COVID-19 Deaths in the United States: Shifts in Hot Spots over the Three Phases of the Pandemic and the Spatiotemporally Varying Impact of Pandemic Vulnerability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.2. Emerging Hot Spot Analysis (Space-Time Pattern Mining)
2.3. Geographically and Temporally Weighted Regression
3. Results
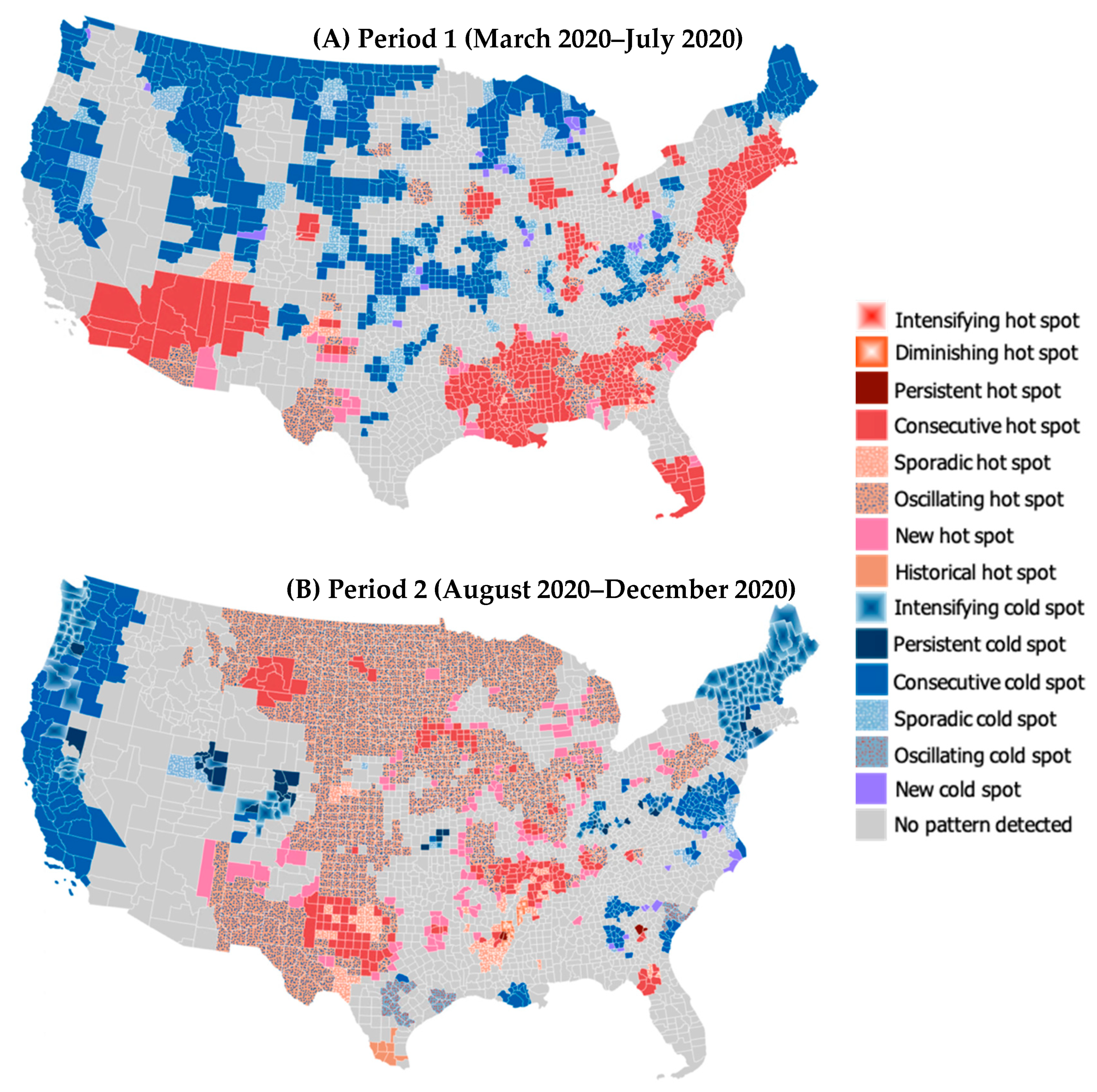
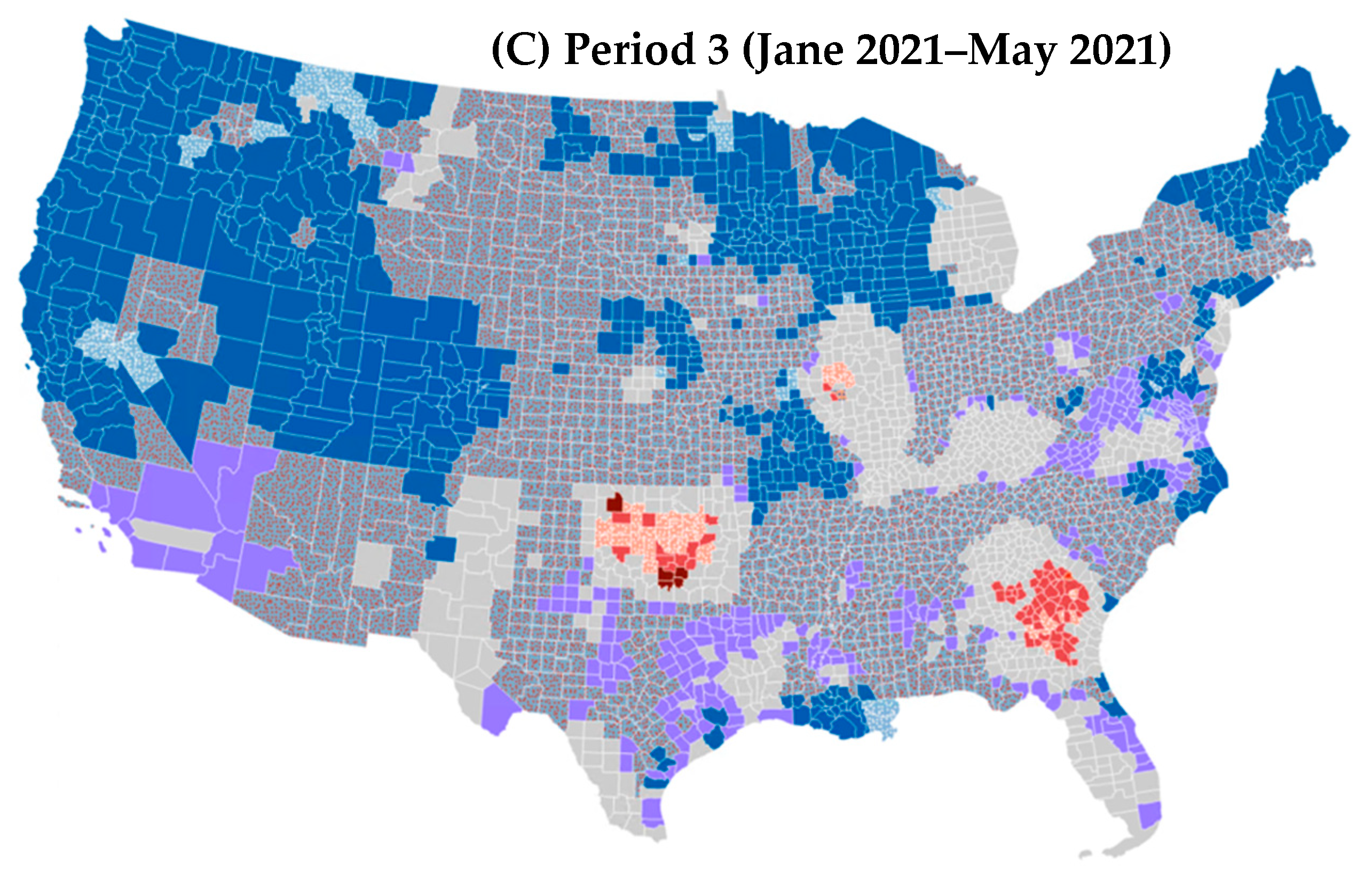
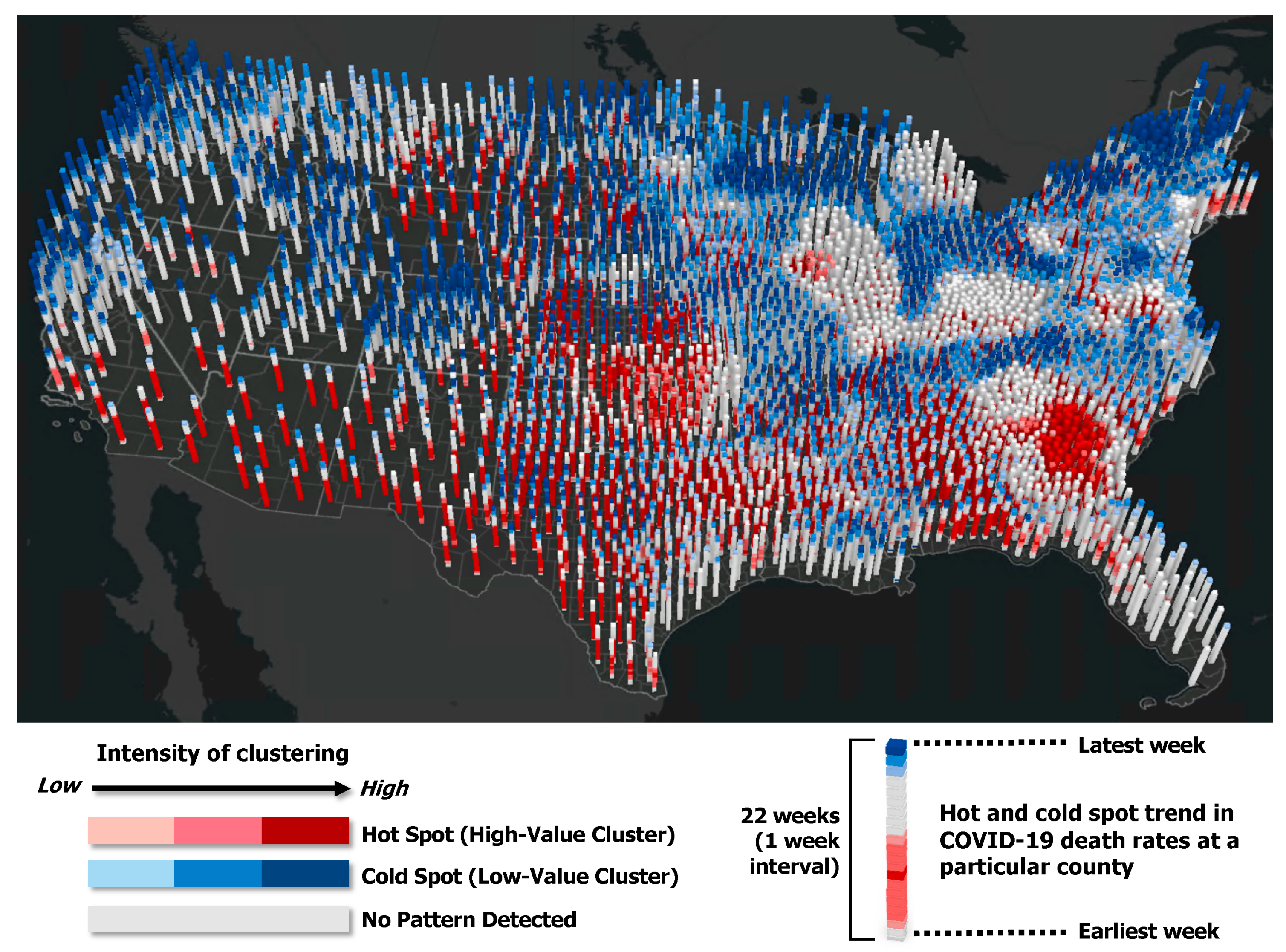
3.1. Spatiotemporal Trend in COVID-19 Mortality Rates
3.2. Results of the Global Regression Model
3.3. Results of the GTWR Model
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.M.; Khan, F.; Pugh, S.; Angulo, F.J.; Schmitt, H.-J.; E Isturiz, R.; Jodar, L.; Swerdlow, D.L. County-level Predictors of Coronavirus Disease 2019 (COVID-19) Cases and Deaths in the United States: What Happened, and Where Do We Go from Here? Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Millett, G.A.; Jones, A.T.; Benkeser, D.; Baral, S.; Mercer, L.; Beyrer, C.; Honermann, B.; Lankiewicz, E.; Mena, L.; Crowley, J.S.; et al. Assessing differential impacts of COVID-19 on Black communities. Ann. Epidemiol. 2020, 47, 37–44. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kuy, S.; Tsai, R.; Bhatt, J.; Chu, Q.D.; Gandhi, P.; Gupta, R.; Gupta, R.; Hole, M.K.; Hsu, B.S.; Hughes, L.S.; et al. Focusing on Vulnerable Populations During COVID-19. Acad. Med. 2020, 95. [Google Scholar] [CrossRef] [PubMed]
- Neelon, B.; Mutiso, F.; Mueller, N.T.; Pearce, J.L.; Benjamin-Neelon, S.E. Spatial and temporal trends in social vulnerability and COVID-19 incidence and death rates in the United States. PLoS ONE 2021, 16, e0248702. [Google Scholar] [CrossRef]
- Yang, W.; Deng, M.; Li, C.; Huang, J. Spatio-Temporal Patterns of the 2019-nCoV Epidemic at the County Level in Hubei Province, China. Int. J. Environ. Res. Public Health 2020, 17, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linard, C.; Tatem, A.J. Large-scale spatial population databases in infectious disease research. Int. J. Health Geogr. 2012, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R. Exploring the neighbourhood-level correlates of Covid-19 deaths in London using a difference across spatial boundaries method. Health Place 2020, 66, 102446. [Google Scholar] [CrossRef] [PubMed]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Anselin, L. Local indicators of spatial association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Fan, J.; Liu, X.; Pan, W.; Douglas, M.W.; Bao, S. Epidemiology of Coronavirus Disease in Gansu Province, China, 2020. Emerg. Infect. Dis. 2020, 26, 1257–1265. [Google Scholar] [CrossRef]
- Gibertoni, D.; Adja KY, C.; Golinelli, D.; Reno, C.; Regazzi, L.; Lenzi, J.; Sanmarchi, F.; Fantini, M.P. Patterns of COVID-19 related excess mortality in the municipalities of Northern Italy during the first wave of the pandemic. Health Place 2021, 67, 102508. [Google Scholar] [CrossRef] [PubMed]
- Fotheringham, A.S.; Charlton, M.E.; Brunsdon, C. Geographically weighted regression: A natural evolution of the expansion method for spatial data analysis. Environ. Plan. A Econ. Space 1998, 30, 1905–1927. [Google Scholar] [CrossRef]
- Huang, J.; Kwan, M.-P.; Kan, Z.; Wong, M.S.; Kwok CY, T.; Yu, X. Investigating the relationship between the built environment and relative risk of COVID-19 in Hong Kong. ISPRS Int. J. Geo-Inf. 2020, 9, 624. [Google Scholar] [CrossRef]
- Karaye, I.M.; Horney, J.A. The impact of social vulnerability on COVID-19 in the U.S.: An analysis of spatially varying relationships. Am. J. Prev. Med. 2020, 59, 317–325. [Google Scholar] [CrossRef]
- Mollalo, A.; Vahedi, B.; Rivera, K.M. GIS-based spatial modeling of COVID-19 incidence rate in the continental United States. Sci. Total Environ. 2020, 728, 138884. [Google Scholar] [CrossRef] [PubMed]
- Hohl, A.; Delmelle, E.M.; Desjardins, M.R.; Lan, Y. Daily surveillance of COVID-19 using the prospective space-time scan statistic in the United States Spatial and Spatio-temporal. Epidemiology 2020, 34, 100354. [Google Scholar]
- Martines, M.R.; Ferreira, R.V.; Toppa, R.H.; Assunção, L.M.; Desjardins, M.R.; Delmelle, E.M. Detecting space–time clusters of COVID-19 in Brazil: Mortality, inequality, socioeconomic vulnerability, and the relative risk of the disease in Brazilian municipalities. J. Geogr. Syst. 2021, 23, 7–36. [Google Scholar] [CrossRef]
- Marvel, S.W.; House, J.S.; Wheeler, M.; Song, K.; Zhou, Y.H.; Wright, F.A.; Chiu, W.A.; Rusyn, I.; Motsinger-Reif, A.; Reif, D.M. The COVID-19 pandemic vulnerability index (PVI) dashboard: Monitoring county-level vulnerability using visualization, statistical modeling, and machine learning. Environ. Health Perspect. 2021, 129, 17701. [Google Scholar] [CrossRef]
- NIEHS 2020 Details for PVI Maps. Available online: https://www.niehs.nih.gov/research/programs/coronavirus/covid19pvi/details/index.cfm (accessed on 11 June 2021).
- Matthews, S.A.; Yang, T.C. Mapping the results of local statistics: Using geographically weighted regression. Demogr. Res. 2012, 26, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Zhai, W. Examining the spatial and temporal relationship between social vulnerability and stay-at-home behaviors in New York City during the COVID-19 pandemic. Sustain. Cities Soc. 2021, 67, 102757. [Google Scholar] [CrossRef]
- Esri 2020 How Emerging Hot Spot Analysis Works. Available online: https://pro.arcgis.com/en/pro-app/2.7/tool-reference/space-time-pattern-mining/learnmoreemerging.htm (accessed on 1 August 2021).
- Huang, B.; Wu, B.; Barry, T. Geographically and temporally weighted regression for spatiotemporal modeling of house prices. Int. J. Geogr. Inf. Sci. 2010, 24, 383–401. [Google Scholar] [CrossRef]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; Kc, R.; Dua, K.; Patel, R.P.; Peterson, G.M.; O’Toole, R.F. Smoking and COVID-19: What we know so far. Respir. Med. 2021, 176, 106237. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Z.W.; Wang, L.; Yuan, M.L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020, 133, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Henry, B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur. J. Intern. Med. 2020, 75, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. Br. Med. J. 2020, 369, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.P.; Ravi, N.; Braneon, C.V. Spatiotemporal associations between social vulnerability, environmental measurements, and COVID-19 in the conterminous United States. GeoHealth 2021, 5, e2021GH000423. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nethery, R.C.; Sabath, B.M.; Braun, D.; Dominici, F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.R.; Bozzuto, C. Estimating and explaining the spread of COVID-19 at the county level in the USA. Commun. Biol. 2021, 4, 60. [Google Scholar] [CrossRef]
- Cordes, J.; Castro, M.C. Spatial analysis of COVID-19 clusters and contextual factors in New York City Spatial and Spatio-temporal. Epidemiology 2020, 34, 100355. [Google Scholar]
- Lau, H.; Khosrawipour, T.; Kocbach, P.; Ichii, H.; Bania, J.; Khosrawipour, V. Evaluating the massive underreporting and undertesting of COVID-19 cases in multiple global epicenters. Pulmonology 2021, 27, 110–115. [Google Scholar] [CrossRef]
- Pierce, J.R., Jr.; Denison, A.V. Place-of-residence errors on death certificates for two contiguous U. S. counties. Popul. Health Metr. 2006, 4, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Openshaw, S. The Modifiable Areal Unit Problem; Geo Books: Norwich, UK, 1984. [Google Scholar]
- Kearney, G.; Jones, K.; Park, Y.M.; Howard, R.; Hylock, R.; Wall, B.; Clay, M.; Schmidt, P. COVID-19: A vaccine priority index mapping tool for rapidly assessing priority populations in North Carolina. 2021, unpublished manuscript. [Google Scholar]
- Cheng, T.; Adepeju, M. Modifiable temporal unit problem (MTUP) and its effect on space-time cluster detection. PLoS ONE 2014, 9, e100465. [Google Scholar] [CrossRef] [Green Version]
- Kwan, M.-P. The uncertain geographic context problem Annals of the Association of American. Geographers 2012, 102, 958–968. [Google Scholar] [CrossRef]
- Kwan, M.-P. The neighborhood effect averaging problem (NEAP): An elusive confounder of the neighborhood effect. Int. J. Environ. Res. Public Health 2018, 15, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kwan, M.-P. An examination of people’s privacy concerns, perceptions of social benefits, and acceptance of COVID-19 mitigation measures that harness location information: A comparative study of the U.S. and South Korea. ISPRS Int. J. Geo-Inf. 2021, 10, 25. [Google Scholar] [CrossRef]
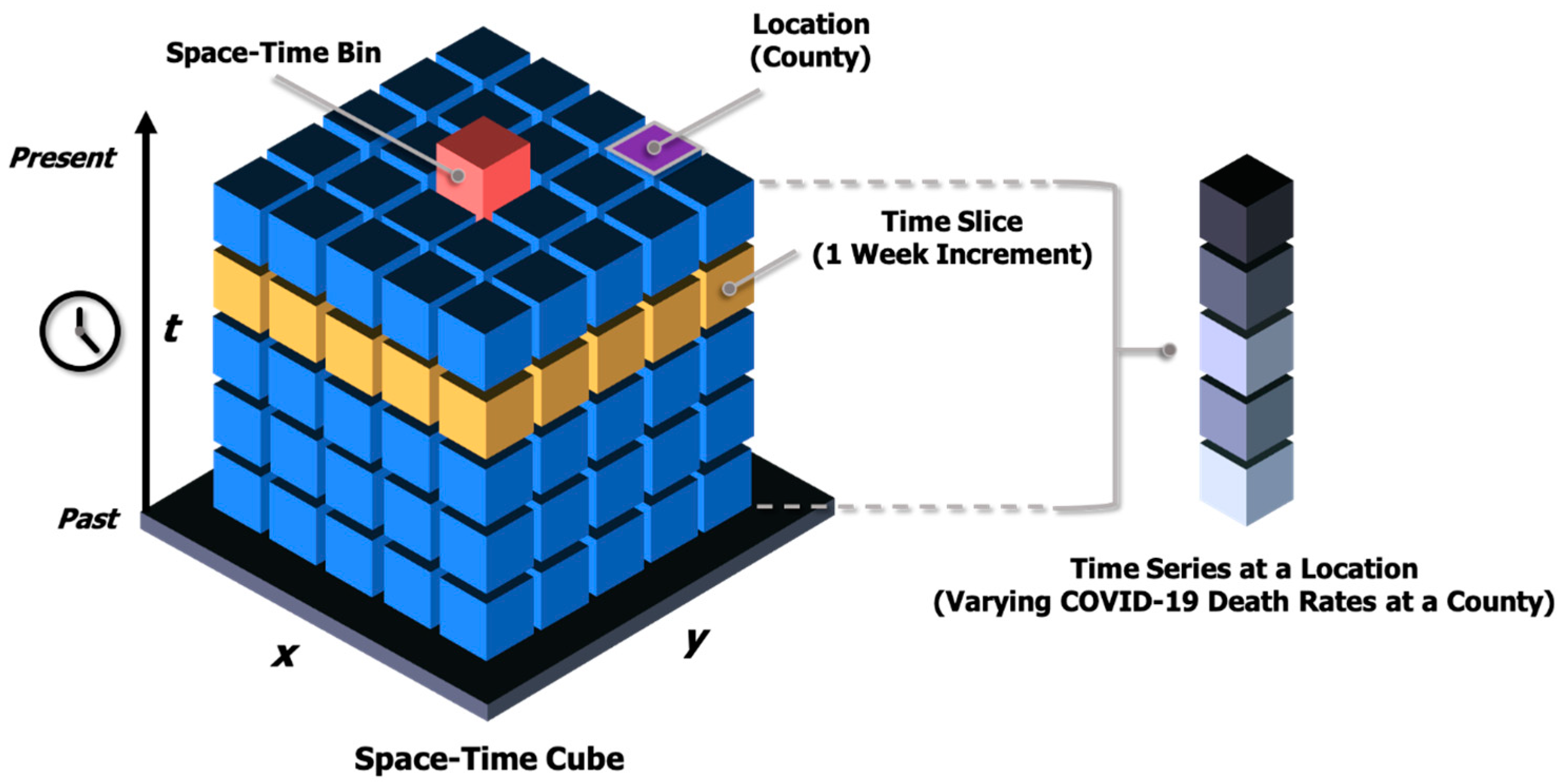
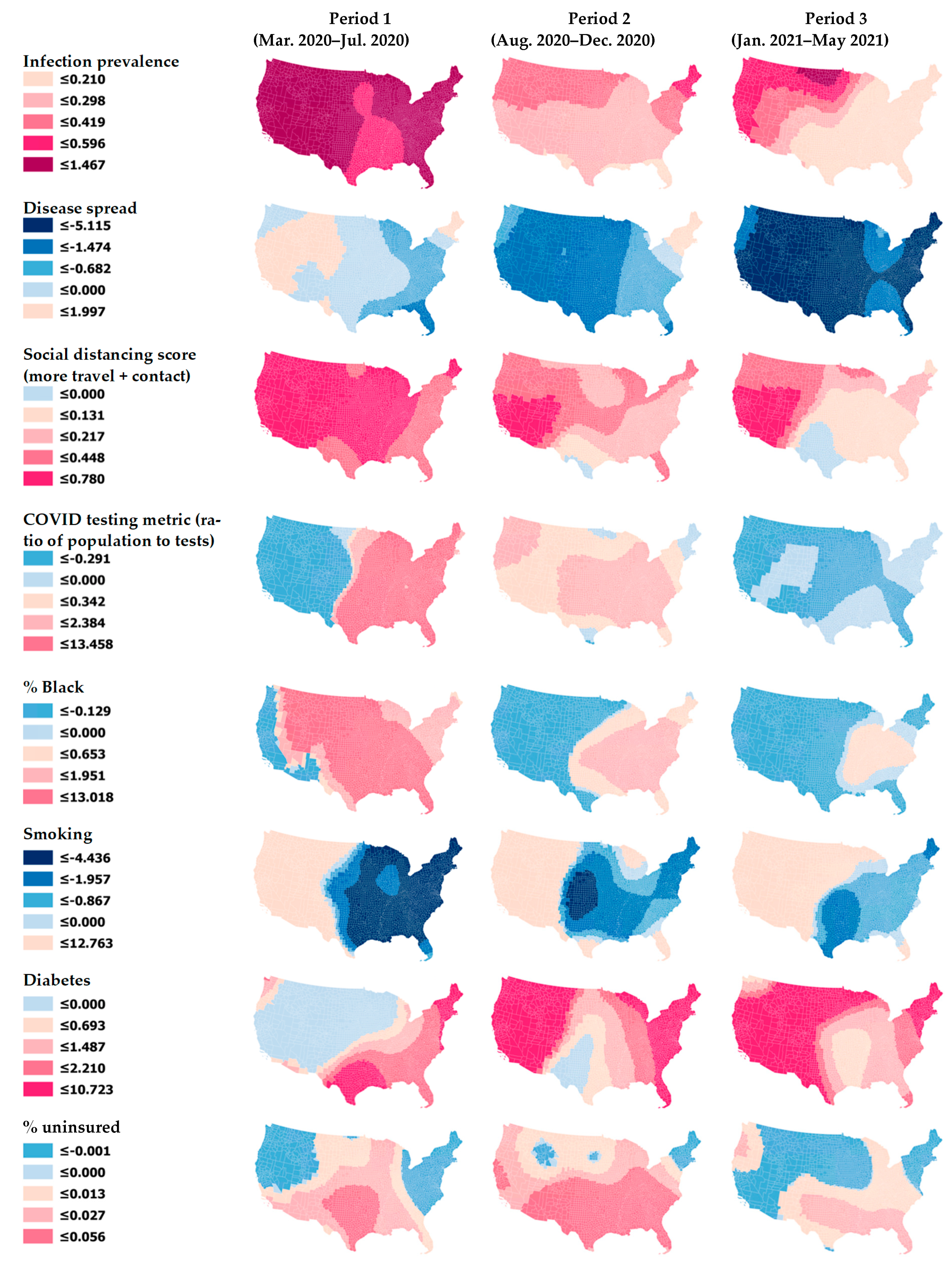
| Data Components in Four Major Domains | Characteristic | Description |
|---|---|---|
| 1. Infection Rate | ||
| Infection prevalence (Transmissible cases) | Dynamic | Cases from the last 14 days divided by population size (counts are obtained from the USA Facts website). The greater this metric, the more likely the virus is to continue to spread. |
| Rate of disease spread | Dynamic | New cases (for the last 14 days) divided by total active cases (counts are obtained from the USA Facts website). The value is close to 1 during exponential growth phase and declines linearly to 0 over the two-week incubation period if there are no new cases. |
| 2. Intervention Measures | ||
| Social distancing score | Dynamic | Changes in overall distance traveled and nonessential visits relative to previous year. A higher score indicates more travel and contact, i.e., less social distancing (derived from Unacast mobile phone data). |
| COVID-19 testing metric (state-level) | Dynamic | Population divided by the number of COVID-19 tests (the inverse of the total tests per population). A higher number indicates that fewer people are getting tested, i.e., lower testing rate (derived from the COVID Tracking Project [Atlantic Monthly Group 2020]). |
| 3. Population Concentration | ||
| Daytime population density | Static | The greater daytime population density, the more likely the disease will spread (derived from 2018 CDC SVI). |
| Baseline traffic | Static | Average traffic volume per meter of major roadways (derived from 2018 Environmental Justice Screening and Mapping Tool of the U.S. Environmental Protection Agency). |
| Residential density (SVI housing type and transportation score) | Static | One of the four themes in the SVI (derived from 2018 CDC SVI). Calculated based on families in multi-unit structures, mobile homes, overcrowding (persons > rooms), no vehicle, and persons in group quarters. |
| 4. Health and Environment | ||
| % Black | Static | Percentage of Black or African American. |
| % Native Americans | Static | Percentage of American Indian or Alaska Native. |
| Air pollution | Static | Average daily levels of particulate matter 2.5 (µg/m3) (derived from 2014 Environmental Public Health Tracking Network). |
| % aged 65 and over | Static | Percentage of population aged 65 and older (derived from 2014 to 2018 ACS). |
| Premature death | Static | Years of potential life lost before age 75 per 100,000 (derived from 2016 to 2018 National Center for Health Statistics: Mortality Files). |
| Smoking | Static | Percentage of adult smokers (derived from 2017 Behavioral Risk Factor Surveillance System). |
| Diabetes | Static | Percentage of adults with diabetes (aged 20 and order) (derived from 2016 U.S. Diabetes Surveillance System). |
| Obesity | Static | Percentage of population aged 20 and older with a body mass index ≥ 30 kg/m2. |
| % uninsured | Static | Percentage of population uninsured (derived from 2018 CDC SVI). |
| SVI socioeconomic status score | Static | One of the four themes in the SVI (derived from 2018 CDC SVI). Calculated based on percent below poverty, percent unemployed, income, and percent with no high school diploma. |
| Hospital beds | Static | Hospital beds in general medical and surgical hospitals (derived from the Homeland Infrastructure Foundation-Level Data). |
| Pattern | Definition |
|---|---|
| Intensifying hot (cold) spot | A location in which at least 90% of time steps present clustering of high (low) values and the intensity of clustering is increasing over time. |
| Diminishing hot (cold) spot | A location in which at least 90% of time steps present clustering of high (low) values and the intensity of clustering is decreasing over time. |
| Persistent hot (cold) spot | A location in which at least 90% of time steps present clustering of high (low) values without an increasing or decreasing trend in the intensity of clustering. |
| Consecutive hot (cold) spot | A location with a single continuous run of clustering of high (low) values for less than 90% of all time steps. |
| Sporadic hot (cold) spot | A location in which clustering of high (low) values is on again and off again over time without a history of clustering of low (high) values for all time steps. |
| Oscillating hot (cold) spot | A location with clustering of high (low) values for the latest time step with a history of clustering of low (high) values during previous time steps. |
| New hot (cold) spot | A location in which clustering of high (low) values has never been identified except for in the latest time step. |
| Historical hot (cold) spot | A location in which clustering of high (low) values has always been identified except for in the latest time step. |
| No pattern detected | A location with no statistically significant hot or cold spot pattern detected. |
| Parameter | Coefficient Estimate | Standard Error | t-Statistic | p-Value |
|---|---|---|---|---|
| Intercept | 0.395 ** | 0.146 | 2.703 | 0.007 |
| Infection prevalence | 0.305 *** | 0.006 | 52.929 | <0.001 |
| Disease spread | −1.594 *** | 0.102 | −15.697 | <0.001 |
| Social distancing score | 0.205 *** | 0.023 | 8.850 | <0.001 |
| COVID-19 testing metric | 0.301 *** | 0.042 | 7.151 | < 0.000 |
| Baseline traffic | 1.739 × 10−4 *** | 4.274 × 10−5 | 4.069 | <0.001 |
| Residential density | 0.106 * | 0.049 | 2.167 | 0.030 |
| % Black | 1.299 *** | 0.102 | 12.779 | <0.001 |
| % Native Americans | 0.434 . | 0.228 | 1.905 | 0.057 |
| Air pollution | 0.084 *** | 0.008 | 9.987 | <0.001 |
| % aged 65 and over | 0.019 *** | 0.003 | 5.876 | <0.001 |
| Premature death | 3.010 × 10−5 *** | 7.577 × 10−6 | 3.973 | <0.001 |
| Smoking | −2.199 *** | 0.526 | −4.183 | <0.001 |
| Diabetes | 1.499 *** | 0.387 | 3.874 | <0.001 |
| % uninsured | 0.028 *** | 0.003 | 9.749 | <0.001 |
| Hospital beds | −11.720 *** | 2.779 | −4.219 | <0.001 |
| Parameter | Mean | Minimum | Lower Quartile | Median | Upper Quartile | Maximum |
|---|---|---|---|---|---|---|
| Intercept | 0.208 | −2.793 | −0.861 | 0.126 | 1.534 | 3.962 |
| Infection prevalence | 0.419 | 0.064 | 0.210 | 0.298 | 0.596 | 1.466 |
| Disease spread | −3.504 | −27.958 | −5.115 | −1.474 | −0.682 | 1.997 |
| Social distancing score | 0.285 | −0.103 | 0.131 | 0.217 | 0.448 | 0.779 |
| COVID-19 testing metric | 1.132 | −12.838 | −0.291 | 0.342 | 2.384 | 13.457 |
| Baseline traffic | 1.454 × 10−4 | −7.520 × 10−4 | −9.066 × 10−5 | 5.327 × 10−5 | 3.842 × 10−4 | 2.282 × 10−3 |
| Residential density | 0.155 | −0.294 | 0.066 | 0.131 | 0.219 | 0.782 |
| % Black | 0.094 | −21.078 | −0.129 | 0.653 | 1.951 | 13.017 |
| % Native Americans | −0.174 | −5.712 | −1.316 | −0.592 | 1.112 | 3.289 |
| Air pollution | 0.090 | −0.062 | 0.055 | 0.088 | 0.134 | 0.205 |
| % aged 65 and over | 0.023 | −0.043 | 0.012 | 0.025 | 0.035 | 0.067 |
| Premature death | 2.838 × 10−5 | −6.394 × 10−5 | −2.276 × 10−6 | 2.749 × 10−5 | 5.431 × 10−5 | 1.360 × 10−4 |
| Smoking | −2.079 | −12.232 | −4.436 | −1.957 | −0.867 | 12.762 |
| Diabetes | 1.515 | −8.721 | 0.693 | 1.487 | 2.210 | 10.722 |
| % uninsured | 0.010 | −0.098 | −0.001 | 0.013 | 0.027 | 0.055 |
| Hospital beds | −1.011 | −45.437 | −11.090 | 0.231 | 6.630 | 57.624 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.M.; Kearney, G.D.; Wall, B.; Jones, K.; Howard, R.J.; Hylock, R.H. COVID-19 Deaths in the United States: Shifts in Hot Spots over the Three Phases of the Pandemic and the Spatiotemporally Varying Impact of Pandemic Vulnerability. Int. J. Environ. Res. Public Health 2021, 18, 8987. https://doi.org/10.3390/ijerph18178987
Park YM, Kearney GD, Wall B, Jones K, Howard RJ, Hylock RH. COVID-19 Deaths in the United States: Shifts in Hot Spots over the Three Phases of the Pandemic and the Spatiotemporally Varying Impact of Pandemic Vulnerability. International Journal of Environmental Research and Public Health. 2021; 18(17):8987. https://doi.org/10.3390/ijerph18178987
Chicago/Turabian StylePark, Yoo Min, Gregory D. Kearney, Bennett Wall, Katherine Jones, Robert J. Howard, and Ray H. Hylock. 2021. "COVID-19 Deaths in the United States: Shifts in Hot Spots over the Three Phases of the Pandemic and the Spatiotemporally Varying Impact of Pandemic Vulnerability" International Journal of Environmental Research and Public Health 18, no. 17: 8987. https://doi.org/10.3390/ijerph18178987
APA StylePark, Y. M., Kearney, G. D., Wall, B., Jones, K., Howard, R. J., & Hylock, R. H. (2021). COVID-19 Deaths in the United States: Shifts in Hot Spots over the Three Phases of the Pandemic and the Spatiotemporally Varying Impact of Pandemic Vulnerability. International Journal of Environmental Research and Public Health, 18(17), 8987. https://doi.org/10.3390/ijerph18178987









