management. World J. Gastroenterol. 2020, 26, 6514–6528. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Armstrong, M.J. Lean NAFLD: A not so benign condition? Hepatol. Commun. 2018, 2, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol. Commun. 2018, 2, 48–57. [Google Scholar] [CrossRef]

| MAFLD (n = 816) | No-MAFLD (n = 171) | p | NAFLD (n = 795) | No-NAFLD (n = 192) | p | p (NAFLD vs. MAFLD) | |
|---|---|---|---|---|---|---|---|
| Age (years) | 56.0 ± 12.3 | 57.3 ± 13.7 | 0.205 | 55.8 ± 12.3 | 58.0 ± 13.5 | 0.033 | 0.763 |
| Women (%) | 38.6 | 45.6 | 0.089 | 39.1 | 42.7 | 0.362 | 0.832 |
| BMI (kg/) | 30.7 ± 5.0 | 25.2± 2.9 | 0.000 | 30.5 ± 5.2 | 26.8 ± 3.8 | 0.000 | 0.319 |
| BMI ≥ 25 kg/ (%) | 92.0 | 49.1 | 0.000 | 88.6 | 68.2 | 0.000 | 0.018 |
| Diabetes (%) | 29.2 | 10.5 | 0.000 | 29.1 | 13.0 | 0.000 | 0.961 |
| Metabolic Syndrome (%) | 60.0 | 18.2 | 0.000 | 59.5 | 25.1 | 0.000 | 0.839 |
| HOMA-IR > 2.5 (%) | 66.9 | 18.7 | 0.000 | 67.2 | 22.9 | 0.000 | 0.912 |
| Prior MACCE (%) | 6.0 | 7.6 | 0.434 | 5.9 | 7.8 | 0.330 | 0.937 |
| AST (UI/L) | 21.0 [17.0–28.0] | 19.0 [16.0–23.0] | 0.000 | 21.0 [17.0–28.0] | 18.0 [16.0–20.0] | 0.000 | 0.569 |
| ALT (UI/L) | 27.0 [19.0–40.0] | 17.0 [14.0–24.0] | 0.000 | 27.0 [19.0–42.0] | 18.0 [14.0–24.0] | 0.000 | 0.705 |
| GGT (UI/L) | 27.0 [17.0–42.0] | 18.0 [12.0–23.0] | 0.000 | 26.0 [17.0–42.0] | 18.0 [13.0–26.0] | 0.000 | 0.778 |
| FIB-4 > 2.67 (%) | 2.6 | 3.4 | 0.588 | 2.6 | 3.1 | 0.713 | 0.964 |
| APRI > 0.7 (%) | 5.7 | 3.0 | 0.187 | 5.8 | 2.7 | 0.115 | 0.932 |
| APRI > 1.0 (%) | 2.3 | 1.3 | 0.448 | 2.6 | 0.0 | 0.037 | 0.704 |
| High waist circumference (%) | 82.5 | 41.2 | 0.000 | 80.2 | 55.0 | 0.000 | 0.246 |
| High blood pressure (%) | 73.6 | 57.6 | 0.000 | 72.4 | 64.4 | 0.028 | 0.861 |
| Low HDL (%) | 39.6 | 12.4 | 0.000 | 39.5 | 15.4 | 0.000 | 0.964 |
| High triglycerides (%) | 42.5 | 11.2 | 0.000 | 42.4 | 14.8 | 0.000 | 0.962 |
| LDL-cholesterol (mg/dL) | 115.0 [85.8–139.0] | 116.0 [91.5–141.0] | 0.548 | 115.5 [87.0–141.7] | 116.0 [91.3–141.0] | 0.744 | 0.986 |
| Statin therapy (%) | 39.6 | 47.6 | 0.055 | 39.4 | 47.6 | 0.038 | 0.959 |
| Diabetes non-insulin therapy (%) | 29.4 | 9.4 | 0.000 | 29.3 | 12.0 | 0.000 | 0.964 |
| Insulin therapy (%) | 2.8 | 1.2 | 0.212 | 2.9 | 1.0 | 0.143 | 0.929 |
| Antiplatelet drugs (%) | 15.4 | 17.4 | 0.493 | 15.2 | 18.2 | 0.305 | 0.902 |
| Blood pressure medications (%) | 61.8 | 53.2 | 0.038 | 61.3 | 56.3 | 0.203 | 0.834 |
| Mediterranean Diet Score > 5 (%) | 36.5 | 51.5 | 0.001 | 36.5 | 49.3 | 0.004 | 0.991 |
| NAFLD to MAFLD (n = 767) | NAFLD to No-MAFLD (n = 28) | No-NAFLD to MAFLD (n = 49) | No-NAFLD to No-MAFLD (n = 143) | p all | p 3 vs. 4 | p 1 vs. 3 | |
|---|---|---|---|---|---|---|---|
| Age (years) | 56.0 ± 12.3 | 49.1 ± 10.8 | 55.0 ± 12.7 | 58.9 ± 13.6 | 0.001 | 0.196 | 0.035 |
| Women (%) | 38.7 | 50.0 | 40.5 | 45.2 | 0.335 | 0.403 | 0.782 |
| BMI (kg/) | 30.7 ± 5.0 | 22.9 ± 1.7 | 30.4 ± 4.1 | 25.6 ± 2.9 | 0.000 | 0.000 | 0.000 |
| BMI ≥ 25 kg/ (%) | 91.8 | - | 95.9 | 58.7 | 0.000 | 0.000 | 0.300 |
| Diabetes (%) | 30.1 | - | 14,3 | 12.6 | 0.000 | 0.760 | 0.018 |
| Metabolic Syndrome (%) | 61.7 | - | 34.7 | 21.8 | 0.000 | 0.073 | 0.000 |
| HOMA-IR > 2.5 (%) | 69.4 | 7.1 | 28.6 | 21.0 | 0.000 | 0.275 | 0.000 |
| Prior MACCE (%) | 6.1 | 0 | 4.1 | 9.1 | 0.238 | 0.259 | 0.559 |
| AST (UI/L) | 21.0 [17.0–28.0] | 21.0 [17.0–27.0] | 19.0 [17.0–21.0] | 18.0 [16.0–22.0] | 0.000 | 0.651 | 0.004 |
| ALT (UI/L) | 27.0 [19.0–42.0] | 25.0 [17.0–43.0] | 20.0 [17.0–27.0] | 16.0 [13.0–22.0] | 0.000 | 0.001 | 0.001 |
| GGT (UI/L) | 27.0 [17.0–42.0] | 18.0 [13.0–34.0] | 24.0 [15.0–51.0] | 17.0 [12.0–22.5] | 0.000 | 0.000 | 0.617 |
| FIB-4 > 2.67 (%) | 2.4 | 7.1 | 5.1 | 2.5 | 0.362 | 0.408 | 0.300 |
| APRI > 0.7 (%) | 5.8 | 8.3 | 5.1 | 1.8 | 0.328 | 0.277 | 0.869 |
| APRI > 1.0 (%) | 2.4 | 7.2 | 0 | 0 | 0.064 | - | 0.314 |
| High waist circumference (%) | 82.6 | 14.3 | 79.6 | 46.5 | 0.000 | 0.000 | 0.586 |
| High blood pressure (%) | 74.0 | 28.6 | 67.3 | 63.4 | 0.000 | 0.617 | 0.302 |
| Low HDL (%) | 40.8 | 3.6 | 19.6 | 14.1 | 0.000 | 0.371 | 0.004 |
| High triglycerides (%) | 43.9 | 3.6 | 21.3 | 12.7 | 0.000 | 0.160 | 0.002 |
| LDL-cholesterol (mg/dL) | 116.0 [91.7–140.4] | 115.0 [85.5–150.7] | 116.5 [89.2–153.2] | 115.0 [85.8–139.0] | 0.901 | 0.486 | 0.689 |
| Statin therapy (%) | 39.7 | 30.8 | 38.8 | 50.7 | 0.0.63 | 0.149 | 0.900 |
| Diabetes non-insulin therapy (%) | 30.4 | 0 | 14.3 | 11.2 | 0.000 | 0.565 | 0.017 |
| Insulin therapy (%) | 3.0 | 0 | 0 | 1.4 | 0.331 | 0.405 | 0.219 |
| Antiplatelet drugs (%) | 15.5 | 7.1 | 14.3 | 15.5 | 0.356 | 0.407 | 0.817 |
| Blood pressure medications (%) | 62.7 | 21.4 | 46.9 | 59.4 | 0.000 | 0.128 | 0.028 |
| Mediterranean Diet Score > 5 (%) | 36.3 | 42.3 | 39.5 | 53.3 | 0.010 | 0.127 | 0.670 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, F.; Ferro, D.; Pastori, D.; Colantoni, A.; Cocomello, N.; Coronati, M.; Angelico, F.; Del Ben, M. Open Issues in the Transition from NAFLD to MAFLD: The Experience of the Plinio Study. Int. J. Environ. Res. Public Health 2021, 18, 8993. https://doi.org/10.3390/ijerph18178993
Baratta F, Ferro D, Pastori D, Colantoni A, Cocomello N, Coronati M, Angelico F, Del Ben M. Open Issues in the Transition from NAFLD to MAFLD: The Experience of the Plinio Study. International Journal of Environmental Research and Public Health. 2021; 18(17):8993. https://doi.org/10.3390/ijerph18178993
Chicago/Turabian StyleBaratta, Francesco, Domenico Ferro, Daniele Pastori, Alessandra Colantoni, Nicholas Cocomello, Mattia Coronati, Francesco Angelico, and Maria Del Ben. 2021. "Open Issues in the Transition from NAFLD to MAFLD: The Experience of the Plinio Study" International Journal of Environmental Research and Public Health 18, no. 17: 8993. https://doi.org/10.3390/ijerph18178993
APA StyleBaratta, F., Ferro, D., Pastori, D., Colantoni, A., Cocomello, N., Coronati, M., Angelico, F., & Del Ben, M. (2021). Open Issues in the Transition from NAFLD to MAFLD: The Experience of the Plinio Study. International Journal of Environmental Research and Public Health, 18(17), 8993. https://doi.org/10.3390/ijerph18178993









