Factors Associated with Insomnia Symptoms in a Longitudinal Study among New York City Healthcare Workers during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Data Collection
2.2. Statistical Analysis
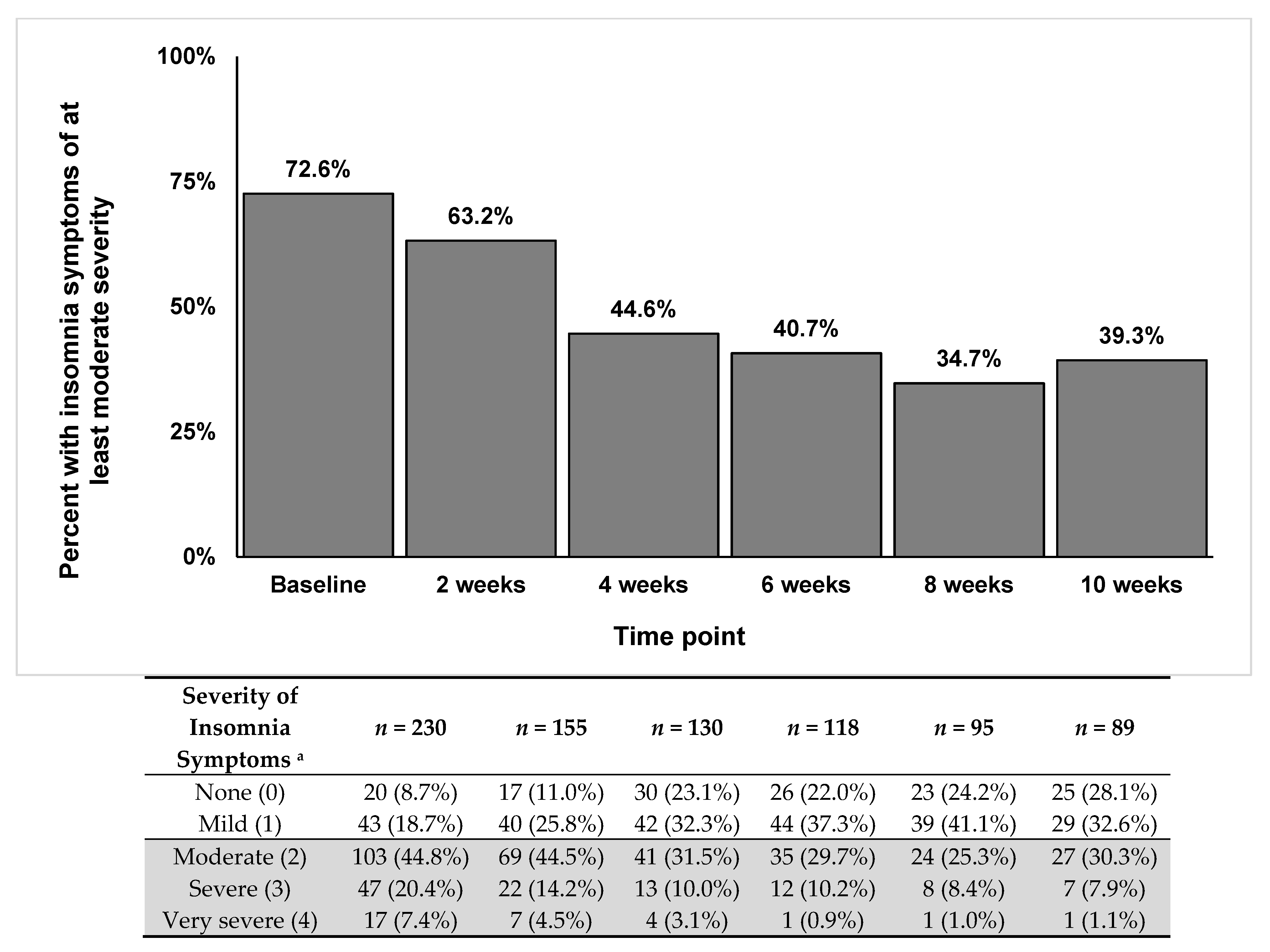
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dzau, V.J.; Kirch, D.; Nasca, T. Preventing a parallel pandemic—A national strategy to protect clinicians’ well-being. N. Engl. J. Med. 2020, 383, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Batra, K.; Singh, T.P.; Sharma, M.; Batra, R.; Schvaneveldt, N. Investigating the Psychological Impact of COVID-19 among Healthcare Workers: A Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 9096. [Google Scholar] [CrossRef] [PubMed]
- Pappa, S.; Ntella, V.; Giannakas, T.; Giannakoulis, V.G.; Papoutsi, E.; Katsaounou, P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, Y.; Nagarajan, R.; Saya, G.K.; Menon, V. Prevalence of psychological morbidities among general population, healthcare workers and COVID-19 patients amidst the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2020, 293, 113382. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chen, C.; Liu, Z.; Luo, X.; Guo, C.; Liu, Z.; Zhang, K.; Liu, H. Prevalence of Sleep Disturbances and Sleep Quality in Chinese Healthcare Workers During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 149. [Google Scholar] [CrossRef]
- Marvaldi, M.; Mallet, J.; Dubertret, C.; Moro, M.R.; Guessoum, S.B. Anxiety, depression, trauma-related, and sleep disorders among healthcare workers duirng the COVID-19 pandemic: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 126, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ding, H.; Zhang, Y.; Zhang, B.; Guo, Y.; Cheung, T.; Hall, B.J.; Shi, T.; Xiang, Y.-T.; Tang, Y. Prevalence of poor psychiatric status and sleep quality among frontline healthcare workers during and after the COVID-19 outbreak: A longitudinal study. Transl. Psychiatry 2021, 11, 223. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, Q.; Liu, Z.; Li, J.; Gong, X.; Liu, J.; Wan, Z.; Yuan, X.; Li, X.; Chen, C. Nurses endured high risks of psychological problems under the epidemic of COVID-19 in a longitudinal study in Wuhan China. J. Psychiatr. Res. 2020, 131, 132–137. [Google Scholar] [CrossRef]
- Neckelmann, D.; Mykletun, A.; Dahl, A.A. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep 2007, 30, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Shechter, A.; Diaz, F.; Moise, N.; Anstey, D.E.; Ye, S.; Agarwal, S.; Birk, J.L.; Brodie, D.; Cannone, D.E.; Chang, B.; et al. Psychological distress, coping behaviors, and preferences for support among New York healthcare workers during the COVID-19 pandemic. Gen. Hosp. Psychiatry 2020, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, N.J.; Bebchuk, J.D.; Jones, C.L.; Lipsitz, S.R.; Catalano, P.J.; Zahner, G.E.; Fitzmaurice, G.M. Goodness-of-fit for GEE: An example with mental health service utilization. Stat. Med. 1999, 18, 213–222. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, W.S.; Cho, A.R.; Kim, T.; Park, J.K. Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Compr. Psychiatry 2018, 87, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Su, T.P.; Lien, T.C.; Yang, C.Y.; Su, Y.L.; Wang, J.H.; Tsai, S.L.; Yin, J.C. Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: A prospective and periodic assessment study in Taiwan. J. Psychiatr. Res. 2007, 41, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Fang, Y.; Guan, Z.; Fan, B.; Kong, J.; Yao, Z.; Liu, X.; Fuller, C.J.; Susser, E.; Lu, J.; et al. The psychological impact of the SARS epidemic on hospital employees in China: Exposure, risk perception, and altruistic acceptance of risk. Canadian journal of psychiatry. Rev. Can. De Psychiatr. 2009, 54, 302–311. [Google Scholar] [CrossRef]
- Lin, C.Y.; Peng, Y.C.; Wu, Y.H.; Chang, J.; Chan, C.H.; Yang, D.Y. The psychological effect of severe acute respiratory syndrome on emergency department staff. Emerg. Med. J. 2007, 24, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.K.; Dunn, R.; Amlot, R.; Rubin, G.J.; Greenberg, N. A Systematic, Thematic Review of Social and Occupational Factors Associated With Psychological Outcomes in Healthcare Employees During an Infectious Disease Outbreak. J. Occup. Environ. Med. 2018, 60, 248–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maunder, R.G.; Lancee, W.J.; Balderson, K.E.; Bennett, J.P.; Borgundvaag, B.; Evans, S.; Fernandes, C.M.; Goldbloom, D.S.; Gupta, M.; Hunter, J.J.; et al. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg. Infect. Dis. 2006, 12, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The pathophysiology of insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, A.L.; Miller, C.B.; Emsley, R.; Sheaves, B.; Freeman, D.; Luik, A.I.; Littlewood, D.L.; Saunders, K.E.; Kanady, J.C.; Carl, J.R. Insomnia as a mediating therapeutic target for depressive symptoms: A sub-analysis of participant data from two large randomized controlled trials of a digital sleep intervention. J. Sleep Res. 2021, 30, e13140. [Google Scholar] [CrossRef] [PubMed]
| (n = 230) | |
| Age (years), median (IQR) | 36 (31–48) |
| Sex, n (%) | |
| Female | 183 (79.6%) |
| Male | 46 (20.0%) |
| Other | 1 (0.4%) |
| Race, n (%) | |
| White | 148 (64.3%) |
| Asian | 26 (11.3%) |
| Black | 21 (9.1%) |
| Other | 20 (8.7%) |
| More than one race | 14 (6.1%) |
| Hawaiian/Pacific Islander | 1 (0.4%) |
| American Indian/Native American | 0 (0%) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 191 (86.8%) |
| Hispanic or Latino | 29 (13.2%) |
| Clinical location, n (%) | |
| COVID-facing | 190 (82.6%) |
| Not COVID-facing | 40 (17.4%) |
| Hours worked in past week (at baseline) a | |
| Median (IQR) | 41–50 h (31–40 h, 51–60 h) |
| Role, n (%) | |
| Registered Nurse | 115 (50.0%) |
| Attending Physician | 50 (21.7%) |
| Resident | 31 (13.5%) |
| Advanced Practice Provider | 13 (5.7%) |
| Fellow | 12 (5.2%) |
| Other | 8 (3.5%) |
| Prefer not to answer | 1 (0.4%) |
| Dichotomized insomnia severity, n (%) | |
| Moderate or severe symptoms (≥2 score) | 167 (72.6%) |
| None or mild symptoms (<2 score) | 63 (27.4%) |
| Completed 10-Week Assessment (n = 89) | Did Not Complete 10-Week Assessment (n = 141) | p-Value c | |
|---|---|---|---|
| Age (years), median (IQR) | 35 (31–44.3) | 41 (32–53) | 0.004 |
| Sex (n%) | 0.408 | ||
| Female | 114 (80.9%) | 69 (77.5%) | |
| Male | 27 (19.1%) | 19 (21.3%) | |
| Other | 0 (0%) | 1 (1.1%) | |
| Race (n%) | 0.334 | ||
| White | 84 (59.6%) | 64 (71.9%) | |
| Asian | 16 (11.3%) | 10 (11.2%) | |
| Black | 14 (9.9%) | 7 (7.9%) | |
| Other | 16 (8.7%) | 4 (4.5%) | |
| More than one race | 10 (7.1%) | 4 (4.5%) | |
| Hawaiian/Pacific Islander | 1 (0.7%) | 0 (0%) | |
| American Indian/Native American | 0 (%) | 0 (0%) | |
| Ethnicity (n%) | 0.302 | ||
| Not Hispanic or Latino | 118 (83.7%) | 73 (82%) | |
| Hispanic or Latino | 23 (16.3%) | 16 (18%) | |
| Clinical location (n%) | 0.020 | ||
| COVID-facing | 123 (87.2%) | 67 (75.3%) | |
| Not COVID-facing | 18 (12.8%) | 22 (24.7%) | |
| Hours worked in past week (at baseline) a | 0.348 | ||
| Median (IQR) | 41–50 h (31–40 h, 51–60 h) | 41–50 h (31–40 h, 51–60 h) | |
| Role (n %) | 0.009 | ||
| Registered Nurse | 78 (55.3%) | 37 (41.6%) | |
| Attending Physician | 19 (13.5%) | 30 (33.7%) | |
| Resident | 23 (16.3%) | 8 (9%) | |
| Advanced Practice Provider | 0 (0%) | 1 (1.1%) | |
| Fellow | 9 (6.4%) | 3 (3.4%) | |
| Other | 11 (7.8%) | 10 (11.2%) | |
| Prefer not to answer | 1 (0.7%) | 0 (0%) | |
| Severity of insomnia symptoms b | 0.160 | ||
| Median (IQR) | 2.00 (2.00, 3.00) | 2.00 (1.00, 3.00) | |
| Dichotomized insomnia severity | 0.088 | ||
| None or mild symptoms (<2 score) | 33 (23.4%) | 30 (33.7%) | |
| Moderate or severe symptoms (≥2 score) | 108 (76.6 %) | 59 (66.3%) |
| Univariable Model | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Variable | B (SE) | OR (95% CI) | p-Value | B (SE) | OR (95% CI) | p-Value |
| Age | −0.03 (0.01) | 0.97 (0.96, 0.99) | 0.005 | −0.02 (0.01) | 0.98 (0.96, 1.00) | 0.031 |
| Role (RN vs. other) | 0.32 (0.21) | 1.38 (0.91, 2.10) | 0.135 | 0.34 (0.23) | 1.40 (0.89, 2.22) | 0.141 |
| Sex (female vs. male) | 0.56 (0.27) | 1.75 (1.03, 3.00) | 0.039 | 0.53 (0.29) | 1.70 (0.96, 3.02) | 0.069 |
| Clinical location (COVID-facing vs. not) | 0.67 (0.21) | 1.95 (1.31, 2.94) | 0.001 | 0.56 (0.21) | 1.75 (1.15, 2.67) | 0.008 |
| Work hours a | 0.13 (0.04) | 1.14 (1.05, 1.25) | 0.003 | 0.15 (0.05) | 1.16 (1.06, 1.27) | 0.002 |
| Race/ethnicity (White, Non-Hispanic/Latino vs. other) | −0.25 (0.22) | 0.78 (0.51, 1.20) | 0.257 | −0.14 (0.23) | 0.87 (0.56, 1.36) | 0.536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.; Chiuzan, C.; Shang, Y.; Ko, G.; Diaz, F.; Shaw, K.; McMurry, C.L.; Cannone, D.E.; Sullivan, A.M.; Lee, S.A.J.; et al. Factors Associated with Insomnia Symptoms in a Longitudinal Study among New York City Healthcare Workers during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 8970. https://doi.org/10.3390/ijerph18178970
Abdalla M, Chiuzan C, Shang Y, Ko G, Diaz F, Shaw K, McMurry CL, Cannone DE, Sullivan AM, Lee SAJ, et al. Factors Associated with Insomnia Symptoms in a Longitudinal Study among New York City Healthcare Workers during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2021; 18(17):8970. https://doi.org/10.3390/ijerph18178970
Chicago/Turabian StyleAbdalla, Marwah, Codruta Chiuzan, Yimeng Shang, Gavin Ko, Franchesca Diaz, Kaitlin Shaw, Cara L. McMurry, Diane E. Cannone, Alexandra M. Sullivan, Sung A. J. Lee, and et al. 2021. "Factors Associated with Insomnia Symptoms in a Longitudinal Study among New York City Healthcare Workers during the COVID-19 Pandemic" International Journal of Environmental Research and Public Health 18, no. 17: 8970. https://doi.org/10.3390/ijerph18178970
APA StyleAbdalla, M., Chiuzan, C., Shang, Y., Ko, G., Diaz, F., Shaw, K., McMurry, C. L., Cannone, D. E., Sullivan, A. M., Lee, S. A. J., Venner, H. K., & Shechter, A. (2021). Factors Associated with Insomnia Symptoms in a Longitudinal Study among New York City Healthcare Workers during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 18(17), 8970. https://doi.org/10.3390/ijerph18178970







