Comparison of Postoperative Pain and Adverse Effects between Variable-Rate Feedback Infusion and Conventional Fixed-Rate Basal Infusion Modes of Patient-Controlled Epidural Analgesia following Open Gastrectomy: A Randomized Controlled Trial
Abstract
1. Introduction
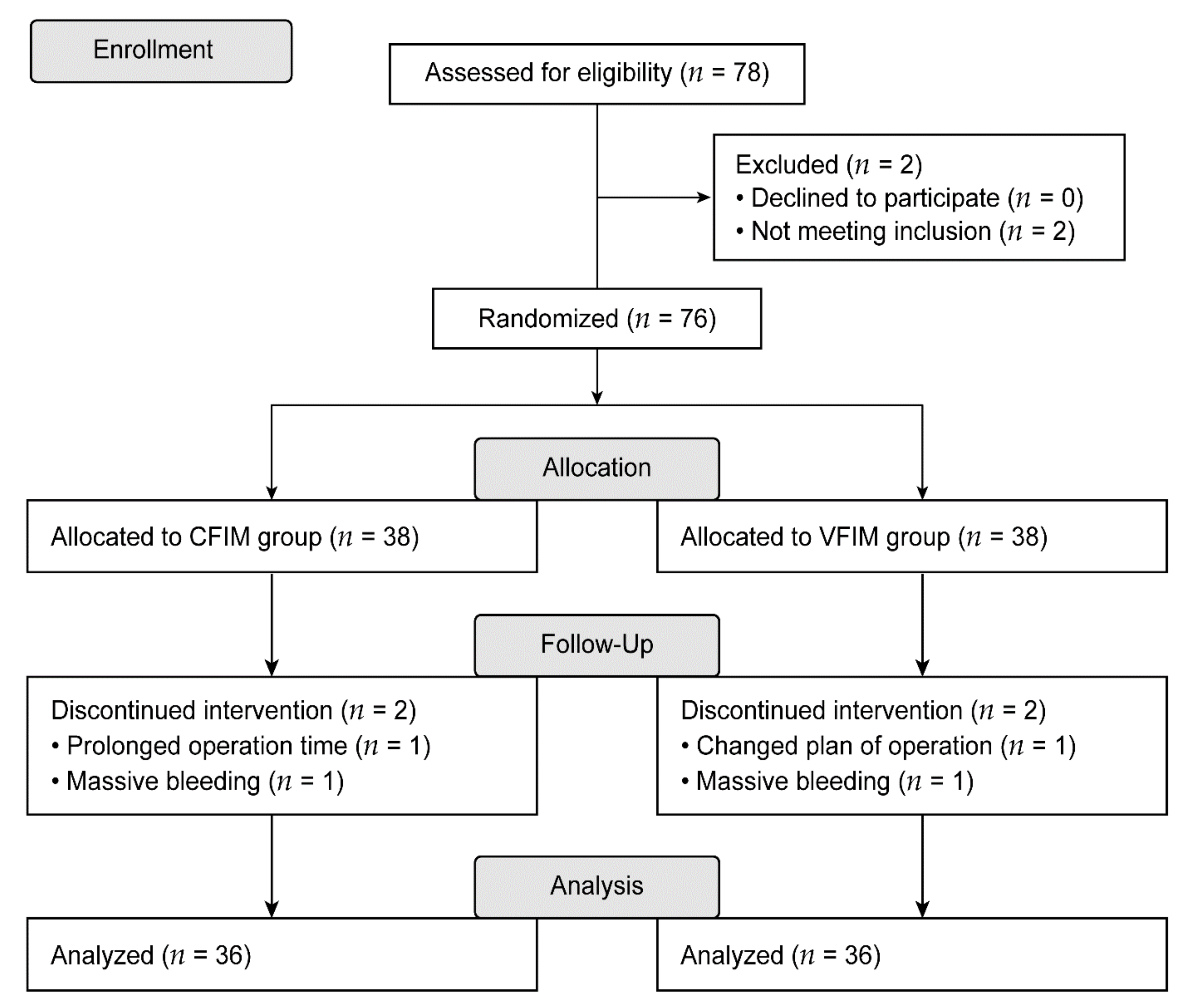
2. Materials and Methods
2.1. Study Population
2.2. Anesthetic Management
2.3. Randomization and Intervention
2.4. Data Collection
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Block, B.M.; Liu, S.S.; Rowlingson, A.J.; Cowan, A.R.; Cowan, J.A., Jr.; Wu, C.L. Efficacy of postoperative epidural analgesia: A meta-analysis. JAMA 2003, 290, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Chen, H.; Liang, J.; Wang, Y. Effect of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain management and short-term outcomes after gastric cancer resection: A retrospective analysis of 3042 consecutive patients between 2010 and 2015. J. Pain Res. 2018, 11, 1743–1749. [Google Scholar] [PubMed]
- Bonnet, F.; Marret, E. Postoperative pain management and outcome after surgery. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Wetterslev, J.; Møiniche, S.; Dahl, J.B. Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, ponv and pain after abdominal surgery. Cochrane Database Syst. Rev. 2000, 7, CD001893. [Google Scholar]
- Grass, J.A. Patient-controlled analgesia. Anesth. Analg. 2005, 101, S44–S61. [Google Scholar] [CrossRef]
- Komatsu, H.; Matsumoto, S.; Mitsuhata, H.; Abe, K.; Toriyabe, S. Comparison of patient-controlled epidural analgesia with and without background infusion after gastrectomy. Anesth. Analg. 1998, 87, 907–910. [Google Scholar]
- Komatsu, H.; Matsumoto, S.; Mitsuhata, H. Comparison of patient-controlled epidural analgesia with and without night-time infusion following gastrectomy. Br. J. Anaesth. 2001, 87, 633–635. [Google Scholar] [CrossRef][Green Version]
- Sng, B.L.; Sia, A.T.; Lim, Y.; Woo, D.; Ocampo, C. Comparison of computer-integrated patient-controlled epidural analgesia and patient-controlled epidural analgesia with a basal infusion for labour and delivery. Anaesth. Intensive Care 2009, 37, 46–53. [Google Scholar] [CrossRef]
- Smythe, M.A.; Zak, M.B.; O’Donnell, M.P.; Schad, R.F.; Dmuchowski, C.F. Patient-controlled analgesia versus patient-controlled analgesia plus continuous infusion after hip replacement surgery. Ann. Pharmacother. 1996, 30, 224–227. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, K.; Tan, P.H.; Chia, Y.Y. Effects of postoperative background pca morphine infusion on pain management and related side effects in patients undergoing abdominal hysterectomy. J. Clin. Anesth. 2011, 23, 124–129. [Google Scholar] [CrossRef]
- Koh, J.C.; Song, Y.; Kim, S.Y.; Park, S.; Ko, S.H.; Han, D.W. Postoperative pain and patient-controlled epidural analgesia-related adverse effects in young and elderly patients: A retrospective analysis of 2435 patients. J. Pain Res. 2017, 10, 897–904. [Google Scholar] [CrossRef]
- Ishida, T.; Naito, T.; Sato, H.; Kawakami, J. Relationship between the plasma fentanyl and serum 4β-hydroxycholesterol based on cyp3a5 genotype and gender in patients with cancer pain. Drug Metab. Pharmacokinet. 2016, 31, 242–248. [Google Scholar] [CrossRef]
- Simon, M.J.G.; Veering, B.T. Factors affecting the pharmacokinetics and neural block characteristics after epidural administration of local anaesthetics. Eur. J. Pain Suppl. 2010, 4, 209–218. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, C.W.; Kang, H.; Park, Y.H.; Choi, G.J.; Jung, Y.H.; Woo, Y.C. A comparison of 2 intravenous patient-controlled analgesia modes after spinal fusion surgery: Constant-rate background infusion versus variable-rate feedback infusion, a randomized controlled trial. Medicine 2019, 98, e14753. [Google Scholar] [CrossRef]
- Jung, K.T.; So, K.Y.; Kim, S.U.; Kim, S.H. The optimizing background infusion mode decreases intravenous patient-controlled analgesic volume and opioid consumption compared to fixed-rate background infusion in patients undergoing laparoscopic cholecystectomy: A prospective, randomized, controlled, double-blind study. Medicina 2021, 57, 42. [Google Scholar]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, A.; Poepping, D.M.; Gerss, J.; Zahn, P.K.; Pogatzki-Zahn, E.M. Sex-related differences of patient-controlled epidural analgesia for postoperative pain. Pain 2012, 153, 238–244. [Google Scholar] [CrossRef]
- Chang, K.Y.; Dai, C.Y.; Ger, L.P.; Fu, M.J.; Wong, K.C.; Chan, K.H.; Tsou, M.Y. Determinants of patient-controlled epidural analgesia requirements: A prospective analysis of 1753 patients. Clin. J. Pain 2006, 22, 751–756. [Google Scholar] [CrossRef]
- Sommer, M.; de Rijke, J.M.; van Kleef, M.; Kessels, A.G.; Peters, M.L.; Geurts, J.W.; Gramke, H.F.; Marcus, M.A. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur. J. Anaesth. 2008, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Svensson, I.; Sjöström, B.; Haljamäe, H. Assessment of pain experiences after elective surgery. J. Pain Symptom Manag. 2000, 20, 193–201. [Google Scholar] [CrossRef]
- Wong, K.; Chong, J.L.; Lo, W.K.; Sia, A.T. A comparison of patient-controlled epidural analgesia following gynaecological surgery with and without a background infusion. Anaesthesia 2000, 55, 212–216. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised international association for the study of pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Dirkwinkel, M.; Gralow, I.; Colak-Ekici, R.; Wolowski, A.; Marziniak, M.; Evers, S. The influence of repetitive painful stimulation on peripheral and trigeminal pain thresholds. J. Neurol. Sci. 2008, 273, 108–111. [Google Scholar] [CrossRef]
- Banozic, A.; Miljkovic, A.; Bras, M.; Puljak, L.; Kolcic, I.; Hayward, C.; Polasek, O. Neuroticism and pain catastrophizing aggravate response to pain in healthy adults: An experimental study. Korean J. Pain 2018, 31, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Lue, Y.J.; Wang, H.H.; Cheng, K.I.; Chen, C.H.; Lu, Y.M. Thermal pain tolerance and pain rating in normal subjects: Gender and age effects. Eur. J. Pain 2018, 22, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Watcha, M.F.; White, P.F. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 1992, 77, 162–184. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Andersen, R.; Krohg, K. Pain as a major cause of postoperative nausea. Can. Anaesth. Soc. J. 1976, 23, 366–369. [Google Scholar] [CrossRef]
- Chia, Y.Y.; Kuo, M.C.; Liu, K.; Sun, G.C.; Hsieh, S.W.; Chow, L.H. Does postoperative pain induce emesis? Clin. J. Pain 2002, 18, 317–323. [Google Scholar] [CrossRef]
| CFIM Group (n = 36) | VFIM Group (n = 36) | p Value | |
|---|---|---|---|
| Age, years | 57.5 [51, 60] | 56.5 [49, 61] | 0.969 |
| Body mass index, kg/m2 | 22.5 ± 3.2 | 23.6 ± 2.8 | 0.114 |
| Male sex | 22 (61%) | 27 (75%) | 0.206 |
| ASA physical status, I/II/III | 21/14/1 | 18/18/0 | 0.343 |
| Comorbidities | |||
| Hypertension | 5 (14%) | 8 (22%) | 0.358 |
| Diabetes mellitus | 6 (17%) | 4 (11%) | 0.496 |
| Subtotal/Total | 25/11 | 26/10 | 0.795 |
| Anesthesia time, min | 182.7 ± 32.8 | 174.4 ± 31.6 | 0.278 |
| Operation time, min | 145.7 ± 32.8 | 138.2 ± 32.6 | 0.403 |
| Fluid intake, mL | 1200 [1050, 1400] | 1200 [1000, 1500] | 0.796 |
| Blood loss, mL | 135 [70, 235] | 100 [77.5, 200] | 0.578 |
| Urine output, mL | 157.5 [110, 280] | 142.5 [97.5, 250] | 0.325 |
| Administered amounts of remifentanil, µg | 524 [432, 681] | 513 [433, 576] | 0.450 |
| Administered amounts of ephedrine, mg | 6 [0, 8] | 4 [0, 12] | 0.862 |
| Mean blood pressure, mmHg | |||
| 0 min | 89.1 ± 15.5 | 85.8 ± 13.7 | 0.346 |
| 30 min | 87.3 ± 12.7 | 86.2 ± 11.8 | 0.707 |
| 60 min | 92.1 ± 11.6 | 87.3 ± 9.9 | 0.060 |
| 90 min | 88.3 ± 12.4 | 85.2 ± 9.0 | 0.253 |
| Heart rate, bpm | |||
| 0 min | 72 [63, 79] | 66 [63, 74] | 0.245 |
| 30 min | 80 [70, 89] | 79 [66, 86] | 0.456 |
| 60 min | 75 [67, 82] | 71 [65, 78] | 0.195 |
| 90 min | 73 [67, 77] | 68 [63, 74] | 0.056 |
| Bispectral index | |||
| 0 min | 43 [36, 56] | 40 [35, 46] | 0.247 |
| 30 min | 40 [33, 46] | 38 [35, 42] | 0.298 |
| 60 min | 38 [30, 46] | 35 [30, 40] | 0.111 |
| 90 min | 35 [28, 43] | 32 [29, 36] | 0.272 |
| CFIM Group (n = 36) | VFIM Group (n = 36) | p Value | |
|---|---|---|---|
| NRS | |||
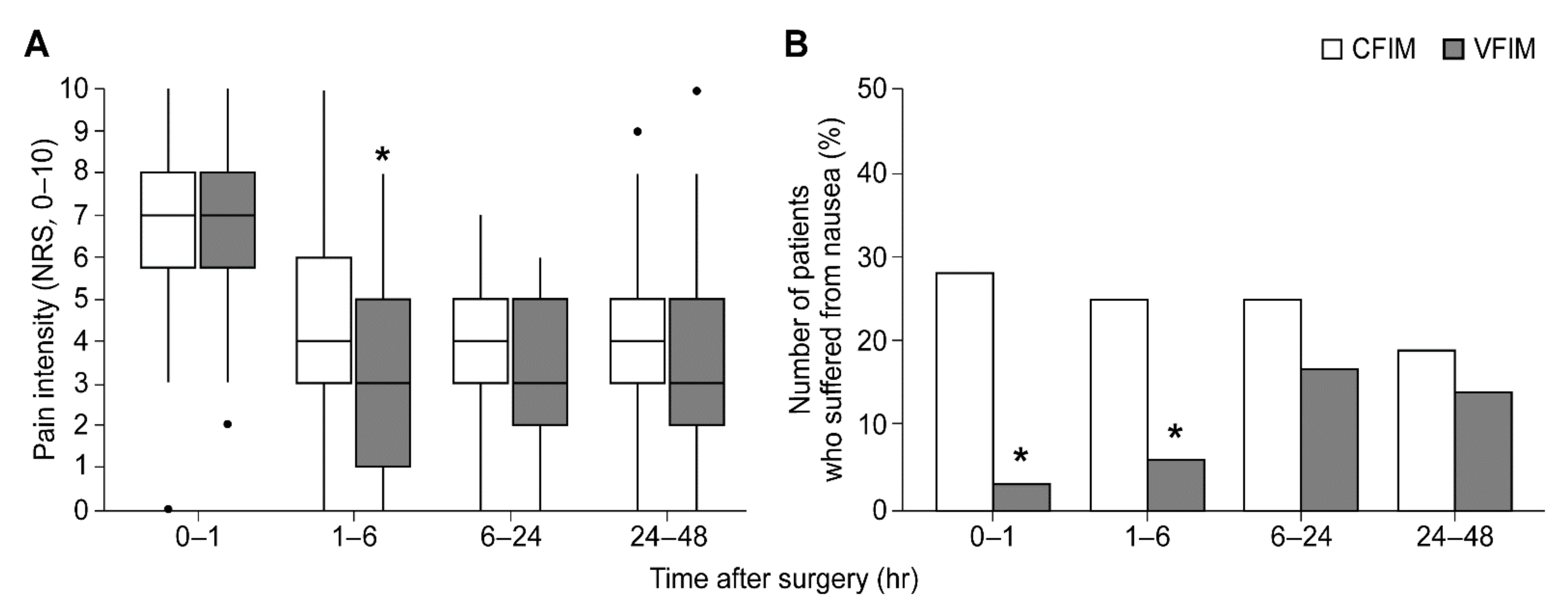
| 0–1 h | 7 [5.5, 8] | 7 [5.5, 8] | 0.613 |
| 1–6 h | 4 [3, 6] | 3 [1, 5] | 0.031 * |
| 6–24 h | 4 [3, 5] | 3 [2, 5] | 0.137 |
| 24–48 h | 4 [3, 5] | 3 [2, 5] | 0.538 |
| Administered dose of PCEA | |||
| 0–1 h | 6.08 [5.37, 7.69] | 6.24 [5.49, 7.35] | 0.503 |
| 1–6 h | 27.4 [19.31, 32.54] | 28.32 [17.19, 36] | 0.305 |
| 6–24 h | 97.74 [86.4, 108.29] | 99.16 [84.04, 122.06] | 0.540 |
| 24–48 h | 215.92 [204.47, 227.84] | 207.15 [166.73, 250] | 0.953 |
| Number of patients who received rescue opioids | |||
| 0–1 h | 28 (78%) | 22 (61%) | 0.125 |
| 1–6 h | 22 (61%) | 19 (53%) | 0.475 |
| 6–24 h | 20 (56%) | 17 (47%) | 0.479 |
| 24–48 h | 14 (39%) | 17 (47%) | 0.475 |
| CFIM Group (n = 36) | VFIM Group (n = 36) | p Value | |
|---|---|---|---|
| Postoperative hospital stays, days | 7 [6, 7.5] | 7 [6, 8] | 0.394 |
| Nausea | |||
| 0–1 h | 10 (28%) | 1 (3%) | 0.003 * |
| 1–6 h | 9 (25%) | 2 (6%) | 0.022 * |
| 6–24 h | 9 (25%) | 6 (17%) | 0.384 |
| 24–48 h | 7 (19%) | 5 (14%) | 0.527 |
| Vomiting | |||
| 0–1 h | 1 (3%) | 0 (0%) | >0.999 |
| 1–6 h | 0 (0%) | 0 (0%) | - |
| 6–24 h | 0 (0%) | 1 (3%) | >0.999 |
| 24–48 h | 0 (0%) | 0 (0%) | - |
| Dizziness | |||
| 0–1 h | 2 (6%) | 1 (3%) | >0.999 |
| 1–6 h | 2 (6%) | 1 (3%) | >0.999 |
| 6–24 h | 2 (6%) | 5 (14%) | 0.429 |
| 24–48 h | 4 (11%) | 4 (11%) | >0.999 |
| Hypotension | |||
| 0–1 h | 2 (6%) | 0 (0%) | 0.493 |
| 1–6 h | 1 (3%) | 1 (3%) | >0.999 |
| 6–24 h | 2 (6%) | 7 (19%) | 0.151 |
| 24–48 h | 1 (3%) | 3 (8%) | 0.614 |
| Tachypnea | |||
| 0–1 h | 0 (0%) | 2 (6%) | 0.493 |
| 1–6 h | 0 (0%) | 1 (3%) | >0.999 |
| 6–24 h | 0 (0%) | 0 (0%) | - |
| 24–48 h | 0 (0%) | 0 (0%) | - |
| Numbness | |||
| 0–1 h | 0 (0%) | 0 (0%) | - |
| 1–6 h | 1 (3%) | 0 (0%) | >0.999 |
| 6–24 h | 1 (3%) | 0 (0%) | >0.999 |
| 24–48 h | 0 (0%) | 0 (0%) | - |
| Pruritus | |||
| 0–1 h | 0 (0%) | 0 (0%) | - |
| 1–6 h | 0 (0%) | 0 (0%) | - |
| 6–24 h | 0 (0%) | 0 (0%) | - |
| 24–48 h | 1 (3%) | 1 (3%) | >0.999 |
| Discontinuation of PCEA | 2 (6%) | 3 (8%) | >0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.K.; Kim, N.Y.; Lee, J.S.; Shin, H.J.; Kim, H.G.; Lee, S.W.; Koh, J.C.; Yoo, Y.C. Comparison of Postoperative Pain and Adverse Effects between Variable-Rate Feedback Infusion and Conventional Fixed-Rate Basal Infusion Modes of Patient-Controlled Epidural Analgesia following Open Gastrectomy: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 8777. https://doi.org/10.3390/ijerph18168777
Jang YK, Kim NY, Lee JS, Shin HJ, Kim HG, Lee SW, Koh JC, Yoo YC. Comparison of Postoperative Pain and Adverse Effects between Variable-Rate Feedback Infusion and Conventional Fixed-Rate Basal Infusion Modes of Patient-Controlled Epidural Analgesia following Open Gastrectomy: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(16):8777. https://doi.org/10.3390/ijerph18168777
Chicago/Turabian StyleJang, Yoo Kyung, Na Young Kim, Jeong Soo Lee, Hye Jung Shin, Hyoung Gyun Kim, Suk Woo Lee, Jae Chul Koh, and Young Chul Yoo. 2021. "Comparison of Postoperative Pain and Adverse Effects between Variable-Rate Feedback Infusion and Conventional Fixed-Rate Basal Infusion Modes of Patient-Controlled Epidural Analgesia following Open Gastrectomy: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 16: 8777. https://doi.org/10.3390/ijerph18168777
APA StyleJang, Y. K., Kim, N. Y., Lee, J. S., Shin, H. J., Kim, H. G., Lee, S. W., Koh, J. C., & Yoo, Y. C. (2021). Comparison of Postoperative Pain and Adverse Effects between Variable-Rate Feedback Infusion and Conventional Fixed-Rate Basal Infusion Modes of Patient-Controlled Epidural Analgesia following Open Gastrectomy: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(16), 8777. https://doi.org/10.3390/ijerph18168777






