Effects of Different Types of Exercise Training on Fine Motor Skills and Testosterone Concentration in Adolescents: A Cluster Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.4. Intervention
2.5. Data Analysis
3. Results
3.1. Cardiovascular Performance
3.2. Static Balance Performance
3.3. Hand–Eye Coordination Performance
3.4. Fine Motor Skills
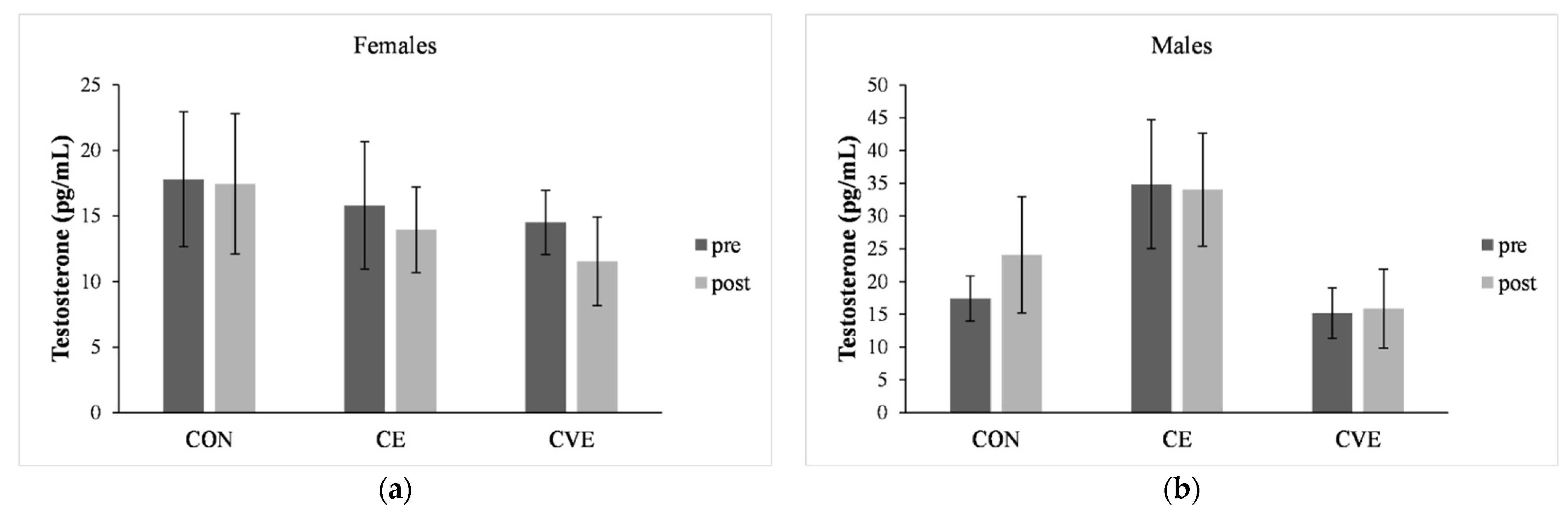
3.5. Testosterone Concentration
3.6. Relationship between Testosterone and Fine Motor Skills
4. Discussion
4.1. Effect of CE and CVE Training on Fine Motor Skills
4.2. Effect of CE and CVE Training on Testosterone
4.3. Association between Testosterone and Fine Motor Skills
4.4. Limitations and Directions for Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biddle, S.J.; Ciaccioni, S.; Thomas, G.; Vergeer, I. Physical activity and mental health in children and adolescents: An updated review of reviews and an analysis of causality. Psychol. Sport Exerc. 2019, 42, 146–155. [Google Scholar] [CrossRef]
- Boreham, C.; Riddoch, C. The physical activity, fitness and health of children. J. Sports Sci. 2001, 19, 915–929. [Google Scholar] [CrossRef]
- Janssen, I.; LeBlanc, A.G. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000, 71, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Young, C.J.; Levine, S.C.; Mix, K.S. The connection between spatial and mathematical ability across development. Front. Psychol. 2018, 9, 755. [Google Scholar] [CrossRef]
- Koutsandréou, F.; Wegner, M.; Niemann, C.; Budde, H. Effects of motor versus cardiovascular exercise training on children’s working memory. Med. Sci. Sports Exerc. 2016, 48, 1144–1152. [Google Scholar] [CrossRef]
- Grissmer, D.; Grimm, K.J.; Aiyer, S.M.; Murrah, W.M.; Steele, J.S. Fine motor skills and early comprehension of the world: Two new school readiness indicators. Dev. Psychol. 2010, 46, 1008–1017. [Google Scholar] [CrossRef]
- Gaul, D.; Issartel, J. Fine motor skill proficiency in typically developing children: On or off the maturation track? Hum. Mov. Sci. 2016, 46, 78–85. [Google Scholar] [CrossRef]
- Geertsen, S.S.; Thomas, R.; Larsen, M.N.; Dahn, I.M.; Andersen, J.N.; Krause-Jensen, M.; Korup, V.; Nielsen, C.M.; Wienecke, J.; Ritz, C.; et al. Motor skills and exercise capacity are associated with objective measures of cognitive functions and academic performance in preadolescent children. PLoS ONE 2016, 11, e0161960. [Google Scholar] [CrossRef] [PubMed]
- Gentier, I.; D’Hondt, E.; Shultz, S.; Deforche, B.; Augustijn, M.; Hoorne, S.; Verlaecke, K.; de Bourdeaudhuij, I.; Lenoir, M. Fine and gross motor skills differ between healthy-weight and obese children. Res. Dev. Disabil. 2013, 34, 4043–4051. [Google Scholar] [CrossRef] [PubMed]
- Payne, V.G.; Isaacs, L.D. Fine motor development. In Human Motor Development: A Lifespan Approach, 9th ed.; Payne, V.G., Isaacs, L.D., Eds.; Routledge (Taylor & Francis Group): New York, NY, USA, 2017; p. 240. [Google Scholar]
- Pozo-Rico, T.; Sandoval, I. Can Academic Achievement in Primary School Students Be Improved Through Teacher Training on Emotional Intelligence as a Key Academic Competency? Front. Psychol. 2020, 10, 2976. [Google Scholar] [CrossRef] [PubMed]
- Steinmayr, R.; Weidinger, A.F.; Schwinger, M.; Spinath, B. The importance of students’ motivation for their academic achievement–replicating and extending previous findings. Front. Psychol. 2019, 10, 1730. [Google Scholar] [CrossRef] [PubMed]
- Budde, H.; Schwarzc, R.; Velasques, B.; Ribeiro, P.; Holzweg, M.; Machado, S.; Brazaitis, M.; Staack, F.; Wegner, M. The need for differentiating between exercise, physical activity, and training. Autoimmun. Rev. 2016, 15, 110–111. [Google Scholar] [CrossRef]
- Gronwald, T.; Törpel, A.; Herold, F.; Budde, H. Perspective of Dose and Response for Individualized Physical Exercise and Training Prescription. J. Funct. Morphol. Kinesiol. 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Herbsleb, M.; de la Cruz, F.; Schumann, A.; Köhler, S.; Puta, C.; Gabriel, H.W.; Reichenbach, J.R.; Bär, K.J. Changes in fMRI activation in anterior hippocampus and motor cortex during memory retrieval after an intense exercise intervention. Biol. Psychol. 2017, 124, 65–78. [Google Scholar] [CrossRef]
- Riebe, D. General Principles of exercise Prescription. In ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Pescatello, L.S., Arena, R., Riebe, D., Thompson, P.D., Eds.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2014; pp. 177–179. [Google Scholar]
- Voelcker-Rehage, C.; Godde, B.; Staudinger, U.M. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 2011, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.A.; Celnik, P.A.; Rothwell, J.C. Cerebellar–Motor Cortex Connectivity: One or Two Different Networks? J. Neurosci. 2020, 40, 4230–4239. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage 2012, 59, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Voelcker-Rehage, C.; Niemann, C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci. Biobehav. Rev. 2013, 37, 2268–2295. [Google Scholar] [CrossRef]
- Black, J.E.; Isaacs, K.R.; Anderson, B.J.; Alcantara, A.A.; Greenough, W.T. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. USA 1990, 87, 5568–5572. [Google Scholar] [CrossRef]
- Wegner, M.; Koedijker, J.M.; Budde, H. The effect of acute exercise and psychosocial stress on fine motor skills and testosterone concentration in the saliva of high school students. PLoS ONE 2014, 9, e92953. [Google Scholar] [CrossRef]
- Grandys, M.; Majerczak, J.; Duda, K.; Zapart-Bukowska, J.; Kulpa, J.; Zoladz, J.A. Endurance training of moderate intensity increases testosterone concentration in young, healthy men. Int. J. Sports Med. 2009, 30, 489–495. [Google Scholar] [CrossRef]
- Hayes, L.D.; Herbert, P.; Sculthorpe, N.F.; Grace, F.M. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr. Connect. 2017, 6, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Akko, D.P.; Koutsandreou, F.; Murillo-Rodriguez, E.; Wegner, M.; Budde, H. The effects of an exercise training on steroid hormones in preadolescent children-A moderator for enhanced cognition? Physiol. Behav. 2020, 227, 113168. [Google Scholar] [CrossRef] [PubMed]
- Niemann, C.; Wegner, M.; Voelcker-Rehage, C.; Holzweg, M.; Arafat, A.M.; Budde, H. Influence of acute and chronic physical activity on cognitive performance and saliva testosterone in preadolescent school children. Ment. Health Phys. Act. 2013, 6, 197–204. [Google Scholar] [CrossRef]
- Fargo, K.N.; Foecking, E.M.; Jones, K.J.; Sengelaub, D.R. Neuroprotective actions of androgens on motoneurons. Front. Neuroendocrinol. 2009, 30, 130–141. [Google Scholar] [CrossRef]
- Byers, J.S.; Huguenard, A.L.; Kuruppu, D.; Liu, N.K.; Xu, X.M.; Sengelaub, D.R. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J. Comp. Neurol. 2012, 520, 2683–2696. [Google Scholar] [CrossRef]
- Oki, K.; Law, T.D.; Loucks, A.B.; Clark, B.C. The effects of testosterone and insulin-like growth factor 1 on motor system form and function. Exp. Gerontol. 2015, 64, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Tammelin, T.; Ekelund, U.; Remes, J.; Näyhä, S. Physical activity and sedentary behaviors among Finnish youth. Med. Sci. Sports Exerc. 2007, 39, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr. Suppl. 2006, 450, 38–46. [Google Scholar] [CrossRef]
- Vasold, K.L.; Parks, A.C.; Phelan, D.; Pontifex, M.B.; Pivarnik, J.M. Reliability and Validity of Commercially Available Low-Cost Bioelectrical Impedance Analysis. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 406–410. [Google Scholar] [CrossRef]
- Rasmussen, A.R.; Wohlfahrt-Veje, C.; de Renzy-Martin, K.T.; Hagen, C.P.; Tinggaard, J.; Mouritsen, A.; Mieritz, M.G.; Main, K.M. Validity of self-assessment of pubertal maturation. Pediatrics 2015, 135, 86–93. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Watkins, D.J.; Afeiche, M.C.; Zhang, Z.; Sánchez, B.N.; Cantonwine, D.; Mercado-García, A.; Blank-Goldenberg, C.; Meeker, J.D.; Téllez-Rojo, M.M.; et al. Validity of Self-Assessed Sexual Maturation Against Physician Assessments and Hormone Levels. J. Pediatr. 2017, 186, 172–178.e3. [Google Scholar] [CrossRef]
- Goodie, J.L.; Larkin, K.T.; Schauss, S. Validation of Polar heart rate monitor for assessing heart rate during physical and mental stress. J. Psychophysiol. 2000, 14, 159–164. [Google Scholar] [CrossRef]
- Freedson, P.S.; Cureton, K.J.; Heath, G.W. Status of field-based fitness testing in children and youth. Prev. Med. 2000, 31, S77–S85. [Google Scholar] [CrossRef]
- Mayorga-Vega, D.; Aguilar-Soto, P.; Viciana, J. Criterion-Related Validity of the 20-M Shuttle Run Test for Estimating Cardiorespiratory Fitness: A Meta-Analysis. J. Sports Sci. Med. 2015, 14, 536–547. [Google Scholar] [PubMed]
- Kemper, H.C.; Van Mechelen, W. Physical Fitness Testing of Children: A European Perspective. Pediatr. Exerc. Sci. 1996, 8, 201–214. [Google Scholar] [CrossRef]
- Sember, V.; Grošelj, J.; Pajek, M. Balance Tests in Pre-Adolescent Children: Retest Reliability, Construct Validity, and Relative Ability. Int. J. Environ. Res. Public Health 2020, 17, 5474. [Google Scholar] [CrossRef]
- Beashel, P.; Taylor, J. Fitness For Health And Performance. In The World of Sport Examined; Beashel, P., Taylor, J., Eds.; Thomas Nelson and Sons: Oxford, UK, 1997; p. 66. [Google Scholar]
- Faber, I.R.; Oosterveld, F.G.; Nijhuis-Van der Sanden, M.W. Does an eye-hand coordination test have added value as part of talent identification in table tennis? A validity and reproducibility study. PLoS ONE 2014, 9, e85657. [Google Scholar] [CrossRef]
- Petermann, F.; Bös, K.; Kastner, J. Movement Assessment Battery for Children-2 (Movement ABC-2): Deutsprachige Adaptation. Manual; Pearson: Frankfurt, Germany, 2010. [Google Scholar]
- Gísladóttir, T.; Haga, M.; Sigmundsson, H. Motor Competence in Adolescents: Exploring Association with Physical Fitness. Sports 2019, 7, 176. [Google Scholar] [CrossRef]
- Crewther, B.T.; Lowe, T.E.; Ingram, J.; Weatherby, R.P. Validating the salivary testosterone and cortisol concentration measures in response to short high-intensity exercise. J. Sports Med. Phys. Fitness. 2010, 50, 85–92. [Google Scholar] [PubMed]
- Vilūnienė, A.; Trinkūnienė, L. Šiuolaikinė kūno kultūros pamoka: žaidimai. In Modern Physical Education Lesson: Games; Lithuanian Sports University: Kaunas, Lithuania, 2014. [Google Scholar]
- Machado, F.A.; Denadai, B.S. Validity of maximum heart rate prediction equations for children and adolescents. Arq. Bras. Cardiol. 2011, 97, 136–140. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.; Nieman, D.C.; Swain, D.P. American college of sports medicine position stand. quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Ferguson, C. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pr. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: New York, NY, USA, 1988; pp. 75–81. [Google Scholar]
- Litleskare, S.; Enoksen, E.; Sandvei, M.; Støen, L.; Stensrud, T.; Johansen, E.; Jensen, J. Sprint Interval Running and Continuous Running Produce Training Specific Adaptations, Despite a Similar Improvement of Aerobic Endurance Capacity—A Randomized Trial of Healthy Adults. Int. J. Environ. Res. Public Health 2020, 17, 3865. [Google Scholar] [CrossRef]
- Lubans, D.R.; Morgan, P.J.; Cliff, D.P.; Barnett, L.M.; Okely, A.D. Fundamental Movement Skills in Children and Adolescents. Sports Med. 2010, 40, 1019–1035. [Google Scholar] [CrossRef]
- Torabi, F.; Farahani, A.; Safakish, S.; Ramezankhani, A.; Dehghan, F. Evaluation of motor proficiency and adiponectin in adolescent students with attention deficit hyperactivity disorder after high-intensity intermittent training. Psychiatry Res. 2018, 261, 40–44. [Google Scholar] [CrossRef]
- Brown, C.G. Improving fine motor skills in young children: An intervention study. Educ. Psychol. Pract. 2010, 26, 269–278. [Google Scholar] [CrossRef]
- Budde, H.; Voelcker-Rehage, C.; Pietrassyk-Kendziorra, S.; Ribeiro, P.; Tidow, G. Acute coordinative exercise improves attentional performance in adolescents. Neurosci. Lett. 2008, 441, 219–223. [Google Scholar] [CrossRef]
- Rinne, P.; Hassan, M.; Fernandes, C.; Han, E.; Hennessy, E.; Waldman, A.; Sharma, P.; Soto, D.; Leech, R.; Malhotra, P.A.; et al. Motor dexterity and strength depend upon integrity of the attention-control system. Proc. Natl. Acad. Sci. USA 2018, 115, E536–E545. [Google Scholar] [CrossRef]
- Budde, H.; Pietrassyk-Kendziorra, S.; Bohm, S.; Voelcker-Rehage, C. Hormonal responses to physical and cognitive stress in a school setting. Neurosci. Lett. 2010, 474, 131–134. [Google Scholar] [CrossRef]
- Budde, H.; Voelcker-Rehage, C.; Pietrassyk-Kendziorra, S.; Machado, S.; Ribeiro, P.; Arafat, A.M. Steroid hormones in the saliva of adolescents after different exercise intensities and their influence on working memory in a school setting. Psychoneuroendocrinology 2010, 35, 382–391. [Google Scholar] [CrossRef]
- Wilkerson, J.E.; Horvath, S.M.; Gutin, B. Plasma testosterone during treadmill exercise. J. Appl. Physiol. 1980, 49, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Best, J.R. Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Dev. Rev. 2010, 30, 331–351. [Google Scholar] [CrossRef]
- Van der Borght, K.; Kóbor-Nyakas, D.É.; Klauke, K.; Eggen, B.J.; Nyakas, C.; Van der Zee, E.; Meerlo, P. Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus 2009, 19, 928–936. [Google Scholar] [CrossRef]
- Miall, R.C.; Reckess, G.Z.; Imamizu, H. The cerebellum coordinates eye and hand tracking movements. Nat. Neurosci. 2001, 4, 638–644. [Google Scholar] [CrossRef]
- Thomas, A.R.; Lacadie, C.; Vohr, B.; Ment, L.R.; Scheinost, D. Fine motor skill mediates visual memory ability with microstructural neuro-correlates in cerebellar peduncles in prematurely born adolescents. Cereb. Cortex 2017, 27, 322–329. [Google Scholar] [CrossRef][Green Version]
- Schutter, D.J.; Meuwese, R.; Bos, M.G.; Crone, E.A.; Peper, J.S. Exploring the role of testosterone in the cerebellum link to neuroticism: From adolescence to early adulthood. Psychoneuroendocrinology 2017, 78, 203–212. [Google Scholar] [CrossRef]
- Duff, S.V.; Aaron, D.H.; Gogola, G.R.; Valero-Cuevas, F.J. Innovative evaluation of dexterity in pediatrics. J. Hand Ther. 2015, 28, 144–150. [Google Scholar] [CrossRef][Green Version]
| Characteristic | CON | CE | CVE |
|---|---|---|---|
| Age (years) | 12.43 (0.62) | 13.15 (0.99) | 12.73 (0.79) |
| BMI (kg/m2) | 19.22 (2.12) | 19.41 (1.95) | 19.54 (2.46) |
| Tanner (stage) | 3.37 (0.68) | 3.29 (0.93) | 3.10 (0.66) |
| Exercise | Aim | Tasks | Modification |
|---|---|---|---|
| Cardiovascular exercise (game) | To catch a ball faster than your competitor | Participants are divided in two groups and successively calculated. Physical Education teacher throws a ball and calls a random number. Those participants (according to the calculation) must run and catch a ball. | Fast walking instead of running; Running sideways |
 | |||
| Coordinative exercise (game) | To complete the task 10 times and do it faster than your competitor | Participants break into pairs. One of the participants jumps over a jumping rope and throws a rope on the ground that forms a loop. The participant stands on one leg in the loop and says “one”. After that, the participant jumps over a rope twice and stands in the loop while says “one, two”, etc. The participant repeats the task until makes a mistake (e.g., losing balance, not forming a loop) and gives the rope to the competitor. | Standing on one leg and clapping hands; Saying letters instead of numbers |
 |
| Variable | CON | CE | CVE |
|---|---|---|---|
| Shuttle run test (score) | |||
| pre | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.3) | 4.0 (3.0, 4.5) |
| post | 5.0 (4.0, 6.0) *** | 3.0 (2.0, 5.0) *** | 6.0 (4.0, 7.0) *** |
| Flamingo balance test (score) | |||
| pre | 7.0 (3.0, 15.0) | 7.0 (5.0, 11.0) | 8.0 (7.0, 12.3) |
| post | 12.0 (5.0, 18.0) ** | 4.0 (3.0, 10.0) ** | 10.0 (7.0, 13.0) |
| Hand–eye coordination test (score) | |||
| pre | 14.0 (11.0, 16.0) | 15.0 (13.0, 20.0) | 15.0 (12.0, 18.0) |
| post | 13.0 (10.0, 18.0) | 18.0 (16.0, 23.0) *** | 19.0 (17.0, 22.0) *** |
| Drawing trail test (score) | |||
| pre | 1.0 (0.0, 3.0) | 2.0 (0.0, 3.0) | 2.0 (1.0, 3.0) |
| post | 2.0 (1.0, 4.0) * | 2.0 (1.0, 4.0) | 1.0 (0.0, 4.0) |
| Factor | df | F | Sig. | Partial Eta Squared | Observed Power |
|---|---|---|---|---|---|
| Time (within-subject) | 1 | 2.638 | 0.107 | 0.021 | 0.364 |
| Group (between-trial) | 2 | 4.986 | 0.008 | 0.074 | 0.804 |
| Time × Group | 2 | 2.620 | 0.077 | 0.041 | 0.513 |
| Variable | CON | CE | CVE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T(Pre) | T(Post) | ΔT | T(Pre) | T(Post) | ΔT | T(Pre) | T(Post) | ΔT | |
| T(pre) | — | — | — | ||||||
| T(post) | 0.30 | — | 0.74 *** | — | 0.57 *** | — | |||
| ΔT | −0.23 | 0.51 *** | — | −0.39 * | 0.26 | — | −0.14 | 0.45 ** | — |
| DTT(pre) | 0.02 | 0.23 | 0.20 | −0.10 | 0.04 | 0.21 | 0.03 | 0.07 | 0.18 |
| DTT(post) | 0.17 | 0.14 | 0.03 | −0.03 | −0.05 | −0.03 | 0.14 | 0.31 * | 0.18 |
| ΔDTT | 0.24 | 0.01 | −0.13 | 0.07 | −0.09 | −0.24 | 0.05 | 0.18 | −0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knatauskaitė, J.; Pukėnas, K.; Trinkūnienė, L.; Budde, H. Effects of Different Types of Exercise Training on Fine Motor Skills and Testosterone Concentration in Adolescents: A Cluster Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 8243. https://doi.org/10.3390/ijerph18168243
Knatauskaitė J, Pukėnas K, Trinkūnienė L, Budde H. Effects of Different Types of Exercise Training on Fine Motor Skills and Testosterone Concentration in Adolescents: A Cluster Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(16):8243. https://doi.org/10.3390/ijerph18168243
Chicago/Turabian StyleKnatauskaitė, Justė, Kazimieras Pukėnas, Laima Trinkūnienė, and Henning Budde. 2021. "Effects of Different Types of Exercise Training on Fine Motor Skills and Testosterone Concentration in Adolescents: A Cluster Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 16: 8243. https://doi.org/10.3390/ijerph18168243
APA StyleKnatauskaitė, J., Pukėnas, K., Trinkūnienė, L., & Budde, H. (2021). Effects of Different Types of Exercise Training on Fine Motor Skills and Testosterone Concentration in Adolescents: A Cluster Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(16), 8243. https://doi.org/10.3390/ijerph18168243






