Pain Reduction after Short Exposure to Virtual Reality Environments in People with Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Virtual Reality Intervention
2.3. Clinical Assessments and Outcome Measures
2.4. Data Analysis
3. Results
3.1. Participant Characteristics
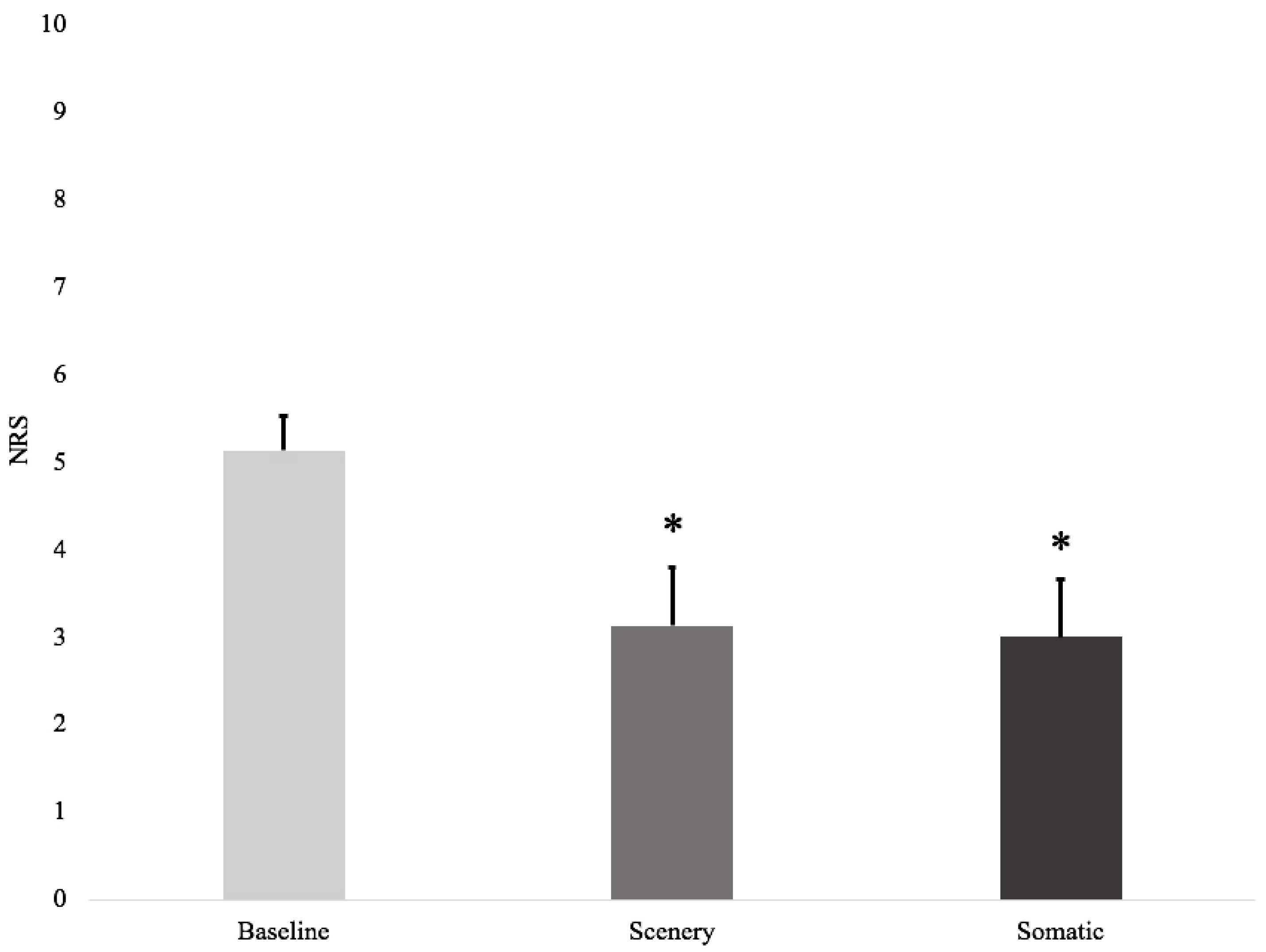
3.2. Effects of VR on Pain
3.3. VR, Immersion, and Presence
4. Discussion
Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yezierski, R.P. Spinal Cord Injury: A Model of Central Neuropathic Pain. Neurosignals 2005, 14, 182–193. [Google Scholar] [CrossRef]
- Siddall, P.J.; Loeser, J.D. Pain Following Spinal Cord Injury. Spinal Cord 2001, 39, 63–73. [Google Scholar] [CrossRef]
- Goossens, D.; Dousse, M.; Ventura, M.; Fattal, C. Chronic Neuropathic Pain in Spinal Cord Injury Patients: What Is the Impact of Social and Environmental Factors on Care Management? Ann. Phys. Rehabil. Med. 2009, 52, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Rhodes, R.E. Exercise Psychology: Physical Activity and Sedentary Behavior; Jones & Bartlett Learning: Burlington, MA, USA, 2017; ISBN 978-1-284-03421-9. [Google Scholar]
- Lenggenhager, B.; Pazzaglia, M.; Scivoletto, G.; Molinari, M.; Aglioti, S.M. The Sense of the Body in Individuals with Spinal Cord Injury. PLoS ONE 2012, 7, e50757. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Molinari, M. The Embodiment of Assistive Devices—From Wheelchair to Exoskeleton. Phys. Life Rev. 2016, 16, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Bors, E. Phantom limbs of patients with spinal cord injury. Arch. Neurol. Psychiatry 1951, 66, 610. [Google Scholar] [CrossRef]
- Curt, A.; Yengue, C.N.; Hilti, L.M.; Brugger, P. Supernumerary Phantom Limbs in Spinal Cord Injury. Spinal Cord 2011, 49, 588–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burke, C.D.; Woodward, I.M. Pain and phantom sensation in spinal cord paralysis. In Handbook of Clinical Neurology; Vinken, P., Bruyn, G., Eds.; North Holland: Amsterdam, The Netherlands, 1969; pp. 26–489. [Google Scholar]
- Hagen, E.M.; Rekand, T. Management of Neuropathic Pain Associated with Spinal Cord Injury. Pain Ther. 2015, 4, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Malloy, K.M.; Milling, L.S. The Effectiveness of Virtual Reality Distraction for Pain Reduction: A Systematic Review. Clin. Psychol. Rev. 2010, 30, 1011–1018. [Google Scholar] [CrossRef]
- Woolf, C.J.; Mannion, R.J. Neuropathic Pain: Aetiology, Symptoms, Mechanisms, and Management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Moseley, G.L.; Butler, D.S. Fifteen Years of Explaining Pain: The Past, Present, and Future. J. Pain 2015, 16, 807–813. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Altschuler, E.L. The Use of Visual Feedback, in Particular Mirror Visual Feedback, in Restoring Brain Function. Brain 2009, 132, 1693–1710. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, B.K.; Gao, K.; Sulea, C.; Wiederhold, M.D. Virtual Reality as a Distraction Technique in Chronic Pain Patients. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Moore, T.; Choo, J. The Impact of Virtual Reality on Chronic Pain. PLoS ONE 2016, 11, e0167523. [Google Scholar] [CrossRef]
- Garrett, B.; Taverner, T.; McDade, P. Virtual Reality as an Adjunct Home Therapy in Chronic Pain Management: An Exploratory Study. JMIR Med. Inform. 2017, 5, e11. [Google Scholar] [CrossRef]
- Dunn, J.; Yeo, E.; Moghaddampour, P.; Chau, B.; Humbert, S. Virtual and Augmented Reality in the Treatment of Phantom Limb Pain: A Literature Review. NeuroRehabilitation 2017, 40, 595–601. [Google Scholar] [CrossRef]
- Rizzo, A.A.; Schultheis, M.; Kerns, K.A.; Mateer, C. Analysis of Assets for Virtual Reality Applications in Neuropsychology. Neuropsychol. Rehabil. 2004, 14, 207–239. [Google Scholar] [CrossRef]
- Weiss, P.L.; Rand, D.; Katz, N.; Kizony, R. Video capture virtual reality as a flexible and effective rehabilitation tool. J. NeuroEng. Rehabil. 2004, 1, 12. [Google Scholar] [CrossRef]
- Villiger, M.; Hepp-Reymond, M.-C.; Pyk, P.; Kiper, D.; Eng, K.; Spillman, J.; Meilick, B.; Estevez, N.; Kollias, S.S.; Curt, A.; et al. Virtual Reality Rehabilitation System for Neuropathic Pain and Motor Dysfunction in Spinal Cord Injury Patients. In Proceedings of the 2011 International Conference on Virtual Rehabilitation, Zurich, Switzerland, 27–29 June 2011; IEEE: Zurich, Switzerland, 2011; pp. 1–4. [Google Scholar]
- Rossi, S.; Tecchio, F.; Pasqualetti, P.; Ulivelli, M.; Pizzella, V.; Romani, G.L.; Passero, S.; Battistini, N.; Rossini, P.M. Somatosensory Processing during Movement Observation in Humans. Clin. Neurophysiol. 2002, 113, 16–24. [Google Scholar] [CrossRef]
- Johnson, S.; Coxon, M. Sound Can Enhance the Analgesic Effect of Virtual Reality. R. Soc. Open Sci. 2016, 3, 150567. [Google Scholar] [CrossRef]
- Sharar, S.R.; Alamdari, A.; Hoffer, C.; Hoffman, H.G.; Jensen, M.P.; Patterson, D.R. Circumplex Model of Affect: A Measure of Pleasure and Arousal During Virtual Reality Distraction Analgesia. Games Health J. 2016, 5, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Botella, C.; Garcia-Palacios, A.; Vizcaíno, Y.; Herrero, R.; Baños, R.M.; Belmonte, M.A. Virtual Reality in the Treatment of Fibromyalgia: A Pilot Study. Cyberpsychol. Behav. Soc. Netw. 2013, 16, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Triberti, S.; Repetto, C.; Riva, G. Psychological Factors Influencing the Effectiveness of Virtual Reality–Based Analgesia: A Systematic Review. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 335–345. [Google Scholar] [CrossRef]
- Pourmand, A.; Davis, S.; Marchak, A.; Whiteside, T.; Sikka, N. Virtual Reality as a Clinical Tool for Pain Management. Curr. Pain Headache Rep. 2018, 22, 3722–3733. [Google Scholar] [CrossRef] [PubMed]
- Mallari, B.; Spaeth, E.K.; Goh, H.; Boyd, B.S. Virtual Reality as an Analgesic for Acute and Chronic Pain in Adults: A Systematic Review and Meta-Analysis. J. Pain Res. 2019, 12, 2053–2085. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.; Chau, B.; Yeo, E.; Ta, P. Virtual Reality for Spinal Cord Injury-Associated Neuropathic Pain: Systematic Review. Ann. Phys. Rehabil. Med. 2019, 62, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.A.; Itani, H.E.S.; Gandoura, N.A. The Neuropathic Pain Scale and Its Reliability and Validity for Diabetic Neuropathy. Diabet. Foot J. Middle East 2015, 1, 12–16. [Google Scholar]
- Witmer, B.G.; Singer, M.J. Measuring Immersion in Virtual Environments; ARI Technical Report 1014; US Army Research Institute for the Behavioral and Social Sciences: Alexandria, VA, USA, 1994. [Google Scholar]
- Witmer, B.G.; Singer, M.J. Measuring Presence in Virtual Environments: A Presence Questionnaire. Presence Teleoperators Virtual Environ. 1998, 7, 225–240. [Google Scholar] [CrossRef]
- Kipping, B.; Rodger, S.; Miller, K.; Kimble, R.M. Virtual Reality for Acute Pain Reduction in Adolescents Undergoing Burn Wound Care: A Prospective Randomized Controlled Trial. Burns 2012, 38, 650–657. [Google Scholar] [CrossRef]
- Maani, C.V.; Hoffman, H.G.; Fowler, M.; Maiers, A.J.; Gaylord, K.M.; DeSocio, P.A. Combining Ketamine and Virtual Reality Pain Control During Severe Burn Wound Care: One Military and One Civilian Patient. Pain Med. 2011, 12, 673–678. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Meyer, W.J.; Ramirez, M.; Roberts, L.; Seibel, E.J.; Atzori, B.; Sharar, S.R.; Patterson, D.R. Feasibility of Articulated Arm Mounted Oculus Rift Virtual Reality Goggles for Adjunctive Pain Control During Occupational Therapy in Pediatric Burn Patients. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 397–401. [Google Scholar] [CrossRef]
- Pozeg, P.; Palluel, E.; Ronchi, R.; Solcà, M.; Al-Khodairy, A.-W.; Jordan, X.; Kassouha, A.; Blanke, O. Virtual Reality Improves Embodiment and Neuropathic Pain Caused by Spinal Cord Injury. Neurology 2017, 89, 1894–1903. [Google Scholar] [CrossRef]
- Jordan, M.; Richardson, E.J. Effects of Virtual Walking Treatment on Spinal Cord Injury–Related Neuropathic Pain: Pilot Results and Trends Related to Location of Pain and at-Level Neuronal Hypersensitivity. Am. J. Phys. Med. Rehabil. 2015, 1, 390–396. [Google Scholar] [CrossRef]
- Oneal, B.J.; Patterson, D.R.; Soltani, M.; Teeley, A.; Jensen, M.P. Virtual Reality Hypnosis in the Treatment of Chronic Neuropathic Pain: A Case Report. Int. J. Clin. Exp. Hypn. 2008, 56, 451–462. [Google Scholar] [CrossRef]
- Murray, C.D.; Pettifer, S.; Howard, T.; Patchick, E.L.; Caillette, F.; Kulkarni, J.; Bamford, C. The Treatment of Phantom Limb Pain Using Immersive Virtual Reality: Three Case Studies. Disabil. Rehabil. 2007, 29, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Crowle, S.; Austwick, G.; Henderson Slater, D. Exploratory Findings with Virtual Reality for Phantom Limb Pain; from Stump Motion to Agency and Analgesia. Disabil. Rehabil. 2009, 31, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Kumru, H.; Soler, D.; Vidal, J.; Navarro, X.; Tormos, J.M.; Pascual-Leone, A.; Valls-Sole, J. The Effects of Transcranial Direct Current Stimulation with Visual Illusion in Neuropathic Pain Due to Spinal Cord Injury: An Evoked Potentials and Quantitative Thermal Testing Study: Effect of Pain Treatment on QTT and CHEPs. Eur. J. Pain 2013, 17, 55–66. [Google Scholar] [CrossRef]
- Patterson, D.R.; Wiechman, S.A.; Jensen, M.; Sharar, S.R. Hypnosis Delivered Through Immersive Virtual Reality for Burn Pain: A Clinical Case Series. Int. J. Clin. Exp. Hypn. 2006, 54, 130–142. [Google Scholar] [CrossRef]
- Rose, T.; Nam, C.S.; Chen, K.B. Immersion of Virtual Reality for Rehabilitation—Review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.-M.; Olga, G.-M.; Desiree, L.; Claudia, P.; Ruben, N. Presence, Involvement and Efficacy of a Virtual Reality Intervention on Pain. Stud. Health Technol. Inform. 2010, 154, 97–101. [Google Scholar] [CrossRef]
- Gupta, A.; Scott, K.; Dukewich, M. Innovative Technology Using Virtual Reality in the Treatment of Pain: Does It Reduce Pain via Distraction, or Is There More to It? Pain Med. 2018, 19, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Henwood, P.; Ellis, J.A. Chronic Neuropathic Pain in Spinal Cord Injury: The Patient’s Perspective. Pain Res. Manag. 2004, 9, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, M.; Norrbrink, C. “But I Know What Works”—Patients’ Experience of Spinal Cord Injury Neuropathic Pain Management. Disabil. Rehabil. 2012, 34, 2139–2147. [Google Scholar] [CrossRef]
- Li, A.; Montaño, Z.; Chen, V.J.; Gold, J.I. Virtual Reality and Pain Management: Current Trends and Future Directions. Pain Manag. 2011, 1, 147–157. [Google Scholar] [CrossRef]
- Austin, P.D.; Siddall, P.J. Virtual Reality for the Treatment of Neuropathic Pain in People with Spinal Cord Injuries: A Scoping Review. J. Spinal Cord Med. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Giusti, E.M.; Manzoni, G.M.; Saviola, D.; Gabrielli, S.; Lacerenza, M.; Pietrabissa, G.; Cattivelli, R.; Spatola, C.A.M.; Rossi, A.; et al. What Is the Role of the Placebo Effect for Pain Relief in Neurorehabilitation? Clinical Implications From the Italian Consensus Conference on Pain in Neurorehabilitation. Front. Neurol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Petrovic, P. Placebo and Opioid Analgesia—Imaging a Shared Neuronal Network. Science 2002, 295, 1737–1740. [Google Scholar] [CrossRef]
- Vase, L.; Skyt, I.; Hall, K.T. Placebo, Nocebo, and Neuropathic Pain. Pain 2016, 157, S98–S105. [Google Scholar] [CrossRef] [PubMed]

| ID | Age | Level of Injury | Time Since Injury (Years) | Injury | ASIA |
|---|---|---|---|---|---|
| 1 | 56 | T4 | 23 | Non-Traumatic | D |
| 2 | 45 | C2 | 1 | Traumatic | D |
| 3 | 44 | C6 | 22 | Traumatic | B |
| 4 | 50 | C5 | 36 | Traumatic | B |
| 5 | 65 | L4 | 5 | Non-Traumatic | D |
| 6 | 58 | C5 | 3 | Traumatic | C |
| 7 | 71 | T10 | 6 | Non-Traumatic | C |
| 8 | 51 | C4 | 9 | Traumatic | C |
| Mean | 55 | - | 13 | - | - |
| SEM | 3 | - | 4 | - | - |
| NRS | ITQ | UQO-PQ | ||||
|---|---|---|---|---|---|---|
| ID | Baseline | Post-Scenery | Post-Somatic | Total | Scenery | Somatic |
| 1 | 4 | 4 | 4 | 55 | 30 | 13 |
| 2 | 5 | 5 | 5 | 74 | 20 | 8 |
| 3 | 7 | 5 | 4 | 69 | 38 | 35 |
| 4 | 5 | 5 | 5 | 68 | 51 | 52 |
| 5 | 4 | 2 | 2 | 40 | 45 | 13 |
| 6 | 6 | 2 | 1 | 35 | 44 | 63 |
| 7 | 4 | 2 | 3 | 55 | 88 | 35 |
| 8 | 6 | 0 | 0 | 37 | 55 | 40 |
| Mean | 5.1 | 3.1 | 3.0 | 54.1 | 46.4 | 32.4 |
| SEM | 0.4 | 0.7 | 0.7 | 5.5 | 7.2 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putrino, D.; Tabacof, L.; Breyman, E.; Revis, J.; Soomro, Z.; Chopra, D.; Delaney, K.; Smeragliuolo, A.; Cortes, M. Pain Reduction after Short Exposure to Virtual Reality Environments in People with Spinal Cord Injury. Int. J. Environ. Res. Public Health 2021, 18, 8923. https://doi.org/10.3390/ijerph18178923
Putrino D, Tabacof L, Breyman E, Revis J, Soomro Z, Chopra D, Delaney K, Smeragliuolo A, Cortes M. Pain Reduction after Short Exposure to Virtual Reality Environments in People with Spinal Cord Injury. International Journal of Environmental Research and Public Health. 2021; 18(17):8923. https://doi.org/10.3390/ijerph18178923
Chicago/Turabian StylePutrino, David, Laura Tabacof, Erica Breyman, Jordan Revis, Zulfi Soomro, Divija Chopra, Kathleen Delaney, Anna Smeragliuolo, and Mar Cortes. 2021. "Pain Reduction after Short Exposure to Virtual Reality Environments in People with Spinal Cord Injury" International Journal of Environmental Research and Public Health 18, no. 17: 8923. https://doi.org/10.3390/ijerph18178923
APA StylePutrino, D., Tabacof, L., Breyman, E., Revis, J., Soomro, Z., Chopra, D., Delaney, K., Smeragliuolo, A., & Cortes, M. (2021). Pain Reduction after Short Exposure to Virtual Reality Environments in People with Spinal Cord Injury. International Journal of Environmental Research and Public Health, 18(17), 8923. https://doi.org/10.3390/ijerph18178923








