3.1. Soil Profile Properties
The very coarse fraction reflects the sedimentary (primary) features of the parent materials because they are unaffected by pedological processes [
28]. The stones are scattered in the Ap horizon, caused by agricultural disturbance, and progressively decrease to zero towards the bottom layer. It is suspected that the parental material was originally sediment in shallow and slow-moving water in fluvial–glacial environment, affected by current activity. A portion of the clay in the Bt horizon is likely to be primary and due to the Würm post glacial warm period. The pedological horizons reflect the soil clay content, which is homogeneous in throughout the three detected horizons. Only a slight increment in clay content is observed in the top layer of the Ap horizon and in the bottom layer of the Ae horizon. These variations are common in soils with high vertical flushing by soil water and assisted by organic colloids from the surface layers. This is a typical condition in a boreal Atlantic strong-weathered soil environment due to the high rainfall rates. The Soil Organic Matter (SOM) content is highest in the Ap horizon and exponentially decreases sharply in the Ae horizon. There is an increase in the organic matter towards the top of the Ap horizon because of the root zone and degrading organic matter accumulating in the soil canopy. The CEC increases both toward the top and the bottom of the profile. Both organic and inorganic colloids appear to affect the CEC and strong multiple correlations are observed between CEC, SOM and clay content:
The clay and organic matter contribution to CEC can be estimated respectively as 14 and 80 Eq·kg−1. The clay minerals have a low CEC and poorly crystalline illitic clay minerals are expected to dominatein the soil profile. The amount of exchangeable Na, K, Mg and Ca (Eq·kg−1) exceeds the CEC; therefore, the soil has an 100% base saturation and is oversaturated with respect to carbonates, condition which theoretically stabilize the formation of pedogenic swelling clay minerals.
The pH ranges from sub-acid to acid and is quite constant along the soil profile. A little increase of the pH is observed in the organic top layer, and a subtle decrement is observed in the clay minerals rich bottom layer of the Bt horizon. The observed pH range is above the pK both of the SOM carboxyl and the clay minerals basal surfaces but is below the pK of Mn, Fe, and Al oxides surfaces. The SOM and the clay minerals are expected to be mostly negatively charged and the oxides are positively charged in the pH range observed in the soil profile. The exchangeable H+ was estimated by pH(KCl) and was constant through the Ap horizon, and increases exponentially below it, in agreement with the observations on the soil colloids, and CEC.
Reducing conditions are evident in the Bt horizon where gray-greenish lens is observed. The relative humidity (RH) can be considered as a broad indication of reducing conditions and correlate with the clay and SOM:
A reducing environmental low oxidative potential is expected to prevail in the bottom of Bt horizon due the high quantity of clay, high relative humidity and low oxygen activity. The pH value is sub-acid for most of the Ap and Ae horizons, but it results in being acid in the Bt, where it is probably buffered by the dissolution and precipitation reactions of oxides.
3.2. Pseudo-Total Elemental Pool
The aqua-regia extracts all the PTEs bound to the soil humic and fulvic compounds, all the PTEs incorporate into soil microfauna and microflora pool, almost all the PTEs bind to soil minerals labile pool, most of the PTEs bound to superior vegetal residues and only a little fraction of the PTEs sinks into primary soil forming minerals. Most of the elements extracted by aqua-regia are therefore the anthropogenic soil PTEs concentration, here mainly due to the smelter plant emission in the atmosphere and its deposition on the top investigated soil layers. The concentration of pseudo-total element pool extracted in the investigated soil reservoirs is reported in
Table 2.
It is evident from
Table 2 that the PTEs load throughout the examined soil profile is inhomogeneous among the different soil layers, and specific for any element. No element studied shows a Gaussian distribution across the profile, as usually observed in most of the soil analysis published in the literature and observed in the field [
23]. It is hypothesized that the PTEs load in any soil layers reflects the adsorbed component in the soil, as well as specific chemical properties of individual PTEs.
When comparing the aqua-regia extracted pool of the trace elements with the averaged soil composition reported in the literature, it is apparent that most of the trace elements in the investigated soil have a concentration closed to the global soil average [
29,
30]. Only in the case of Cd is the soil averaged value exceeded by the aqua-regia’ extract soil-to-crust enrichment factor, which is close to two. It should be noted that the trace metal concentration in the aqua-regia extractable pools and the soil average concentration reported in the literature are not directly comparable, because the global average soil composition quoted in the literature results from a total (HF) digestion and not by aqua-regia extraction. The aqua-regia soil extract and does not allow us to estimate the PTEs occluded in most of the silica primary minerals, and it is believed to be almost everywhere strongly sensitive to anthropogenic soil PTEs load [
23,
25].
The correlation matrix among the elements of the aqua-regia pool is shown in
Table 3, and it allowsus to broadly distinguish four groups of the elements with similar distribution in the soil profile. The groups are (a) Na; (b) Al, Fe, Mg, K and Si; (c) Ni, Co, Cu, Zn, Cd, Pb, Mn and Ca; and (d) Cr, P and S.
The concentration of Na has a complex distribution throughout the soil profile and its pseudo-total concentration does not correlate with any other elements. It is reasonable to suppose that the concentration of Na in the soil is affected by the sea aerosol input for the sample site from on-land marine transfer. The concentration of K, Fe, Mg, Al and Si was almost constant within the soil profile, and a concentration peak of these elements are observed in the Bt horizon. Elements K, Mg, Al, Fe and Si results strongly correlate to the clay fraction content, and therefore is reasonable believe that the soil clay minerals are the main source of the aqua-regia extracted pool of these soil chemical element. The group of the bivalent cation elements Ni, Co, Cu, Zn, Cd, Pb, Mn and Ca has complex patterns in the distribution of the concentration along soil profile. Correlations among the concentration of these elements in the soil profile are observed, and correlation varies from weak to strong. The concentrations of the elements of this group are correlated with SOM and for some elements even with the soil clay fraction, such as Ca, Co and Ni. The group of elements stable as anions (Cr, P and S) has a pseudo-total concentration almost monotonically decreasing across the soil profile. Positive strong correlations between pseudo-total pool and organic matter are observed, and it is reasonable to assume that these anionic species are strongly bounds to the SOM colloids.
3.3. Soil Enrichment Factor
The soil to crust enrichment factor is the ratio between the element concentration in the soil and the element concentration in the crust. The soil enrichment factor (SEF) in the investigated soil was calculated by assuming that the pseudo-total pool of the measured elements is due to the anthropicfluxes and to the perturbation of the natural biogeochemical cycles, dividing the aqua-regia extracted pools values for the average crust concentration of the PTEs. The SEF calculated for the soil is reported in
Table 4.
The enrichment factor for Ca, Mn, Cd, Co, Cr, Cu, Ni, Pb, Zn, P and S in the studied soil result correlates with the USA soil-to-crust enrichment factor.
If the SEF of the S is disregarded, a strong correlation is observed between the pseudo-total SEF in Clelland and the soil enrichment in the USA:
This result is in agreement with previous investigation on anthropic Zn, Pb and Cd in NWItaly [
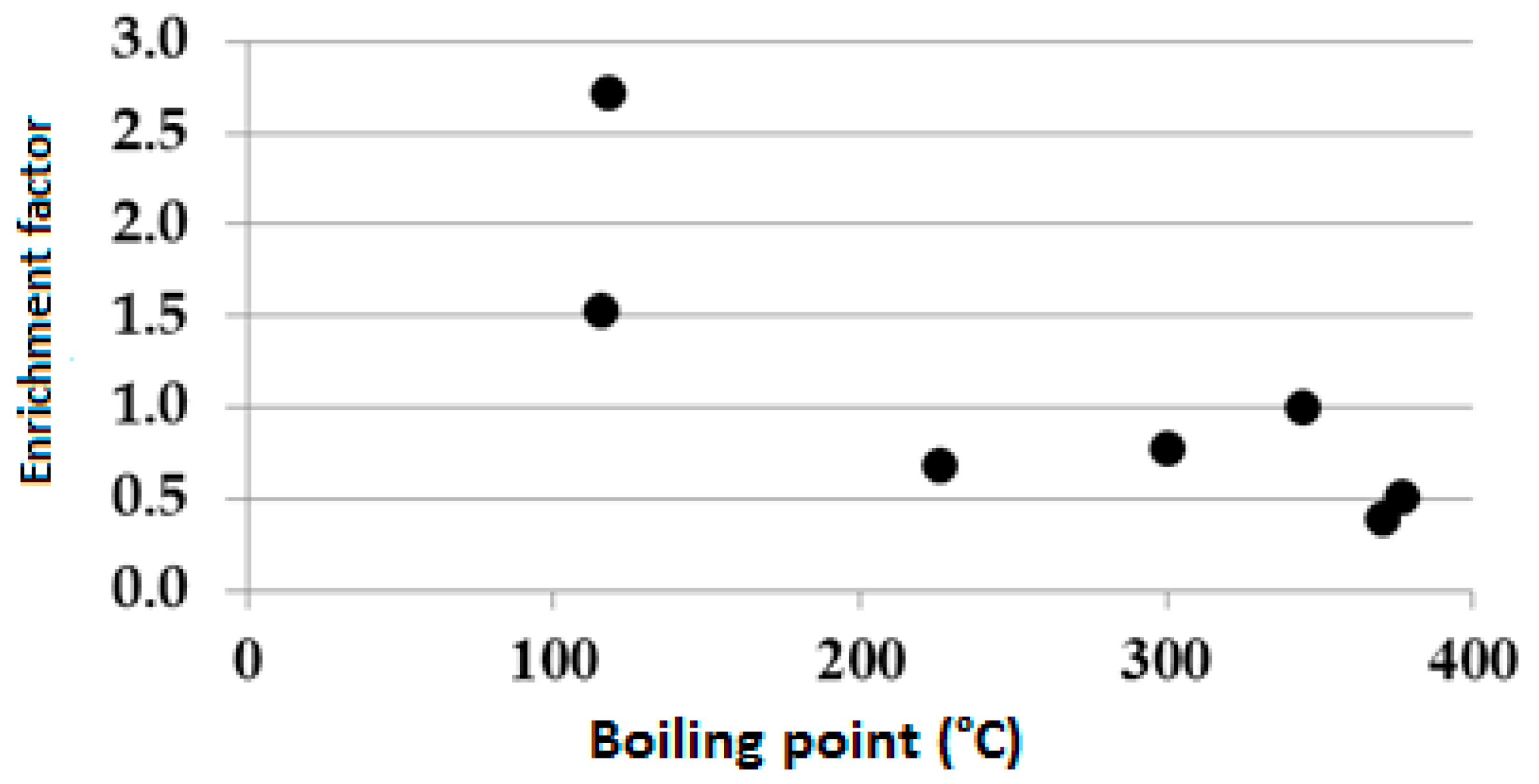
30]. The SEF of Cd, Co, Cr, Cu, Mn, Ni, Pb and Zn observed in the soil does not correlate with the ionic potential (IP) of the element; instead, it negatively correlates with the boiling temperature of the element (
Figure 2). The correlation observed between SEF and element boiling temperature suggests that the SEF of the PTEs is due to the smelter plant activity rather than soil chemical pedological processes both in Clelland, NW Italy, the USA and in Canadian soils [
13,
15,
16,
18,
31].
3.4. Residence Time of Element in the Reservoirs
The residence times of the pollutants in the reservoirs of the soil profile are shown in
Table 5.
The average residence time (year) is in the following order:
The residence time in the top soil profile is maximum, decreases downward following organic matter distribution and increases again in proximity to the Bt and beyond.
The soil properties which control the residence time have been investigated by forward multiple regression analysis. The main soil property which controls the residence time of Na is the clay content. The main soil property which controls the residence time of the bivalent cations, i.e., Ca, Co, Ni, Cu, Zn, Cd, Pb and Mn, is the SOM, while for the anions (Si, P and S), it is both the organic matter and clay fraction:
Assuming that the SOM is the dominant soil property controllingresidence time (RTE) of the elements in the topsoil layer, the RTE can be described by the following simple linear model:
The parameter α has been calculated by multiple regression for Ca, Co, Ni, Cu, Zn, Cd, Pb, Mn, Si, P and S; the results are shown in
Table 6, and the slopes of the linear regression results α has been correlated withthe IP of the element.
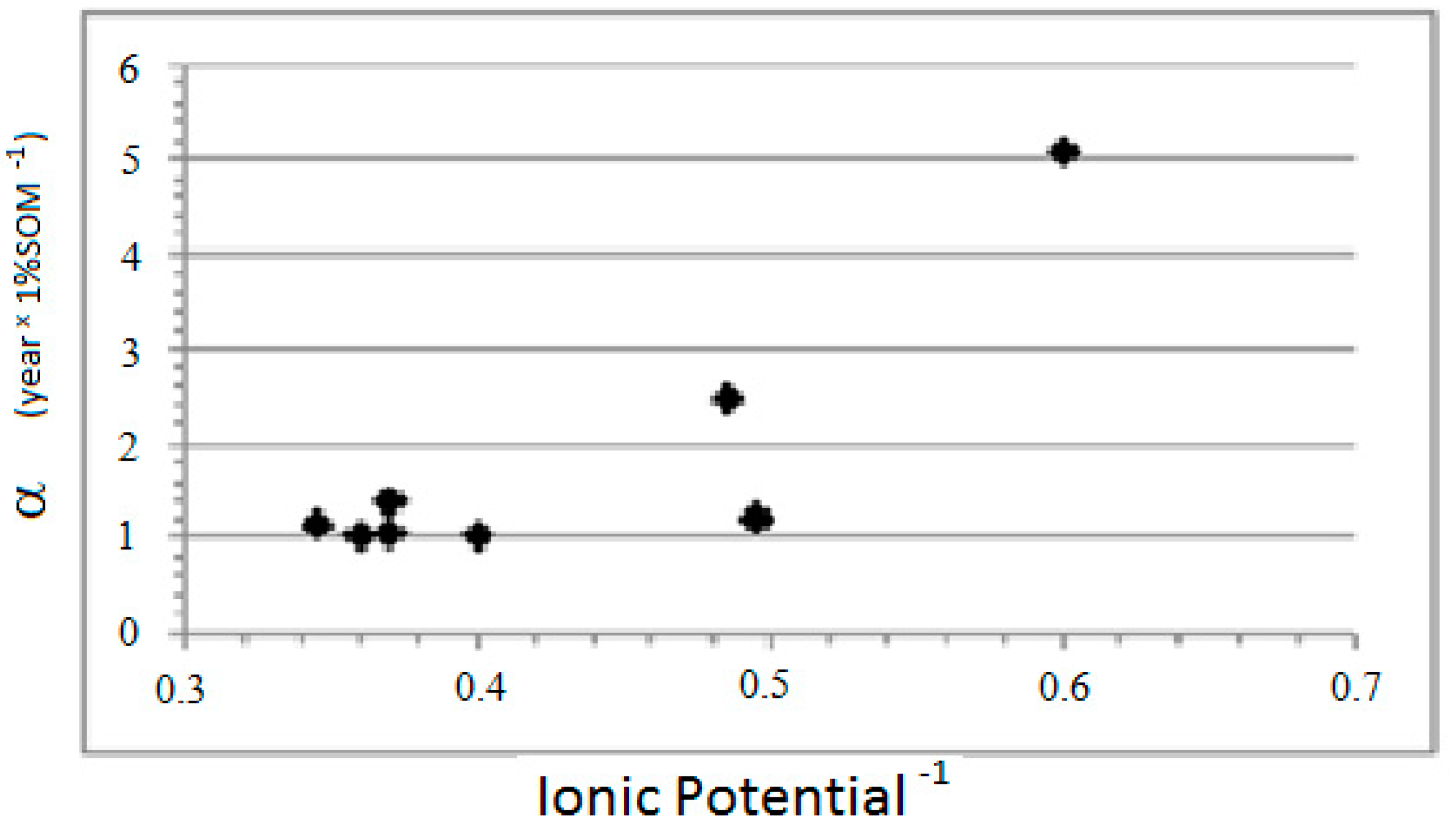
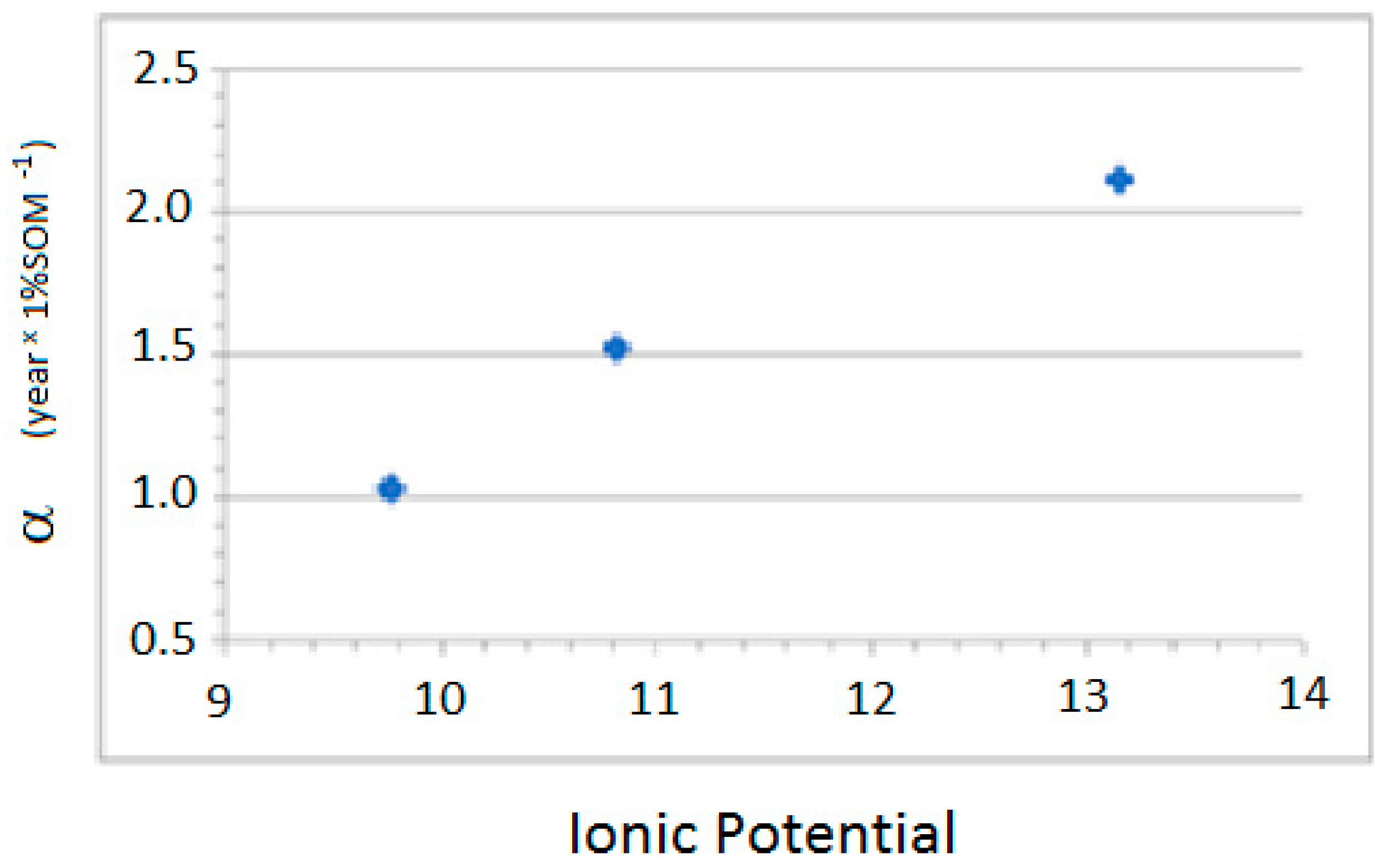
The results differ between divalent cations and anions. For the divalent cations, the constant α is inversely proportional to the IP of the metal, while, for the anions, it is directly proportional to the IP (
Figure 3 and
Figure 4).
The regressions observed between parameter α and the IP of the elements are as follows:
3.5. The Downward Migration Rate of the Pollutants
The calculated downward migration rates are listed in
Table 7. The downward migration rates increase in the following order:
As a general rule in the profile, the downward migration rates have a minimum in the top layer, increase to a maximum in the Ae horizon and decrease again approaching the Bt horizon.
It has been assumed that the abundance of the adsorbing phases should be linearly correlated with the logarithm of the downward migration rates, V
(E), of the element. This has been tested by multiple regression analysis. In
Table 8, the correlation matrix between ln(V
E) and the soil properties is shown. Most of the PTEs correlate both with SOM and CEC, but the correlation with clay content alone is poor.
All the soil properties correlated to the lnV(E) have been investigated by using the forward multiple regression method. The meaningful multiple regression funds are listed below.
ln(VNa) = (2.12 ± 0.53) − (0.0663 ± 0.0210) × Clay%; n = 8, r2 = 0.624, F = 1.96 × 10−2
ln(VCo) = (1.033 ± 0.101) − (0.177 ± 0.016) × SOM; n = 8, r2 = 0.951, F = 3.81 × 10−5
ln(VNi) = (1.164 ± 0.130) − (0.211 ± 0.021) × SOM; n = 8, r2 = 0.944, F = 5.69 × 10−5
ln(VCu) = (0.824 ± 0.278) − (0.160 ± 0.045) × SOM; n = 8, r2 = 0.676, F = 1.22 × 10−2
ln(VZn) = (0.625 ± 0.265) − (0.160 ± 0.043) × SOM; n = 8, r2 = 0.698, F = 9.76 × 10−3
ln(VCd) = (1.259 ± 0.637) − (0.281 ± 0.104) × SOM; n = 8, r2 = 0.550, F = 3.48 × 10−2
ln(VPb) = (1.018 ± 0.571) − (0.365 ± 0.093) × SOM; n = 8, r2 = 0.720, F = 7.76 × 10−3
ln (VMn) = (0.615 ± 0.224) − (0.120 ± 0.036) × SOM; n = 8, r2 = 0.645, F = 1.64 × 10−2
ln (VCa) = (1.093 ± 0.130) − (0.201 ± 0.021) × SOM; n = 8, r2 = 0.938; F = 7.73 × 10−5
ln(VS) = (0.911 ± 0.223) − (0.240 ± 0.036) × SOM; n = 8, r2 = 0.880, F = 5.73 × 10−4
ln (VP) = (0.687 ± 0.252) − (0.173 ± 0.041) × SOM; n = 8, r2 = 0.748; F = 5.53 × 10−3
The ln(V
Na) correlates with clay content only. For all bivalent cations and all anions, the ln(V
E) the result correlates linearly with SOM (
Table 9).
Forward multiple regression analysis demonstrates that the bulk clay fraction is not meaningfully correlated to ln(V
E) when SOM and clay fraction are considered together. It is reasonable to assume that the downward migration rate of the considered PTEs is sensitive of the mineralogical composition to the clay fraction, as previously demonstrated for
137Cs [
15,
31,
32]. Indeed, the Gibbs free energy of the adsorption of cations on clay minerals is very different among the several mineralogical species [
24,
33].
It has been suggested that the above-reported correlation could be expressed as a linear function of the SOM following the model:
Following the proposed linear model, ln(V0) is the logarithm of the downward migration rate in absence of organic matter, and KdSOM is the fraction of the mobile element retained in the layer because of the adsorption of the element into the organic matter. The values of ln(V0) and KdSOM have been estimated by linear regression above.
It is reasonable to assume both the parameters ln(V0) and KdSOM could be related to chemical properties of the elements.
With respect to anions, no meaningful correlation was found between element properties and ln(V
0) or Kd
SOM. With respect to bivalent cations Co, Ni, Cu, Zn, Mn, Cd, Ca and Pb, the parameter ln(V
0) is linearly correlated to the ΔG* of the adsorption of the cation on bentonite for the divalent cations, as shown in the following equation:
The downward migration rate (V
0) of the element therefore decreases exponentially as the adsorption constant on bentonite increases. The parameter Kd
SOM for the bivalent cations Co, Ni, Cu, Zn, Mn, Cd, Ca and Pb is resulted inversely proportional to the ionic radius of the element following the linear model:
Assuming that the soil properties that control the downward migration rates of the elements do not change with time and are in the steady state, the proposed box and flux model allowsus to predict the evolution with time of the element concentration throughout the soil profile. This assumption of the steady state of the soil properties controlling the PTEs leaching is reasonable when the concentration of the SOM pool in the soil profile has reached the steady state. It is not true if the climate change impact on land biomass and plant productivity are considered.
It is evident from the applied model that the time needed to decreases at half the concentration of the pollutants in the ploughed layer ranges from 25 years for the slowest mobile element (S) to 10 years for the faster moving (Na). The distribution of the pollutant throughout the soil profile calculated by the proposed box and flux model does not follow a Gaussian shape distribution, unlike in the case of the usual convective–advective physical models. It is because some soil reservoirs selectively accumulate some PTEs with respect to others PTEs. The reason for this phenomenon is that the proposed box and flux model is sensitive to the inhomogeneity commonly observed in the layered soil profile.