Inequalities in Exposure to Ambient Air Neurotoxicants and Disparities in Markers of Neurodevelopment in Children by Maternal Nativity Status
Abstract
:1. Introduction
2. Methods
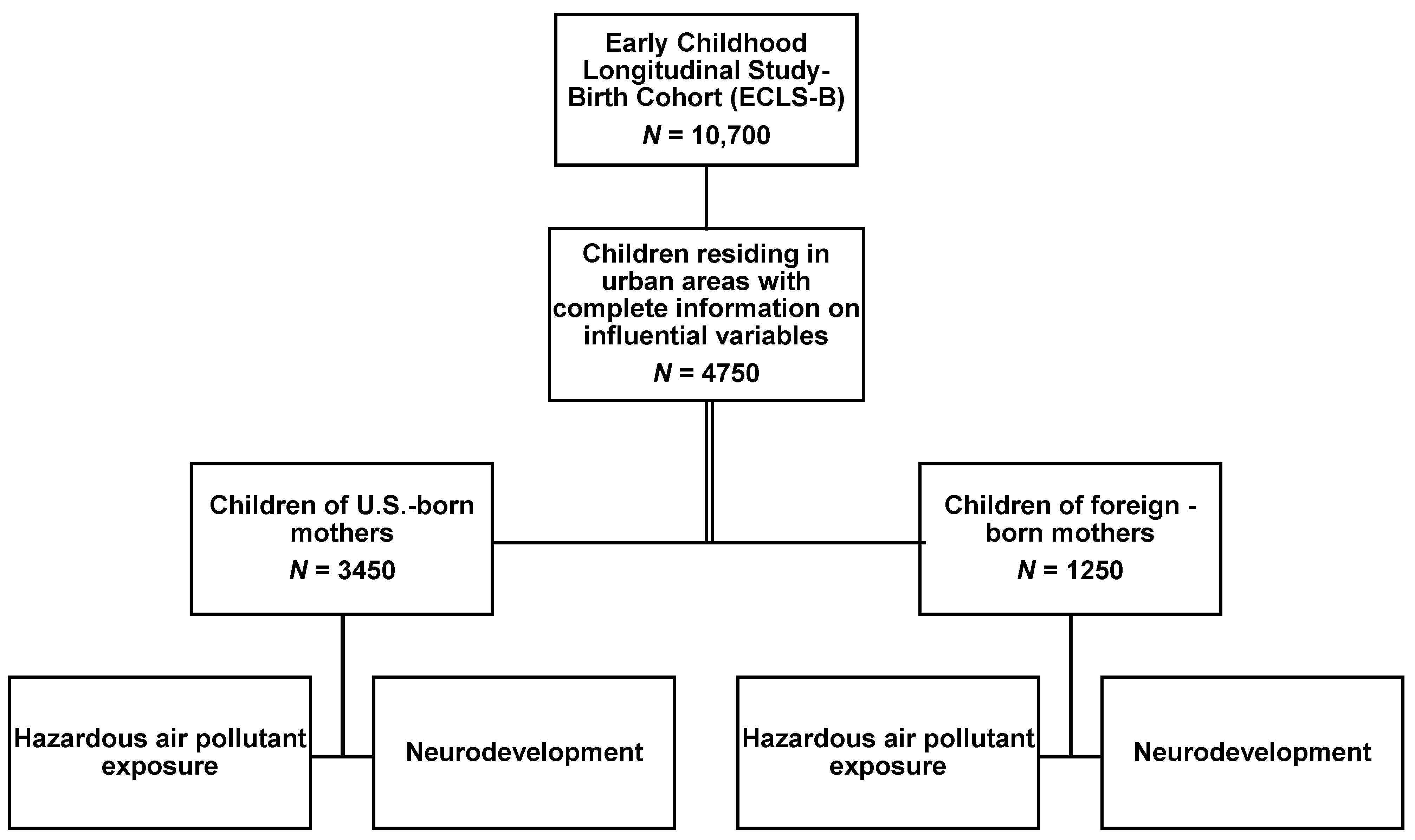
2.1. Study Population
2.2. Outcome Assessment
2.3. Exposure Assessment
2.4. Identification of Influential Variables
2.5. Statistical Analysis
3. Results
3.1. Diversity in the Demographic Characteristics of the Population
3.2. Assessing Air Pollutant Exposure profiles
3.3. Maternal Nativity Status Associated with Measures of Cognitive Development
3.4. Air Pollutant Exposure Assessment
3.5. Interaction between Isophorone Exposure, Maternal Nativity on Neurodevelopment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickens, H.W. The role of race/ethnicity and social class in minority health status. Health Serv. Res. 1995, 30 Pt 2, 151–162. [Google Scholar]
- Montgomery, L.E.; Carter-Pokras, O. Health Status by Social Class and/or Minority Status: Implications for Environmental Equity Research. Toxicol. Ind. Health 1993, 9, 729–773. [Google Scholar] [CrossRef] [PubMed]
- Danso, K. Nativity and Health Disparities: Predictors of Immigrant Health. Soc. Work Public Health 2016, 31, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, H.; Holmes, S.M.; Madrigal, D.S.; Young, M.-E.D.; Beyeler, N.; Quesada, J. Immigration as a Social Determinant of Health. Annu. Rev. Public Health 2015, 36, 375–392. [Google Scholar] [CrossRef]
- McGee, S.A.; Claudio, L. Nativity as a Determinant of Health Disparities among Children. J. Immigr. Minor. Health 2018, 20, 517–528. [Google Scholar] [CrossRef]
- Mendoza, F.S. Health Disparities and Children in Immigrant Families: A Research Agenda. Pediatrics 2009, 124 (Suppl. 3), S187–S195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcazar, A.J.; Grineski, S.E.; Collins, T. The Hispanic health paradox across generations: The relationship of child generational status and citizenship with health outcomes. Public Health 2015, 129, 691–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, G.; Brotanek, J. The healthy immigrant effect: A greater understanding might help us improve the health of all children. Arch. Pediatr. Adolesc. Med. 2005, 159, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Passel, J.S.; D’Vera Cohn, D. US Population Projections, 2005–2050; Pew Research Center: Washington, DC, USA, 2008. [Google Scholar]
- Morello-Frosch, R.; Zuk, M.; Jerrett, M.; Shamasunder, B.; Kyle, A.D. Understanding The Cumulative Impacts Of Inequalities In Environmental Health: Implications For Policy. Health Aff. 2011, 30, 879–887. [Google Scholar] [CrossRef]
- Volk, H.E.; Perera, F.; Braun, J.M.; Kingsley, S.L.; Gray, K.; Buckley, J.; Clougherty, J.E.; Croen, L.A.; Eskenazi, B.; Herting, M.; et al. Prenatal air pollution exposure and neurodevelopment: A review and blueprint for a harmonized approach within ECHO. Environ. Res. 2021, 196, 110320. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Apelberg, B.J.; Buckley, T.J.; White, R.H. Socioeconomic and racial disparities in cancer risk from air toxics in Maryland. Environ. Health Perspect. 2005, 113, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Brown, P. Race, Class, and Environmental Health: A Review and Systematization of the Literature. Environ. Res. 1995, 69, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Liévanos, R.S. Race, deprivation, and immigrant isolation: The spatial demography of air-toxic clusters in the continental United States. Soc. Sci. Res. 2015, 54, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.; Dadvand, P. Pre-natal brain development as a target for urban air pollution. Basic Clin. Pharmacol. Toxicol. 2019, 125, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- US EPA (Environmental Protection Agency). Hazardous Air Pollutants. Available online: https://www.epa.gov/haps (accessed on 20 February 2020).
- Schell, L.M.; Denham, M. Environmental Pollution in Urban Environments and Human Biology. Annu. Rev. Anthr. 2003, 32, 111–134. [Google Scholar] [CrossRef]
- Von Ehrenstein, O.S.; Aralis, H.; Cockburn, M.; Ritz, B. In Utero Exposure to Toxic Air Pollutants and Risk of Childhood Autism. Epidemiology 2014, 25, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Windham, G.C.; Zhang, L.; Gunier, R.; Croen, L.A.; Grether, J.K. Autism Spectrum Disorders in Relation to Distribution of Hazardous Air Pollutants in the San Francisco Bay Area. Environ. Health Perspect. 2006, 114, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Aghaei, M.; Janjani, H.; Yousefian, F.; Jamal, A.; Yunesian, M. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ. Res. 2019, 173, 135–156. [Google Scholar] [CrossRef]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Melnyk, S.; James, S.J.; Palmer, R.F.; Austin, C.; et al. Prenatal air pollution influences neurodevelopment and behavior in autism spectrum disorder by modulating mitochondrial physiology. Mol. Psychiatry 2021, 26, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, F.M.; Tulve, N.S. A systematic review and meta-analysis examining the interrelationships between chemical and non-chemical stressors and inherent characteristics in children with ADHD. Environ. Res. 2020, 180, 108884. [Google Scholar] [CrossRef] [PubMed]
- Raz, R.; Roberts, A.; Lyall, K.; Hart, J.E.; Just, A.C.; Laden, F.; Weisskopf, M.G. Autism Spectrum Disorder and Particulate Matter Air Pollution before, during, and after Pregnancy: A Nested Case–Control Analysis within the Nurses’ Health Study II Cohort. Environ. Health Perspect. 2015, 123, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Engle, R.; Mora-Tiscareño, A.; Styner, M.; Gómez-Garza, G.; Zhu, H.; Jewells, V.; Torres-Jardón, R.; Romero, L.; Monroy-Acosta, M.E.; et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn. 2011, 77, 345–355. [Google Scholar] [CrossRef]
- Lopuszanska, U.; Samardakiewicz, M. The Relationship Between Air Pollution and Cognitive Functions in Children and Adolescents: A Systematic Review. Cogn. Behav. Neurol. 2020, 33, 157–178. [Google Scholar] [CrossRef]
- Alemany, S.; Vilor-Tejedor, N.; García-Esteban, R.; Bustamante, M.; Dadvand, P.; Esnaola, M.; Mortamais, M.; Forns, J.; Van Drooge, B.L.; Álvarez-Pedrerol, M.; et al. Traffic-Related Air Pollution, APOE ε4 Status, and Neurodevelopmental Outcomes among School Children Enrolled in the BREATHE Project (Catalonia, Spain). Environ. Health Perspect. 2018, 126, 087001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Garciduenas, L.; Torres-Jardón, R.; Kulesza, R.J.; Park, S.-B.; D’Angiulli, A. Air pollution and detrimental effects on children’s brain. The need for a multidisciplinary approach to the issue complexity and challenges. Front. Hum. Neurosci. 2014, 8, 613. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Casanova, I.; Stein, A.D.; Barraza-Villarreal, A.; Feregrino, R.G.; DiGirolamo, A.; Hernandez-Cadena, L.; Rivera, J.A.; Romieu, I.; Ramakrishnan, U. Prenatal exposure to environmental pollutants and child development trajectories through 7 years. Int. J. Hyg. Environ. Health 2018, 221, 616–622. [Google Scholar] [CrossRef]
- Dellefratte, K.; Stingone, J.A.; Claudio, L. Combined association of BTEX and material hardship on ADHD-suggestive behaviours among a nationally representative sample of US children. Paediatr. Peerinat. Epidemiol. 2019, 33, 482–489. [Google Scholar] [CrossRef]
- Stingone, J.; Pandey, O.P.; Claudio, L.; Pandey, G. Using machine learning to identify air pollution exposure profiles associated with early cognitive skills among U.S. children. Environ. Pollut. 2017, 230, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.M.; Hsu, H.-H.L.; Coull, B.A.; Bellinger, D.C.; Kloog, I.; Schwartz, J.; Wright, R.; Wright, R.J. Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ. Int. 2016, 87, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.S.; Rauh, V.A.; Bansal, R.; Hao, X.; Toth, Z.; Nati, G.; Walsh, K.; Miller, R.L.; Arias, F.; Semanek, D.; et al. Effects of Prenatal Exposure to Air Pollutants (Polycyclic Aromatic Hydrocarbons) on the Development of Brain White Matter, Cognition, and Behavior in Later Childhood. JAMA Psychiatry 2015, 72, 531–540. [Google Scholar] [CrossRef]
- Stingone, J.A.; McVeigh, K.H.; Claudio, L. Early-life exposure to air pollution and greater use of academic support services in childhood: A population-based cohort study of urban children. Environ. Health 2017, 16, 2. [Google Scholar] [CrossRef] [Green Version]
- Perera, F.P.; Rauh, V.; Whyatt, R.M.; Tsai, W.-Y.; Tang, D.; Diaz, D.; Hoepner, L.; Barr, D.; Tu, Y.-H.; Camann, D.; et al. Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons on Neurodevelopment in the First 3 Years of Life among Inner-City Children. Environ. Health Perspect. 2006, 114, 1287–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stingone, J.A.; McVeigh, K.H.; Claudio, L. Association between prenatal exposure to ambient diesel particulate matter and perchloroethylene with children’s 3rd grade standardized test scores. Environ. Res. 2016, 148, 144–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoni, S.; Lucchini, R.G. Manganese exposure: Cognitive, motor and behavioral effects on children: A review of recent findings. Curr. Opinion Pediatr. 2013, 25, 255. [Google Scholar] [CrossRef] [Green Version]
- Kalkbrenner, A.E.; Daniels, J.L.; Chen, J.-C.; Poole, C.; Emch, M.; Morrissey, J. Perinatal Exposure to Hazardous Air Pollutants and Autism Spectrum Disorders at Age 8. Epidemiology 2010, 21, 631–641. [Google Scholar] [CrossRef] [Green Version]
- von Ehrenstein, O.S.; Heck, J.E.; Park, A.S.; Cockburn, M.; Escobedo, L.; Ritz, B. In Utero and early-life exposure to ambient air toxics and childhood brain tumors: A population-based case–control study in California, USA. Environ. Health Perspect. 2016, 124, 1093. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, A.H.; Hvidtfeldt, U.A.; Sørensen, M.; Puett, R.; Ketzel, M.; Brandt, J.; Christensen, J.H.; Geels, C.; Raaschou-Nielsen, O. Components of particulate matter air-pollution and brain tumors. Environ. Int. 2020, 144, 106046. [Google Scholar] [CrossRef]
- Grineski, S.E.; Collins, T.W. Geographic and social disparities in exposure to air neurotoxicants at U.S. public schools. Environ. Res. 2018, 161, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Lett, L.A.; Stingone, J.A.; Claudio, L. The Combined Influence of Air Pollution and Home Learning Environment on Early Cognitive Skills in Children. Int. J. Environ. Res. Public Health 2017, 14, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, R.; Grineski, S.; Collins, T. Children’s exposure to vehicular air pollution in the United States: Environmental injustices at the intersection of race/ethnicity and language. Environ. Sociol. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Bethel, J.; Green, J.L.; Nord, C.; Kalton, G.; West, J. Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) Methodology Report for the 9-Month Data Collection (2001–2002); US Department of Education National Center for Education Statistics: Washington, DC, USA, 2005; Volume 2. Available online: https://eric.ed.gov/?id=ED485636 (accessed on 20 February 2020).
- Wong, M.S.; Jones-Smith, J.C.; Colantuoni, E.; Thorpe, R.J., Jr.; Bleich, S.N.; Chan, K.S. The Longitudinal Association between Early Childhood Obesity and Fathers’ Involvement in Caregiving and Decision-Making. Obesity 2017, 25, 1754–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, J.E.; Bates, L.; Yabiku, S.T. Mother’s age at arrival in the United States and early cognitive development. Early Child. Res. Q. 2009, 24, 367–380. [Google Scholar] [CrossRef]
- Stoner, A.M.; Anderson, S.E.; Buckley, T.J. Ambient Air Toxics and Asthma Prevalence among a Representative Sample of US Kindergarten-Age Children. PLoS ONE 2013, 8, e75176. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Robinson, W. On the Causal Interpretation of Race in Regressions Adjusting for Confounding and Mediating Variables. Epidemiology 2014, 25, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.V.; Jun, H.-J.; Kawachi, I.; Wright, R.J. Contribution of Race/Ethnicity and Country of Origin to Variations in Lifetime Reported Asthma: Evidence for a Nativity Advantage. Am. J. Public Health 2009, 99, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Messer, L.C.; Laraia, B.A.; Kaufman, J.S.; Eyster, J.; Holzman, C.; Culhane, J.; Elo, I.; Burke, J.G.; O’Campo, P. The Development of a Standardized Neighborhood Deprivation Index. J. Urban Health 2006, 83, 1041–1062. [Google Scholar] [CrossRef] [Green Version]
- Glick, J.E.; White, M.J. Academic trajectories of immigrant youths: Analysis within and across cohorts. Demography 2003, 40, 759–783. [Google Scholar] [CrossRef] [PubMed]
- Coll, C.G.E.; Marks, A.K.E. The Immigrant Paradox in Children and Adolescents: Is Becoming American a Developmental Risk? American Psychological Association: Washington, DC, USA, 2012. [Google Scholar]
- Sunyer, J.; Esnaola, M.; Alvarez-Pedrerol, M.; Forns, J.; Rivas, I.; López-Vicente, M.; Suades-González, E.; Foraster, M.; Garcia-Esteban, R.; Basagaña, X.; et al. Association between Traffic-Related Air Pollution in Schools and Cognitive Development in Primary School Children: A Prospective Cohort Study. PLoS Med. 2015, 12, e1001792. [Google Scholar] [CrossRef]
- Forns, J.; Dadvand, P.; Esnaola, M.; Alvarez-Pedrerol, M.; López-Vicente, M.; Garcia-Esteban, R.; Cirach, M.; Basagaña, X.; Guxens, M.; Sunyer, J. Longitudinal association between air pollution exposure at school and cognitive development in school children over a period of 3.5 years. Environ. Res. 2017, 159, 416–421. [Google Scholar] [CrossRef]
- Brockmeyer, S.; D’Angiulli, A. How air pollution alters brain development: The role of neuroinflammation. Transl. Neurosci. 2016, 7, 24–30. [Google Scholar] [CrossRef]
- Mage, D.; Ozolins, G.; Peterson, P.; Webster, A.; Orthofer, R.; Vandeweerd, V.; Gwynne, M. Urban air pollution in megacities of the world. Atmos. Environ. 1996, 30, 681–686. [Google Scholar] [CrossRef]
- Rauh, V.A.; Landrigan, P.J.; Claudio, L. Housing and health: Intersection of poverty and environmental exposures. Ann. N. Y. Acad. Sci. 2008, 1136, 276–288. [Google Scholar] [CrossRef]
- Mohai, P.; Lantz, P.M.; Morenoff, J.; House, J.S.; Mero, R.P. Racial and Socioeconomic Disparities in Residential Proximity to Polluting Industrial Facilities: Evidence From the Americans’ Changing Lives Study. Am. J. Public Health 2009, 99 (Suppl. 3), S649–S656. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Etzel, R.A. Textbook of Children’s Environmental Health; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Mikati, I.; Benson, A.; Luben, T.; Sacks, J.; Richmond-Bryant, J. Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status. Am. J. Public Health 2018, 108, 480–485. [Google Scholar] [CrossRef]
- Zidek, J.V.; Wong, H.; Le, N.; Burnett, R. Causality, measurement error and multicollinearity in epidemiology. Environmetrics 1996, 7, 441–451. [Google Scholar] [CrossRef]
- Rivas, I.; Querol, X.; Wright, J.; Sunyer, J. How to protect school children from the neurodevelopmental harms of air pollution by interventions in the school environment in the urban context. Environ. Int. 2018, 121, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Payne-Sturges, D.C.; Marty, M.A.; Perera, F.; Miller, M.; Swanson, M.; Ellickson, K.; Cory-Slechta, D.A.; Ritz, B.; Balmes, J.; Anderko, L.; et al. Healthy Air, Healthy Brains: Advancing Air Pollution Policy to Protect Children’s Health. Am. J. Public Health 2019, 109, 550–554. [Google Scholar] [CrossRef] [PubMed]

| Maternal Nativity Status | |||
|---|---|---|---|
| Demographic Factor | Total Population (n = 4750) (%) | U.S.-Born (n = 3450) (%) | Foreign-Born (n = 1250) (%) |
| Maternal Race | |||
| White, non-Hispanic | 47.20 | 45.05 | 2.16 |
| Black, non-Hispanic | 17.88 | 16.28 | 1.59 |
| Hispanic | 18.23 | 9.12 | 9.12 |
| Asian, non-Hispanic | 16.19 | 1.68 | 14.51 |
| Other, non-Hispanic | 0.49 | 0.34 | 0.16 |
| Maternal Education | |||
| High school and below | 39.20 | 39.20 | 39.00 |
| Some college | 27.80 | 30.70 | 19.60 |
| Bachelor’s Degree & above | 33.00 | 30.10 | 41.40 |
| Birth weight | |||
| Normal | 75.20 | 71.34 | 86.60 |
| Moderately low | 15.20 | 17.44 | 8.42 |
| Very low | 9.54 | 11.21 | 5.02 |
| SES Index Quintile | |||
| First | 14.80 | 13.88 | 17.22 |
| Second | 17.40 | 17.74 | 16.33 |
| Third | 19.60 | 21.44 | 14.39 |
| Fourth | 19.80 | 21.75 | 14.63 |
| Fifth | 28.30 | 25.19 | 37.43 |
| NDI mean (SD) | −0.11 (1.02) | −0.11 (1.00) | −0.12 (1.09) |
| Characteristic | Model 1 | Model 2 | Model 3 a | Model 4 a | Model 5 a |
|---|---|---|---|---|---|
| Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | |
| Nativity status | |||||
| U.S-born | reference | reference | reference | ||
| Foreign-born | −0.37 [−0.46, −0.28] | −0.17 [−0.29, −0.06] | −0.17 [−0.27, −0.06] | ||
| Isophorone | |||||
| ≤7.90 ng/m3 | reference | reference | reference | ||
| ≥7.90 ng/m3 | −0.14 [−0.24, −0.04] | −0.05 [−0.13, 0.004] | −0.04 [−0.12, 0.04] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya, F.; Stingone, J.A.; Claudio, L. Inequalities in Exposure to Ambient Air Neurotoxicants and Disparities in Markers of Neurodevelopment in Children by Maternal Nativity Status. Int. J. Environ. Res. Public Health 2021, 18, 7512. https://doi.org/10.3390/ijerph18147512
Araya F, Stingone JA, Claudio L. Inequalities in Exposure to Ambient Air Neurotoxicants and Disparities in Markers of Neurodevelopment in Children by Maternal Nativity Status. International Journal of Environmental Research and Public Health. 2021; 18(14):7512. https://doi.org/10.3390/ijerph18147512
Chicago/Turabian StyleAraya, Faven, Jeanette A. Stingone, and Luz Claudio. 2021. "Inequalities in Exposure to Ambient Air Neurotoxicants and Disparities in Markers of Neurodevelopment in Children by Maternal Nativity Status" International Journal of Environmental Research and Public Health 18, no. 14: 7512. https://doi.org/10.3390/ijerph18147512
APA StyleAraya, F., Stingone, J. A., & Claudio, L. (2021). Inequalities in Exposure to Ambient Air Neurotoxicants and Disparities in Markers of Neurodevelopment in Children by Maternal Nativity Status. International Journal of Environmental Research and Public Health, 18(14), 7512. https://doi.org/10.3390/ijerph18147512







