Racial Residential Segregation and Race Differences in Ideal Cardiovascular Health among Young Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Sample
2.2. Dependent Variable
2.3. Independent Variables
2.4. Covariates
2.5. Statistical Analysis
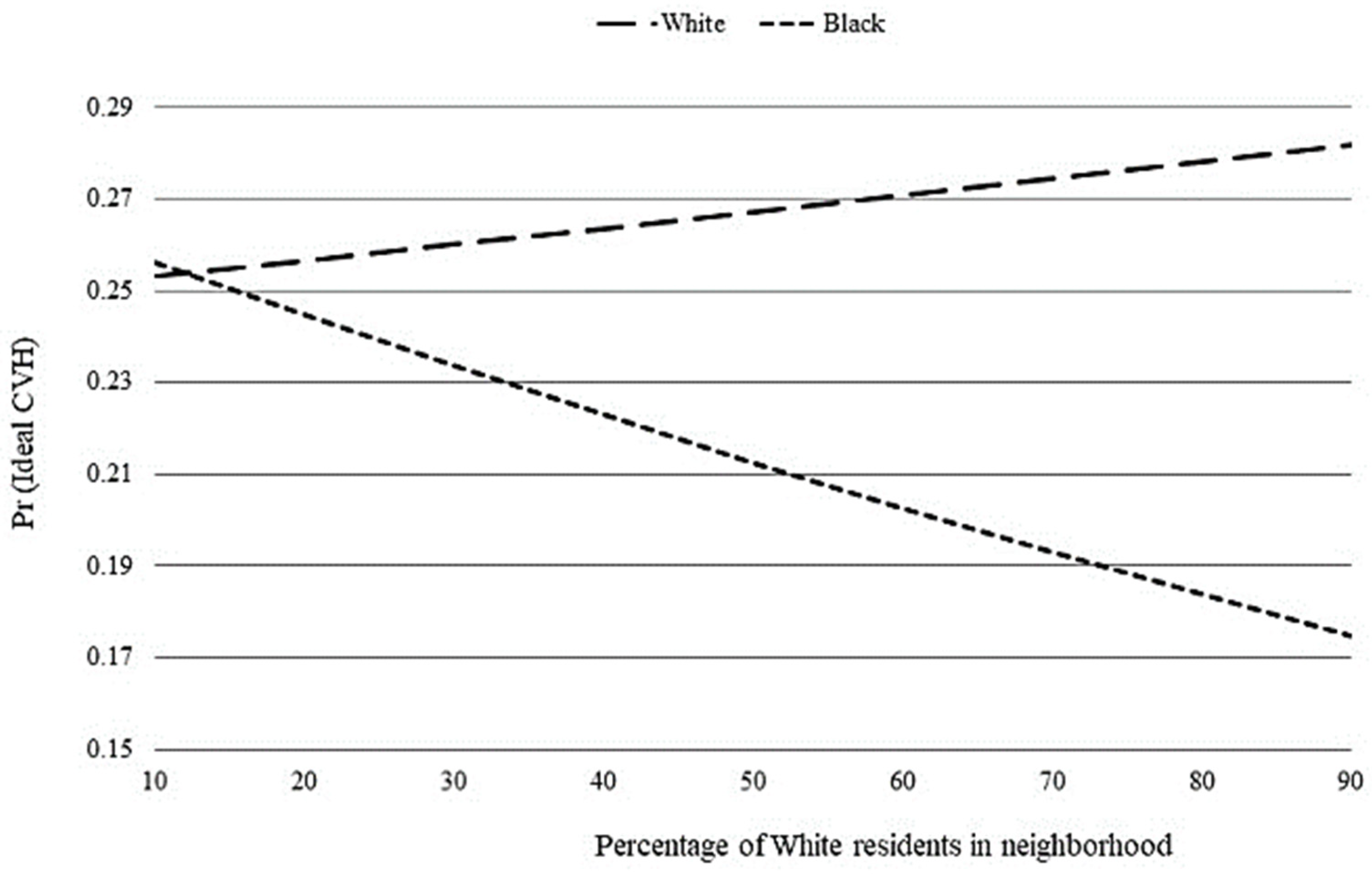
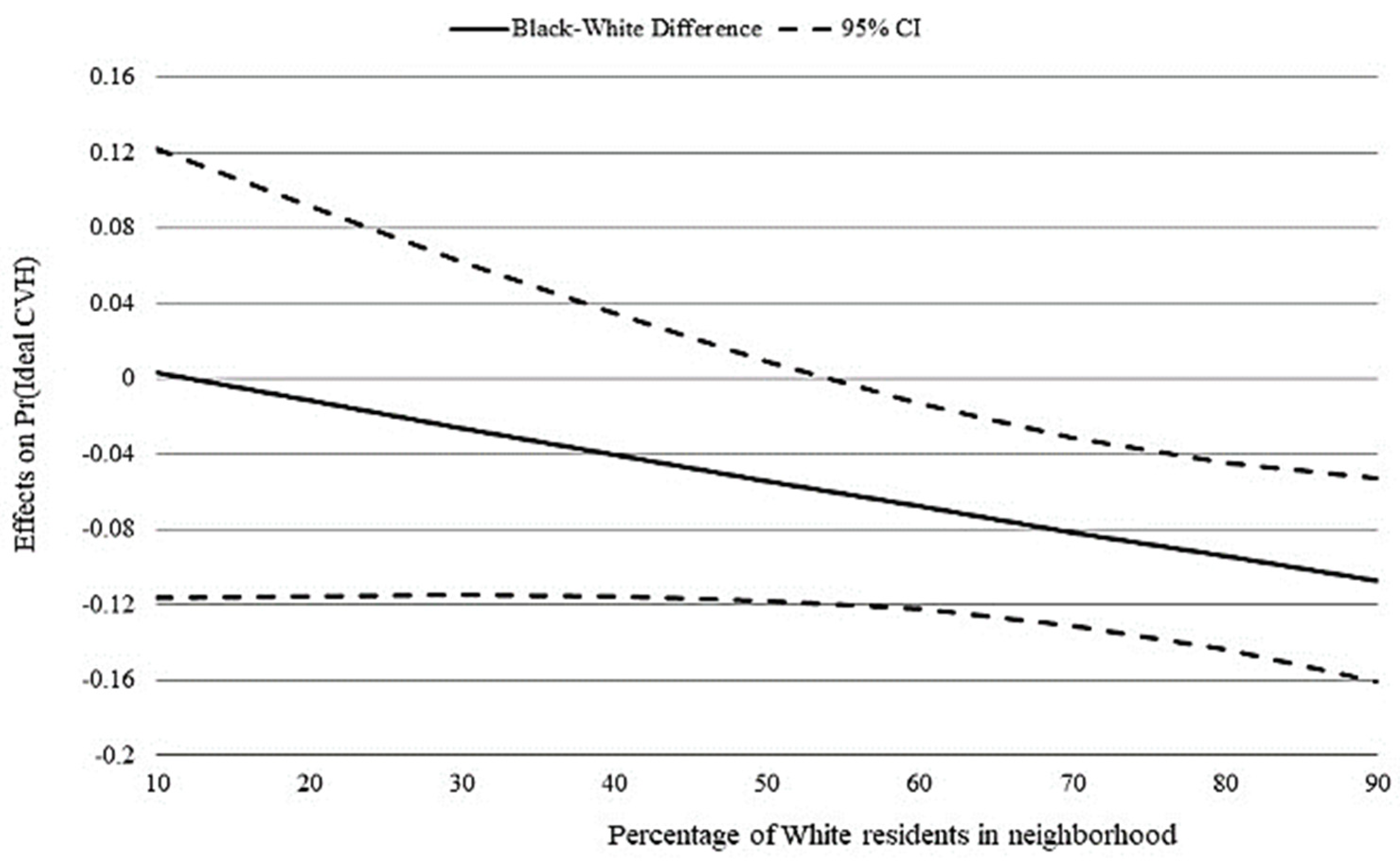
3. Results
4. Discussion
- Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention; National Center for Health Statistics. Underlying Cause of Death 1999–2018 on CDC WONDER Online Database, Released 2020. Data Are from the Multiple Cause of Death Files, 1999–2018, as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions through the Vital Statistics Cooperative Program. Available online: http://wonder.cdc.gov/ucd-icd10.html (accessed on 9 April 2020).
- Heron, M. Deaths: Leading Causes for 2017; National Vital Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2019; Volume 68.
- Baker, P.; Shand, T. Men’s health: Time for a new approach to policy and practice? J. Glob. Health 2017, 7, 010306. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American heart association’s strategic impact goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- McClurkin, M.A.; Yingling, L.R.; Ayers, C.; Cooper-McCann, R.; Suresh, V.; Nothwehr, A.; Barrington, D.S.; Powell-Wiley, T.M. Health Insurance Status as a Barrier to Ideal Cardiovascular Health for U.S. Adults: Data from the National Health and Nutrition Examination Survey (NHANES). PLoS ONE 2015, 10, e0141534. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cogswell, M.E.; Flanders, W.D.; Hong, Y.; Zhang, Z.; Loustalot, F.; Gillespie, C.; Merritt, R.; Hu, F.B. Trends in Cardiovascular Health Metrics and Associations With All-Cause and CVD Mortality Among US Adults. JAMA 2012, 307, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Mujahid, M.S.; Moore, L.V.; Petito, L.C.; Kershaw, K.N.; Watson, K.; Roux, A.V.D. Neighborhoods and racial/ethnic differences in ideal cardiovascular health (the Multi-Ethnic Study of Atherosclerosis). Health Place 2017, 44, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.J.; Fesahazion, R.G.; Parker, L.; Wilder, T.; Rooks, R.N.; Bowie, J.V.; Bell, C.N.; Szanton, S.L.; LaVeist, T.A. Accelerated Health Declines among African Americans in the USA. J. Urban Health 2016, 93, 808–819. [Google Scholar] [CrossRef]
- Williams, D.R.; Collins, C. Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Rep. 2001, 116, 404–416. [Google Scholar] [CrossRef]
- Riley, A.R. Neighborhood Disadvantage, Residential Segregation, and Beyond—Lessons for Studying Structural Racism and Health. J. Racial Ethn. Health Disparities 2017, 5, 357–365. [Google Scholar] [CrossRef]
- Williams, D.R. Race and health: Basic questions, emerging directions. Ann. Epidemiol. 1997, 7, 322–333. [Google Scholar] [CrossRef]
- Bailey, Z.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef]
- LaVeist, T.; Pollack, K.; Thorpe, R.; Fesahazion, R.; Gaskin, D. Place, Not Race: Disparities Dissipate In Southwest Baltimore When Blacks And Whites Live Under Similar Conditions. Health Aff. 2011, 30, 1880–1887. [Google Scholar] [CrossRef]
- Kershaw, K.N.; Osypuk, T.; Do, D.P.; de Chavez, P.J.; Roux, A.V.D. Neighborhood-Level Racial/Ethnic Residential Segregation and Incident Cardiovascular Disease. Circulation 2015, 131, 141–148. [Google Scholar] [CrossRef]
- Kershaw, K.N.; Albrecht, S.S. Racial/ethnic residential segregation and cardiovascular disease risk. Curr. Cardiovasc. Risk Rep. 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Thorpe, R.J.; Kelley, E.; Bowie, J.V.; Griffith, D.M.; Bruce, M.; LaVeist, T. Explaining Racial Disparities in Obesity Among Men: Does Place Matter? Am. J. Men Health 2014, 9, 464–472. [Google Scholar] [CrossRef]
- Thorpe, R.J.; Kennedy-Hendricks, A.; Griffith, D.; Bruce, M.A.; Coa, K.; Bell, C.N.; Young, J.; Bowie, J.V.; LaVeist, T.A. Race, Social and Environmental Conditions, and Health Behaviors in Men. Fam. Community Health 2015, 38, 297–306. [Google Scholar] [CrossRef]
- Harris, K.M.; Gordon-Larsen, P.; Chantala, K.; Udry, J.R. Longitudinal Trends in Race/Ethnic Disparities in Leading Health Indicators From Adolescence to Young Adulthood. Arch. Pediatr. Adolesc. Med. 2006, 160, 74–81. [Google Scholar] [CrossRef]
- Stroud, C.; Walker, L.R.; Davis, M.; Irwin, C.E. Investing in the Health and Well-Being of Young Adults. J. Adolesc. Health 2015, 56, 127–129. [Google Scholar] [CrossRef]
- Liu, K.; Daviglus, M.L.; Loria, C.M.; Colangelo, L.A.; Spring, B.; Moller, A.C.; Lloyd-Jones, D. Healthy Lifestyle through Young Adulthood and the Presence of Low Cardiovascular Disease Risk Profile in Middle Age. Circulation 2012, 125, 996–1004. [Google Scholar] [CrossRef]
- Unger, E.; Diez-Roux, A.V.; Lloyd-Jones, D.; Mujahid, M.S.; Nettleton, J.A.; Bertoni, A.; Badon, S.E.; Ning, H.; Allen, N.B. Association of Neighborhood Characteristics with Cardiovascular Health in the Multi-Ethnic Study of Atherosclerosis. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 524–531. [Google Scholar] [CrossRef]
- Lawrence, E.; Hummer, R.A.; Harris, K.M. The Cardiovascular Health of Young Adults: Disparities along the Urban-Rural Continuum. Ann. Am. Acad. Polit. Soc. Sci. 2017, 672, 257–281. [Google Scholar] [CrossRef]
- Lawrence, E.M.; Hummer, R.A.; Domingue, B.W.; Harris, K.M. Wide educational disparities in young adult cardiovascular health. SSM Popul. Health 2018, 5, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.M. Why Do College Graduates Behave More Healthfully Than Those Who Are Less Educated? J. Health Soc. Behav. 2017, 58, 291–306. [Google Scholar] [CrossRef]
- Harris, K.M.; Udry, J.R. National Longitudinal Study of Adolescent to Adult Health (Add Health), 1994–2008 [Public Use]. In ICPSR Data Holdings; Inter-University Consortium for Political and Social Research (ICPSR): Ann Arbor, MI, USA, 2008. [Google Scholar]
- Steinberger, J.; Daniels, S.R.; Hagberg, N.; Isasi, C.R.; Kelly, A.S.; Lloyd-Jones, D.; Pate, R.R.; Pratt, C.; Shay, C.M.; Towbin, J.A.; et al. Cardiovascular Health Promotion in Children: Challenges and Opportunities for 2020 and Beyond: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e236–e255. [Google Scholar] [CrossRef]
- White, K.; Borrell, L.N. Racial/ethnic residential segregation: Framing the context of health risk and health disparities. Health Place 2011, 17, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Perreira, K.M.; Deeb-Sossa, N.; Harris, K.M.; Bollen, K. What Are We Measuring? An Evaluation of the CES-D Across Race/Ethnicity and Immigrant Generation*. Soc. Forces 2005, 83, 1567–1601. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC.: College Station, TX, USA, 2017. [Google Scholar]
- Bruce, M.A.; Wilder, T.; Norris, K.C.; Beech, B.M.; Griffith, D.M.; Thorpe, J.R.J. Perspective: Cardiovascular Disease among Young African American Males. Ethn. Dis. 2017, 27, 363–366. [Google Scholar] [CrossRef][Green Version]
- Mayne, S.L.; Hicken, M.T.; Merkin, S.S.; E Seeman, T.; Kershaw, K.N.; Do, D.P.; Hajat, A.; Roux, A.V.D. Neighbourhood racial/ethnic residential segregation and cardiometabolic risk: The multiethnic study of atherosclerosis. J. Epidemiol. Community Health 2018, 73, 26–33. [Google Scholar] [CrossRef]
- Gooding, H.C.; Milliren, C.; Shay, C.M.; Richmond, T.K.; Field, A.E.; Gillman, M.W. Achieving Cardiovascular Health in Young Adulthood—Which Adolescent Factors Matter? J. Adolesc. Health 2016, 58, 119–121. [Google Scholar] [CrossRef]
- Griffith, D.M. Biopsychosocial Approaches to Men’s Health Disparities Research and Policy. Behav. Med. 2016, 42, 211–215. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 5080) | White (n = 4001) | Black (n = 1079) | ||
|---|---|---|---|---|
| Variables | % or Mean (SD) | % or Mean (SD) | % or Mean (SD) | p |
| Ideal cardiovascular health | 26.9 | 28.0 | 20.9 | 0.014 *** |
| Percent White in neighborhood | 76.1 (±1.306) | 81.4 (±0.833) | 47.7 (±2.154) | <0.001 *** |
| Neighborhood context | ||||
| Population density (persons/square km.) | 1692 (±159) | 1587 (±146) | 2259 (±432) | 0.123 |
| Urbanicity | 0.173 | |||
| Metropolitan area | 84.0 | 85.1 | 78.3 | |
| Micropolitan area | 10.2 | 9.5 | 14.0 | |
| Small town/Rural area | 5.8 | 5.4 | 8.7 | |
| Social status | ||||
| Age | 28.4 (±0.124) | 28.4 (±0.131) | 28.7 (±0.227) | 0.116 |
| Education | 0.007 *** | |||
| Less than high school | 10.1 | 9.4 | 13.9 | |
| High school diploma | 20.9 | 20.0 | 26.1 | |
| Some college | 43.0 | 43.0 | 42.7 | |
| College degree or more | 26.0 | 27.6 | 17.3 | |
| Income-to-needs | 4.5 (±0.093) | 4.7 (±0.097) | 3.5 (±0.138) | <0.001 *** |
| Risk behavior | ||||
| Binge drinking | 79.6 | 81.0 | 71.6 | <0.001 *** |
| Stressful life event | ||||
| Financial strain | 22.8 | 21.1 | 32.0 | <0.001 *** |
| Arrest experience | 42.9 | 41.5 | 50.6 | 0.006 *** |
| Underemployed | 29.5 | 28.6 | 33.9 | 0.077 |
| Medical care | ||||
| Insurance status | 73.1 | 74.5 | 65.2 | <0.001 *** |
| Routine checkup | 63.8 | 61.8 | 75.0 | <0.001 *** |
| Unmet healthcare need | 25.8 | 25.5 | 27.5 | 0.398 |
| Psychosocial factors | ||||
| Perceived stress | 4.5 (±0.064) | 4.4 (±0.063) | 5.0 (±0.168) | 0.001 *** |
| Depressive symptoms | 2.3 (±0.051) | 2.2 (±0.047) | 2.9 (±0.151) | <0.001 *** |
| Model 1 b | Model 2 b | Model 3 c | Model 4 c | |
|---|---|---|---|---|
| Variable | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Black d | 0.67 (0.497, 0.924) | 0.58 (0.415, 0.820) | 0.70 (0.502, 0.970) | 1.10 (0.525, 2.314) |
| Percent White in neighborhood e | 0.99 (0.991, 1.001) | 1.00 (0.995, 1.004) | 1.00 (0.995, 1.008) | |
| Black × percent White in neighborhood f | 0.99 (0.982, 1.002) | |||
| Constant | 0.39 (0.353, 0.428) | 0.55 (0.370, 0.821) | 0.21 (0.037, 1.137) | 0.17 (0.028, 1.005) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baxter, S.L.K.; Chung, R.; Frerichs, L.; Thorpe, R.J., Jr.; Skinner, A.C.; Weinberger, M. Racial Residential Segregation and Race Differences in Ideal Cardiovascular Health among Young Men. Int. J. Environ. Res. Public Health 2021, 18, 7755. https://doi.org/10.3390/ijerph18157755
Baxter SLK, Chung R, Frerichs L, Thorpe RJ Jr., Skinner AC, Weinberger M. Racial Residential Segregation and Race Differences in Ideal Cardiovascular Health among Young Men. International Journal of Environmental Research and Public Health. 2021; 18(15):7755. https://doi.org/10.3390/ijerph18157755
Chicago/Turabian StyleBaxter, Samuel L. K., Richard Chung, Leah Frerichs, Roland J. Thorpe, Jr., Asheley C. Skinner, and Morris Weinberger. 2021. "Racial Residential Segregation and Race Differences in Ideal Cardiovascular Health among Young Men" International Journal of Environmental Research and Public Health 18, no. 15: 7755. https://doi.org/10.3390/ijerph18157755
APA StyleBaxter, S. L. K., Chung, R., Frerichs, L., Thorpe, R. J., Jr., Skinner, A. C., & Weinberger, M. (2021). Racial Residential Segregation and Race Differences in Ideal Cardiovascular Health among Young Men. International Journal of Environmental Research and Public Health, 18(15), 7755. https://doi.org/10.3390/ijerph18157755







