Perioperative Hypothermia in Children
Abstract
1. Definition
2. History
3. Incidence
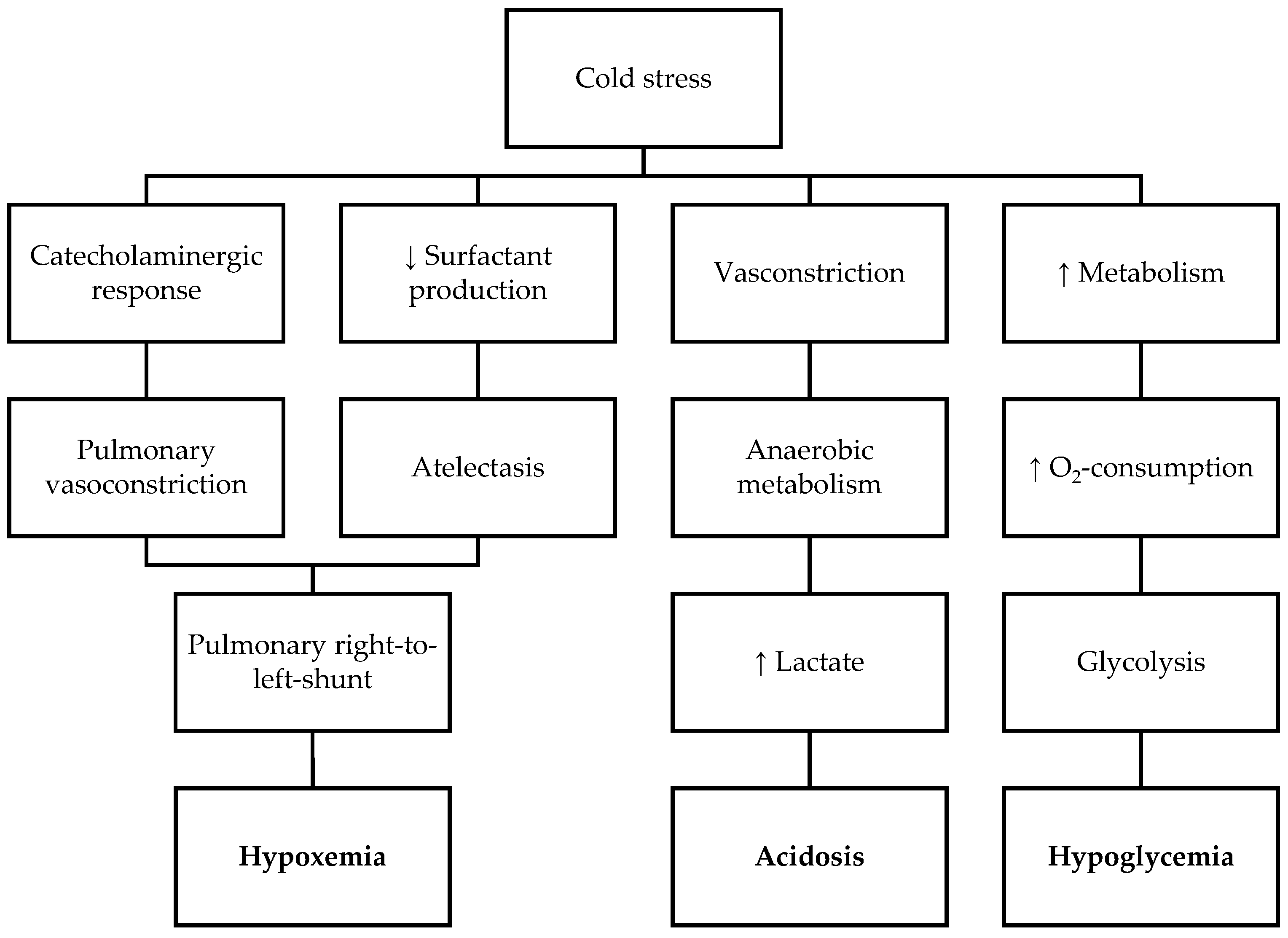
4. Development
5. Risk Factors
6. Adverse Events
6.1. Neonates and Preterm Infants
6.2. Infants and Children
7. Monitoring of Core Temperature
7.1. Core Sites
7.2. Other Sites
7.3. Recommendations
7.4. Perspectives
8. Prevention
8.1. Warming Therapy during Transport
8.2. Warming before Induction of Anaesthesia
8.3. Warming Therapy during Anaesthesia
8.4. Infusion Warming
8.5. Warming Therapy after Anaesthesia
9. Risks of Active Warming Therapy
9.1. Risks of Forced-Air Warming
9.1.1. Thermal Softening of Tracheal Tubes
9.1.2. Burns
9.2. Noise
9.3. Risks of Conductive Warming
9.4. Risks of Infusion Warming
9.4.1. Risk of Burns
9.4.2. Risks of Infections and Haemolysis
9.4.3. Air Embolism
9.5. Overheating
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torossian, A.; Becke, K.; Bein, B.; Bräuer, A.; Gantert, D.; Greif, R.; Höcker, J.; Horn, E.P.; Kimberger, O.; Klar, E.; et al. S3 Leitlinie “Vermeidung von perioperativer Hypothermie”: Aktualisierung 2019. Available online: https://www.awmf.org/uploads/tx_szleitlinien/001-018l_S3_Vermeidung_perioperativer_Hypothermie_2019-08.pdf (accessed on 5 May 2021).
- von Bibra, E.F.; Harless, E. Die Wirkung des Schwefeläthers in Chemischer und Physiologischer Beziehung; Verlag von Carl Heyder: Erlangen, Germany, 1847. [Google Scholar]
- France, G.G. Hypothermia in the newborn: Body temperatures following anaesthesia. Br. J. Anaesth. 1957, 29, 390–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farman, J.V. Heat losses in infants undergoing surgery in air-conditioned theatres. Br. J. Anaesth. 1962, 34, 543–557. [Google Scholar] [CrossRef][Green Version]
- Bering, E.A.; Matson, D.D. A technic for the prevention of severe hypothermia during surgery of infants. Ann. Surg. 1953, 137, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Calvert, D.G. Inadvertent hypothermia in paediatric surgery and a method for its prevention. Anaesthesia 1962, 17, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.B.; Shaw, A.; Etchells, A.H. Contact mattress to prevent heat loss in neonatal and paediatric surgery. Br. J. Anaesth. 1973, 45, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Görges, M.; Afshar, K.; West, N.; Pi, S.; Bedford, J.; Whyte, S.D. Integrating intraoperative physiology data into outcome analysis for the ACS Pediatric National Surgical Quality Improvement Program. Paediatr. Anaesth. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.; Christensen, R.; Voepel-Lewis, T. Perioperative Hypothermia in the Pediatric Population: Prevalence, Risk Factors and Outcomes. J. Anesth. Clin. Res. 2010, 1, 102. [Google Scholar] [CrossRef]
- Sim, R.; Hall, N.J.; de Coppi, P.; Eaton, S.; Pierro, A. Core temperature falls during laparotomy in infants with necrotizing enterocolitis. Eur. J. Pediatr. Surg. 2012, 22, 45–49. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Cao, R.; Li, G.; Deng, L.; Li, J. The low fresh gas flow anesthesia and hypothermia in neonates undergoing digestive surgeries: A retrospective before-after study. BMC Anesthesiol. 2020, 20, 223. [Google Scholar] [CrossRef]
- Ongun, E.A.; Dursun, O.; Kazan, M.S.; Nur, B.; Mihci, E. Early postoperative follow-up after craniosynostosis surgery. Turk. J. Med. Sci. 2018, 48, 584–591. [Google Scholar] [CrossRef]
- Thompson, D.R.; Zurakowski, D.; Haberkern, C.M.; Stricker, P.A.; Meier, P.M.; Bannister, C.; Benzon, H.; Binstock, W.; Bosenberg, A.; Brzenski, A.; et al. Endoscopic Versus Open Repair for Craniosynostosis in Infants Using Propensity Score Matching to Compare Outcomes: A Multicenter Study from the Pediatric Craniofacial Collaborative Group. Anesth. Analg. 2018, 126, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Brozanski, B.S.; Piazza, A.J.; Chuo, J.; Natarajan, G.; Grover, T.R.; Smith, J.R.; Mingrone, T.; McClead, R.E.; Rakesh, R.; Rintoul, N.; et al. STEPP IN: Working Together to Keep Infants Warm in the Perioperative Period. Pediatrics 2020, 145, e20191121. [Google Scholar] [CrossRef] [PubMed]
- Schroeck, H.; Lyden, A.K.; Benedict, W.L.; Ramachandran, S.K. Time Trends and Predictors of Abnormal Postoperative Body Temperature in Infants Transported to the Intensive Care Unit. Anesthesiol. Res. Prac. 2016, 2016, 7318137. [Google Scholar] [CrossRef]
- Kim, P.; Taghon, T.; Fetzer, M.; Tobias, J.D. Perioperative hypothermia in the pediatric population: A quality improvement project. Am. J. Med. Qual. 2013, 28, 400–406. [Google Scholar] [CrossRef]
- Nemeth, M.; Lovric, M.; Asendorf, T.; Bräuer, A.; Miller, C. Intraoperative zero-heat-flux thermometry overestimates esophageal temperature by 0.26 °C: An observational study in 100 infants and young children. J. Clin. Monit. Comput. 2020. [Google Scholar] [CrossRef]
- Hubbard, R.; Edmonds, K.; Rydalch, E.; Pawelek, O.; Griffin, E.; Gautam, N. Anesthetic management of catheter-based patent ductus arteriosus closure in neonates weighing <3 kg: A Retrospective Ob-servational Study. Paediatr. Anaesth. 2020, 30, 506–510. [Google Scholar] [CrossRef]
- Galante, D. Intraoperative hypothermia. Relation between general and regional anesthesia, upper- and lower-body warming: What strategies in pediatric anesthesia? Paediatr. Anaesth. 2007, 17, 821–823. [Google Scholar] [CrossRef]
- Sessler, D.I. Temperature monitoring and perioperative thermoregulation. Anesthesiology 2008, 109, 318–338. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative thermoregulation and heat balance. Lancet 2016, 387, 2655–2664. [Google Scholar] [CrossRef]
- Knobel, R.; Holditch-Davis, D. Thermoregulation and heat loss prevention after birth and during neonatal intensive-care unit stabilization of extremely low-birthweight infants. J. Obstet. Gynecol. Neonatal Nurs. 2007, 36, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. BioMed Res. Int. 2016, 2016, 2365609. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Maya, Y.; Wagner, M.; Arias-Loza, P.; Werner, R.A.; Herrmann, K.; Higuchi, T. Activation of brown adipose tissue in hypothyroidism. Ann. Med. 2015, 47, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.J.; Scopes, J.W. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature 1965, 206, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Houstĕk, J.; Vízek, K.; Pavelka, S.; Kopecký, J.; Krejcová, E.; Hermanská, J.; Cermáková, M. Type II iodothyronine 5′-deiodinase and uncoupling protein in brown adipose tissue of human newborns. J. Clin. Endocrinol. Metab. 1993, 77, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.J.; Pikaar, M.E.; Badger, P.; McIntosh, N. Temperature control in very low birthweight infants during first five days of life. ADC Fetal Neonatal Ed. 1997, 76, F47–F50. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, B.; Sessler, D.I. Thermoregulatory thresholds for vasoconstriction in pediatric patients anesthetized with halothane or halothane and caudal bupivacaine. Anesthesiology 1992, 76, 387–392. [Google Scholar] [CrossRef]
- Plattner, O.; Semsroth, M.; Sessler, D.I.; Papousek, A.; Klasen, C.; Wagner, O. Lack of nonshivering thermogenesis in infants anesthetized with fentanyl and propofol. Anesthesiology 1997, 86, 772–777. [Google Scholar] [CrossRef]
- Sessler, D.I. Forced-air warming in infants and children. Paediatr. Anaesth. 2013, 23, 467–468. [Google Scholar] [CrossRef]
- Sedin, G. Neonatal Heat Transfer, Routes of Heat Loss and Heat Gain. In Thermoregulation of Sick and Low Birth Weight Neonates: Temperature Control Temperature Monitoring Thermal Environment; Okken, A., Koch, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 21–36. ISBN 978-3-642-79936-5. [Google Scholar]
- Matsukawa, T.; Hanagata, K.; Ozaki, M.; Iwashita, H.; Koshimizu, M.; Kumazawa, T.I.M. midazolam as premedication produces a concentration-dependent decrease in core temperature in male volunteers. Br. J. Anaesth. 1997, 78, 396–399. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative heat balance. Anesthesiology 2000, 92, 578–596. [Google Scholar] [CrossRef]
- Bräuer, A. Perioperative Temperature Management; Cambridge University Press: Cambridge, UK, 2017; ISBN 9781107535770. [Google Scholar]
- Lai, L.-L.; See, M.-H.; Rampal, S.; Ng, K.-S.; Chan, L. Significant factors influencing inadvertent hypothermia in pediatric anesthesia. J. Clin. Monit. Comput. 2019, 33, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.; Ohlson, K.B.; Johnson, L.; Cannon, B.; Lindahl, S.G.; Nedergaard, J. Halothane selectively inhibits nonshivering thermogenesis. Possible implications for thermoregulation during anesthesia of infants. Anesthesiology 1995, 82, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Tander, B.; Baris, S.; Karakaya, D.; Ariturk, E.; Rizalar, R.; Bernay, F. Risk factors influencing inadvertent hypothermia in infants and neonates during anesthesia. Paediatr. Anaesth. 2005, 15, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, D.; Williams, L.; Lloyd, C.; McCoy, D.S.; Miller Walters, E.; Guzzetta, C.E.; Baumgart, S.; Sill, A.; Mueller-Burke, D.; Short, B.L. Perioperative hypothermia in NICU infants: Its occurrence and impact on infant outcomes. Adv. Neonatal. Care 2014, 14, 154–164. [Google Scholar] [CrossRef]
- World Health Organization. Maternal; Newborn Health/Safe Motherhood. In Thermal Protection of the Newborn: A Practical Guide; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Engorn, B.M.; Kahntroff, S.L.; Frank, K.M.; Singh, S.; Harvey, H.A.; Barkulis, C.T.; Barnett, A.M.; Olambiwonnu, O.O.; Heitmiller, E.S.; Greenberg, R.S. Perioperative hypothermia in neonatal intensive care unit patients: Effectiveness of a thermoregulation intervention and associated risk factors. Paediatr. Anaesth. 2017, 27, 196–204. [Google Scholar] [CrossRef]
- Feltes, T.F.; Bacha, E.; Beekman, R.H., 3rd; Cheatham, J.P.; Feinstein, J.A.; Gomes, A.S.; Hijazi, Z.M.; Ing, F.F.; de Moor, M.; Morrow, W.R.; et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2607–2652. [Google Scholar] [CrossRef]
- Sun, Z.; Honar, H.; Sessler, D.I.; Dalton, J.E.; Yang, D.; Panjasawatwong, K.; Deroee, A.F.; Salmasi, V.; Saager, L.; Kurz, A. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology 2015, 122, 276–285. [Google Scholar] [CrossRef]
- Mullany, L.C.; Katz, J.; Khatry, S.K.; LeClerq, S.C.; Darmstadt, G.L.; Tielsch, J.M. Risk of mortality associated with neonatal hypothermia in southern Nepal. Arch Pediatr. Adolesc. Med. 2010, 164, 650–656. [Google Scholar] [CrossRef]
- Brindle, M.E.; McDiarmid, C.; Short, K.; Miller, K.; MacRobie, A.; Lam, J.Y.K.; Brockel, M.; Raval, M.V.; Howlett, A.; Lee, K.-S.; et al. Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2020, 44, 2482–2492. [Google Scholar] [CrossRef]
- Sessler, D.I. Complications and treatment of mild hypothermia. Anesthesiology 2001, 95, 531–543. [Google Scholar] [CrossRef]
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef] [PubMed]
- Moler, F.W.; Silverstein, F.S.; Holubkov, R.; Slomine, B.S.; Christensen, J.R.; Nadkarni, V.M.; Meert, K.L.; Clark, A.E.; Browning, B.; Pemberton, V.L.; et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N. Engl. J. Med. 2015, 372, 1898–1908. [Google Scholar] [CrossRef]
- Moler, F.W.; Silverstein, F.S.; Holubkov, R.; Slomine, B.S.; Christensen, J.R.; Nadkarni, V.M.; Meert, K.L.; Browning, B.; Pemberton, V.L.; Page, K.; et al. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N. Engl. J. Med. 2017, 376, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Juszczak, E.; Linsell, L.; Azzopardi, D.; Cowan, F.; Marlow, N.; Edwards, D. Neonatal ECMO study of temperature (NEST): A randomized controlled trial. Pediatrics 2013, 132, e1247–e1256. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, J.S.; Ward, R.E.; Lacroix, J.; Hébert, P.C.; Barnes, M.A.; Bohn, D.J.; Dirks, P.B.; Doucette, S.; Fergusson, D.; Gottesman, R.; et al. Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 2008, 358, 2447–2456. [Google Scholar] [CrossRef]
- Deshpande, S.A.; Platt, M.P. Association between blood lactate and acid-base status and mortality in ventilated babies. ADC Fetal Neonatal Ed. 1997, 76, F15–F20. [Google Scholar] [CrossRef]
- Miller, S.S.; Lee, H.C.; Gould, J.B. Hypothermia in very low birth weight infants: Distribution, risk factors and outcomes. J. Perinatol. 2011, 31, S49–S56. [Google Scholar] [CrossRef]
- Görges, M.; West, N.C.; Cheung, W.; Zhou, G.; Miyanji, F.; Whyte, S.D. Preoperative warming and undesired surgical and anesthesia outcomes in pediatric spinal surgery—A retrospective cohort study. Paediatr. Anaesth. 2016, 26, 866–875. [Google Scholar] [CrossRef]
- Linam, W.M.; Margolis, P.A.; Staat, M.A.; Britto, M.T.; Hornung, R.; Cassedy, A.; Connelly, B.L. Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect. Control Hosp. Epidemiol. 2009, 30, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Amin, R.; Arca, M.J.; Datta, A. Effects of intraoperative temperatures on postoperative infections in infants and neonates. J. Pediatr. Surg. 2020, 55, 80–85. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative Temperature Monitoring. Anesthesiology 2021, 134, 111–118. [Google Scholar] [CrossRef]
- Rubinstein, E.H.; Sessler, D.I. Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology 1990, 73, 541–545. [Google Scholar] [CrossRef]
- Burgess, G.E.; Cooper, J.R.; Marino, R.J.; Peuler, M.J. Continuous monitoring of skin temperature using a liquid-crystal thermometer during anesthesia. South. Med. J. 1978, 71, 516–518. [Google Scholar] [CrossRef]
- Bloch, E.C.; Ginsberg, B.; Binner, R.A. The esophageal temperature gradient in anesthetized children. J. Clin. Monit. 1993, 9, 73–77. [Google Scholar] [CrossRef]
- Wang, M.; Singh, A.; Qureshi, H.; Leone, A.; Mascha, E.J.; Sessler, D.I. Optimal Depth for Nasopharyngeal Temperature Probe Positioning. Anesth. Analg. 2016, 122, 1434–1438. [Google Scholar] [CrossRef]
- Pasquier, M.; Paal, P.; Kosinski, S.; Brown, D.; Podsiadlo, P.; Darocha, T. Esophageal Temperature Measurement. N. Engl. J. Med. 2020, 383, e93. [Google Scholar] [CrossRef]
- Whitby, J.D.; Dunkin, L.J. Oesophageal temperature differences in children. Br. J. Anaesth. 1970, 42, 1013–1015. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, J.; Jung, J.-Y.; Shim, J.W.; Jung, H.S. Simple calculation of the optimal insertion depth of esophageal temperature probes in children. J. Clin. Monit. Comput. 2020, 34, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.V.; Braitman, L.E. Nasal temperature can be used as a reliable surrogate measure of core temperature. J. Clin. Monit. Comput. 2008, 22, 309–314. [Google Scholar] [CrossRef]
- Lee, J.; Lim, H.; Son, K.-G.; Ko, S. Optimal nasopharyngeal temperature probe placement. Anesth. Analg. 2014, 119, 875–879. [Google Scholar] [CrossRef]
- Snoek, A.P.; Saffer, E. Agreement between lower esophageal and nasopharyngeal temperatures in children ventilated with an endotracheal tube with leak. Pediatr. Anesth. 2016, 26, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Matsukawa, T.; Ozaki, M.; Sessler, D.I.; Nishiyama, T.; Kumazawa, T. The accuracy and precision of four infrared aural canal thermometers during cardiac surgery. Acta Anaesthesiol. Scand. 1998, 42, 1222–1226. [Google Scholar] [CrossRef]
- Langham, G.E.; Maheshwari, A.; Contrera, K.; You, J.; Mascha, E.; Sessler, D.I. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology 2009, 111, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Greenes, D.S.; Fleisher, G.R. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr. Adolesc. Med. 2001, 155, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.; Najafi, N.; Poelaert, J. Intra-operative temperature monitoring with cutaneous zero-heat- flux-thermometry in comparison with oesophageal temperature: A prospective study in the paediatric population. Paediatr. Anaesth. 2019, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Evron, S.; Weissman, A.; Toivis, V.; Shahaf, D.B.; You, J.; Sessler, D.I.; Ezri, T. Evaluation of the Temple Touch Pro, a Novel Noninvasive Core-Temperature Monitoring System. Anesth. Analg. 2017, 125, 103–109. [Google Scholar] [CrossRef]
- Conway, A.; Bittner, M.; Phan, D.; Chang, K.; Kamboj, N.; Tipton, E.; Parotto, M. Accuracy and precision of zero-heat-flux temperature measurements with the 3M™ Bair Hugger™ Temperature Monitoring System: A systematic review and meta-analysis. J. Clin. Monit. Comput. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cassey, J.G.; King, R.A.; Armstrong, P. Is there thermal benefit from preoperative warming in children? Paediatr. Anaesth. 2010, 20, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, A.; Perl, T.; Uyanik, Z.; English, M.J.M.; Weyland, W.; Braun, U. Perioperative thermal insulation: Minimal clinically important differences? Br. J. Anaesth. 2004, 92, 836–840. [Google Scholar] [CrossRef]
- NICE. Hypothermia: Prevention and Management in Adults Having Surgery: Clinical Guideline. Available online: www.nice.org.uk/guidance/cg65 (accessed on 23 March 2021).
- Matsukawa, T.; Sessler, D.I.; Sessler, A.M.; Schroeder, M.; Ozaki, M.; Kurz, A.; Cheng, C. Heat flow and distribution during induction of general anesthesia. Anesthesiology 1995, 82, 662–673. [Google Scholar] [CrossRef]
- Horn, E.-P.; Bein, B.; Böhm, R.; Steinfath, M.; Sahili, N.; Höcker, J. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia 2012, 67, 612–617. [Google Scholar] [CrossRef]
- Eich, C.; Zink, W.; Schwarz, S.; Radke, O.C.; Bräuer, A. A combination of convective and conductive warming ensures pre- and post-bypass normothermia in paediatric cardiac anaesthesia. Appl. Cardiopulm. Pathophysiol. 2009, 13, 3–10. [Google Scholar]
- Triffterer, L.; Marhofer, P.; Sulyok, I.; Keplinger, M.; Mair, S.; Steinberger, M.; Klug, W.; Kimberger, O. Forced-Air Warming During Pediatric Surgery: A Randomized Comparison of a Compressible with a Noncompressible Warming System. Anesth. Analg. 2016, 122, 219–225. [Google Scholar] [CrossRef]
- Witt, L.; Dennhardt, N.; Eich, C.; Mader, T.; Fischer, T.; Bräuer, A.; Sümpelmann, R. Prevention of intraoperative hypothermia in neonates and infants: Results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr. Anaesth. 2013, 23, 469–474. [Google Scholar] [CrossRef]
- Kurz, A.; Kurz, M.; Poeschl, G.; Faryniak, B.; Redl, G.; Hackl, W. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth. Analg. 1993, 77, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Grote, R.; Wetz, A.; Bräuer, A.; Menzel, M. Short interruptions between pre-warming and intraoperative warming are associated with low intraoperative hypothermia rates. Acta Anaesthesiol. Scand. 2020, 64, 489–493. [Google Scholar] [CrossRef]
- Bräuer, A.; Scheithauer, S. Prävention der unbeabsichtigten perioperativen Hypothermie. Krankenh.hyg. Up2Date 2016, 11, 291–303. [Google Scholar] [CrossRef]
- Bissonnette, B.; Sessler, D.I.; LaFlamme, P. Passive and active inspired gas humidification in infants and children. Anesthesiology 1989, 71, 350–354. [Google Scholar] [CrossRef]
- Sümpelmann, R.; Becke, K.; Zander, R.; Witt, L. Perioperative fluid management in children: Can we sum it all up now? Curr. Opin. Anaesthesiol. 2019, 32, 384–391. [Google Scholar] [CrossRef]
- Fillies, T.; Homann, C.; Meyer, U.; Reich, A.; Joos, U.; Werkmeister, R. Perioperative complications in infant cleft repair. Head Face Med. 2007, 3, 9. [Google Scholar] [CrossRef]
- Shorrab, A.A.; El-Sawy, M.E.; Othman, M.M.; Hammouda, G.E. Prevention of hypothermia in children under combined epidural and general anesthesia: A comparison between upper- and lower-body warming. Paediatr. Anaesth. 2007, 17, 38–43. [Google Scholar] [CrossRef]
- Beebe, D.S.; Beck, D.; Belani, K.G. Clinical management of infants and newborn babies undergoing major surgery utilizing a rapid infusion device. Pediatr. Anesth. 1994, 4, 115–121. [Google Scholar] [CrossRef]
- Bissonnette, B.; Paut, O. Active warming of saline or blood is ineffective when standard infusion tubing is used: An experimental study. Can. J. Anesth. 2002, 49, 270–275. [Google Scholar] [CrossRef][Green Version]
- Schnoor, J.; Weber, I.; Macko, S.; Heussen, N.; Rossaint, R. Heating capabilities of the Hotline and Autoline at low flow rates. Paediatr. Anaesth. 2006, 16, 410–416. [Google Scholar] [CrossRef]
- Schmidt, J.H.; Weyland, W.; Fritz, U.; Bräuer, A.; Rathgeber, J.; Braun, U. Experimentelle Untersuchung zur Effektivität verschiedener Infusions- und Blutwärme-verfahren. Anaesthesist 1996, 45, 1067–1074. [Google Scholar] [CrossRef]
- Perl, T.; Kunze-Szikszay, N.; Bräuer, A.; Quintel, M.; Röhrig, A.L.; Kerpen, K.; Telgheder, U. Aluminium release by coated and uncoated fluid-warming devices. Anaesthesia 2019, 74, 708–713. [Google Scholar] [CrossRef]
- Ayala, J.L.; Coe, A. Thermal softening of tracheal tubes: An unrecognized hazard of the Bair Hugger active patient warming system. Br. J. Anaesth. 1997, 79, 543–545. [Google Scholar] [CrossRef]
- Bräuer, A.; Quintel, M. Forced-air warming: Technology, physical background and practical aspects. Curr. Opin. Anaesthesiol. 2009, 22, 769–774. [Google Scholar] [CrossRef]
- Mehta, S.P. Burn injuries from warming devices in the operating room. ASA Monit. 2013, 77, 16–17. [Google Scholar]
- Azzam, F.J.; Krock, J.L. Thermal burns in two infants associated with a forced air warming system. Anesth. Analg. 1995, 81, 661. [Google Scholar] [CrossRef]
- Siddik-Sayyid, S.M.; Abdallah, F.W.; Dahrouj, G.B. Thermal burns in three neonates associated with intraoperative use of Bair Hugger warming devices. Paediatr. Anaesth. 2008, 18, 337–339. [Google Scholar] [CrossRef]
- Truell, K.D.; Bakerman, P.R.; Teodori, M.F.; Maze, A. Third-degree burns due to intraoperative use of a Bair Hugger warming device. Ann. Thorac. Surg. 2000, 69, 1933–1934. [Google Scholar] [CrossRef]
- Golden, S.; Bachmann, C. Forced air warmer burn can occur with poor circulation. APSF Newsl. 2006, 20, 87. [Google Scholar]
- Wagner, K.; Swanson, E.; Raymond, C.J.; Smith, C.E. Comparison of two convective warming systems during major abdominal and orthopedic surgery. Can. J. Anesth. 2008, 55, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.D. Noise in the operating room. Anesthesiology 2014, 121, 894–898. [Google Scholar] [CrossRef]
- Crino, M.H.; Nagel, E.L. Thermal burns caused by warming blankets in the operating room. Anesthesiology 1968, 29, 149–150. [Google Scholar] [CrossRef]
- Dewar, D.J.; Fraser, J.F.; Choo, K.L.; Kimble, R.M. Thermal injuries in three children caused by an electrical warming mattress. Br. J. Anaesth. 2004, 93, 586–589. [Google Scholar] [CrossRef]
- Acikel, C.; Kale, B.; Celikoz, B. Major thermal burn due to intraoperative heating blanket malfunction. Burns 2002, 28, 283–284. [Google Scholar] [CrossRef]
- Sieunarine, K.; White, G.H. Full-thickness burn and venous thrombosis following intravenous infusion of microwave-heated crystalloid fluids. Burns 1996, 22, 568–569. [Google Scholar] [CrossRef]
- Arrandale, L.; Ng, L. Superficial burn caused by a Hotline fluid warmer infusion set. Anaesthesia 2009, 64, 101–102. [Google Scholar] [CrossRef] [PubMed]
- In-line blood/solution warmers. Health Devices 1996, 25, 352–390.
- Clarke, P.A.; Thornton, M.J. Failure of a water-bath design intravenous fluid warmer. Can. J. Anaesth. 2009, 56, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Szerb, J. Failure of an iv fluid warming device. Can. J. Anaesth. 2007, 54, 324–325. [Google Scholar] [CrossRef][Green Version]
- Mittnacht, A.J.C.; Lin, H.-M.; Liu, X.; Wax, D. New-onset intra-operative hyperthermia in a large surgical patient population: A retrospective observational study. Eur. J. Anaesthesiol. 2020, 38, 487–493. [Google Scholar] [CrossRef]



| Authors | Based on | Formula |
|---|---|---|
| Whitby et al. [64] | Age (years) | 2 × age (years)/3 + 10 [cm] from corniculate cartilages |
| Hong et al. [65] | Height (cm) |
|
| Pre-OP | Intra-OP (General Anaesthesia) | Post-OP |
|---|---|---|
| Continuous methods:
| Serial measurements:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemeth, M.; Miller, C.; Bräuer, A. Perioperative Hypothermia in Children. Int. J. Environ. Res. Public Health 2021, 18, 7541. https://doi.org/10.3390/ijerph18147541
Nemeth M, Miller C, Bräuer A. Perioperative Hypothermia in Children. International Journal of Environmental Research and Public Health. 2021; 18(14):7541. https://doi.org/10.3390/ijerph18147541
Chicago/Turabian StyleNemeth, Marcus, Clemens Miller, and Anselm Bräuer. 2021. "Perioperative Hypothermia in Children" International Journal of Environmental Research and Public Health 18, no. 14: 7541. https://doi.org/10.3390/ijerph18147541
APA StyleNemeth, M., Miller, C., & Bräuer, A. (2021). Perioperative Hypothermia in Children. International Journal of Environmental Research and Public Health, 18(14), 7541. https://doi.org/10.3390/ijerph18147541






