Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism: A Case Report with 5 Years Follow-Up and Review of the Literature
Abstract
1. Introduction
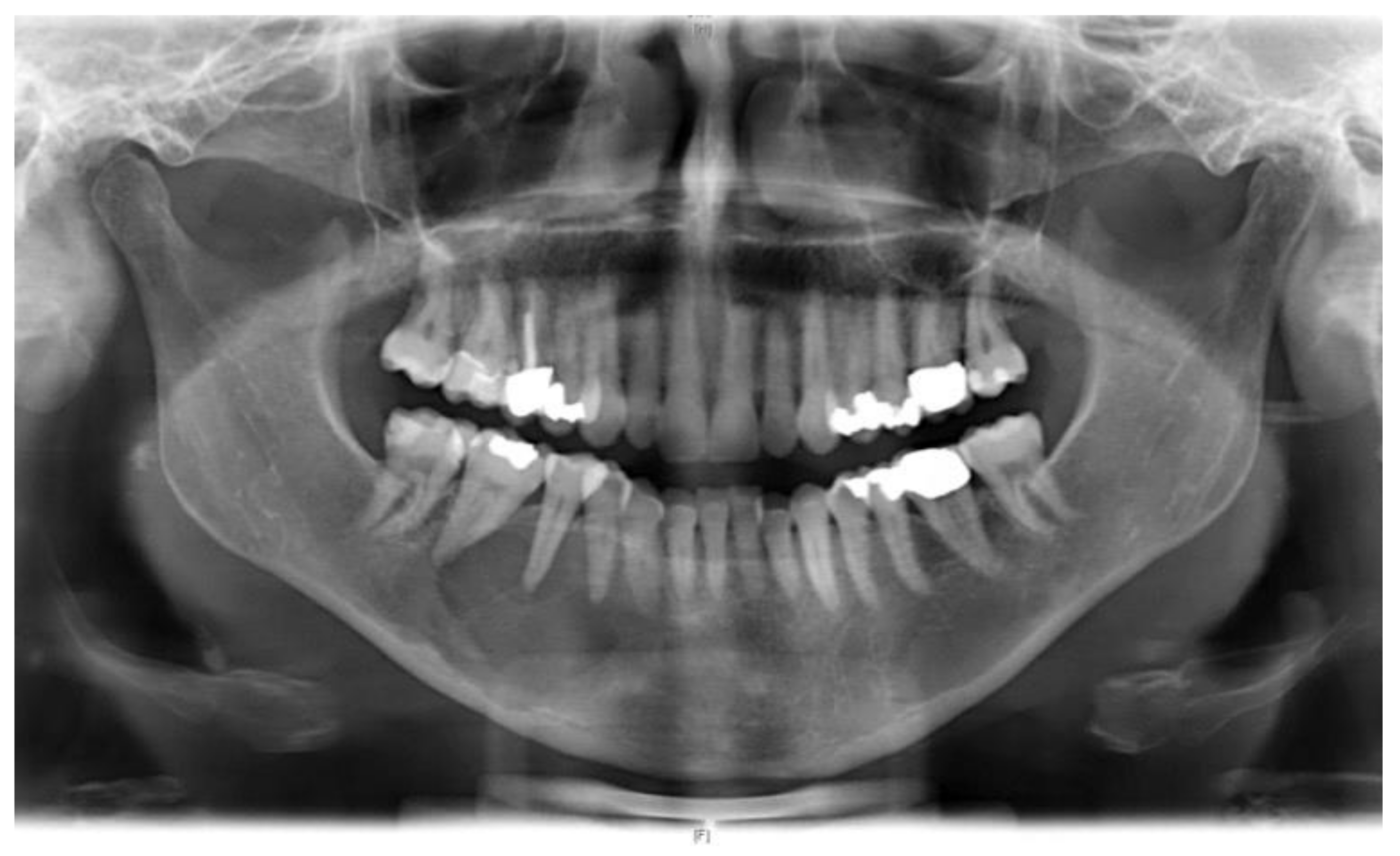
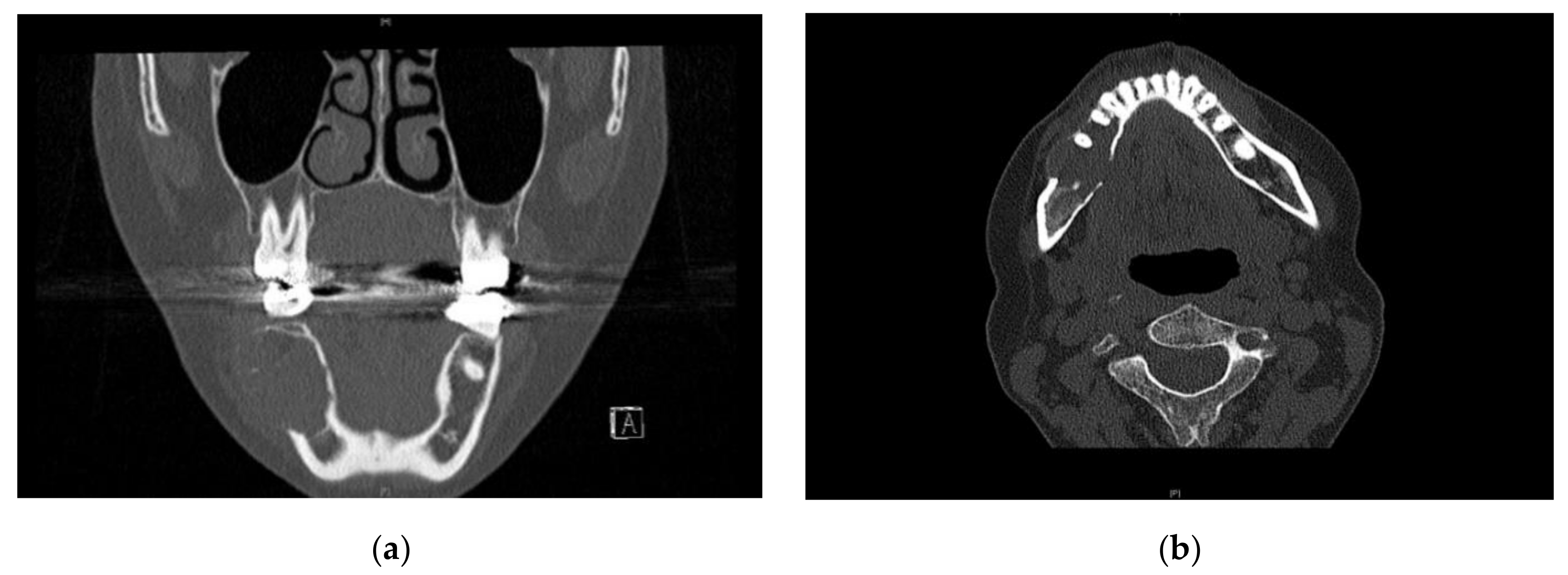
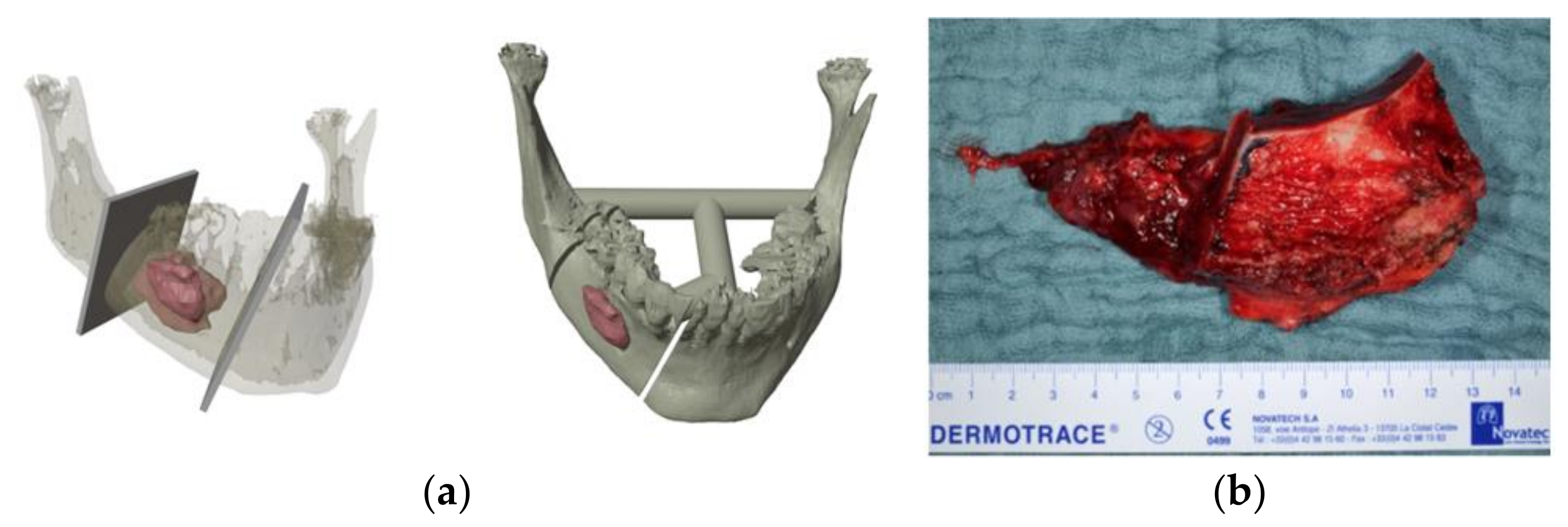
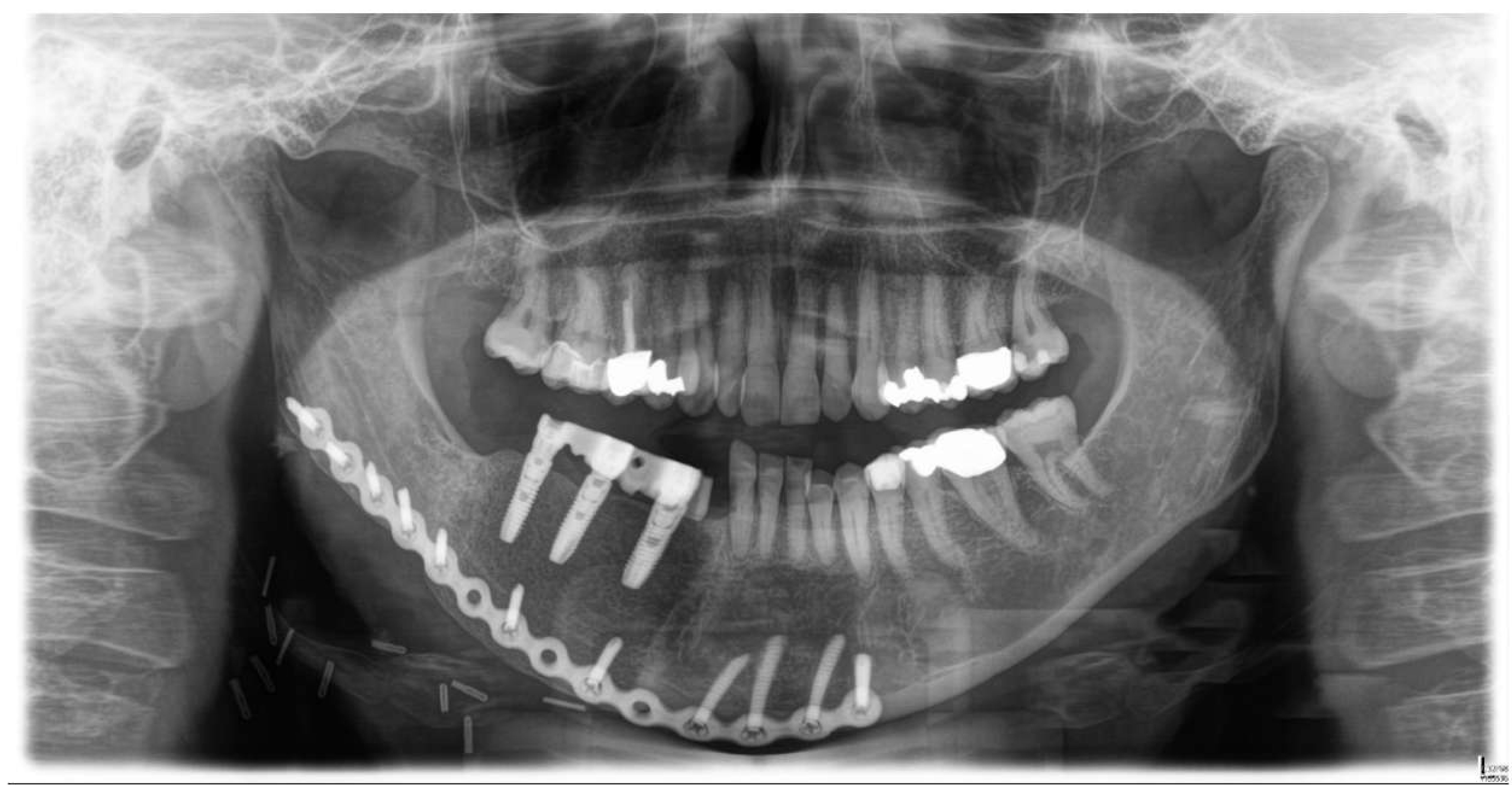
2. Case Presentation
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPT | hyperparathyroidism |
| PTH | parathyroid hormone |
| NR | normal range |
| DCIA | deep circumflex iliac artery |
References
- Olvi, L.G.; Santini-Araujo, E. “Brown Tumor” of Hyperparathyroidism. In Tumors and Tumor-Like Lesions of Bone: For Surgical Pathologists, Orthopedic Surgeons and Radiologists; Santini-Araujo, E., Kalil, R.K., Bertoni, F., Park, Y.-K., Eds.; Springer: London, UK, 2015; pp. 815–825. ISBN 978-1-4471-6578-1. [Google Scholar]
- Huang, R.; Zhuang, R.; Liu, Y.; Li, T.; Huang, J. Unusual Presentation of Primary Hyperparathyroidism: Report of Three Cases. BMC Med. Imaging 2015, 15, 23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, M.D.; Silverberg, S.J. Primary Hyperparathyroidism. Nat. Rev. Endocrinol. 2018, 14, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hedgeman, E.; Lipworth, L.; Lowe, K.; Saran, R.; Do, T.; Fryzek, J. International Burden of Chronic Kidney Disease and Secondary Hyperparathyroidism: A Systematic Review of the Literature and Available Data. Int. J. Nephrol. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Manjunatha, B.S.; Purohit, S.; Harsh, A.; Vangala, N. A Complex Case of Brown Tumors as Initial Manifestation of Primary Hyperparathyroidism in a Young Female. J. Oral Maxillofac. Pathol. 2019, 23, 477. [Google Scholar] [CrossRef]
- Zou, H.; Song, L.; Jia, M.; Wang, L.; Sun, Y. Brown Tumor of Multiple Facial Bones Associated with Primary Hyperparathyroidism: A Clinical Case Report. Medicine 2018, 97, e11877. [Google Scholar] [CrossRef]
- Xie, C.; Tsakok, M.; Taylor, N.; Partington, K. Imaging of Brown Tumours: A Pictorial Review. Insights Imaging 2019, 10, 75. [Google Scholar] [CrossRef]
- Taylor, G.I.; Townsend, P.; Corlett, R. Superiority of the Deep Circumflex Iliac Vessels as the Supply for Free Groin Flaps. Clinical Work. Plast. Reconstr. Surg. 1979, 64, 745–759. [Google Scholar] [CrossRef]
- Larson, C.B. Rating Scale for Hip Disabilities. Clin. Orthop. Relat. Res. 1963, 31, 85–93. [Google Scholar] [CrossRef]
- Jović, N.; Kozomara, R.; Stosić, S.; Broćić, M.; Hrvacević, R.; Ilić, S. Brown tumor of the maxilla in patient with secondary hyperparathyroidism. Vojn. Pregl. 2004, 61, 683–687. [Google Scholar] [CrossRef]
- Triantafillidou, K.; Zouloumis, L.; Karakinaris, G.; Kalimeras, E.; Iordanidis, F. Brown Tumors of the Jaws Associated with Primary or Secondary Hyperparathyroidism. A Clinical Study and Review of the Literature. Am. J. Otolaryngol. 2006, 27, 281–286. [Google Scholar] [CrossRef]
- Pérez-Guillermo, M.; Acosta-Ortega, J.; García-Solano, J.; Ramos-Freixá, J. Cytologic Aspect of Brown Tumor of Hyperparathyroidism. Report of a Case Affecting the Hard Palate. Diagn. Cytopathol. 2006, 34, 291–294. [Google Scholar] [CrossRef]
- Leal, C.T.S.; Lacativa, P.G.S.; Gomes, E.M.S.; Nunes, R.C.; de S. Costa, F.L.F.; Gandelmann, I.H.A.; Cavalcante, M.A.A.; Farias, M.L.F. Surgical Approach and Clinical Outcome of a Deforming Brown Tumor at the Maxilla in a Patient with Secondary Hyperparathyroidism Due to Chronic Renal Failure. Arq. Bras. Endocrinol. Metab. 2006, 50, 963–967. [Google Scholar] [CrossRef]
- Jakubowski, J.M.; Velez, I.; McClure, S.A. Brown Tumor as a Result of Hyperparathyroidism in an End-Stage Renal Disease Patient. Case Rep. Radiol. 2011, 2011, 415476. [Google Scholar] [CrossRef]
- Nair, P.P.; Gharote, H.P.; Thomas, S.; Guruprasad, R.; Singh, N. Brown Tumour of the Jaw. BMJ Case Rep. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Nair, P.; Hegde, K.; Neelakantan, S. A Bilaterally Recurring Exophytic Mass on the Lower Jaw. Case Rep. 2011, 2011, bcr0920103362. [Google Scholar] [CrossRef] [PubMed]
- Praveen, A.H.; Thriveni, R. Maxillary and Mandibular Hyperparathyroidism. Natl. J. Maxillofac. Surg. 2012, 3, 51–54. [Google Scholar] [CrossRef]
- Arunkumar, K.; Kumar, S.; Deepa, D. Brown Tumor in Mandible as a First Sign of Vitamin D Deficiency: A Rare Case Report and Review. Indian J. Endocr. Metab. 2012, 16, 310. [Google Scholar] [CrossRef]
- Pechalova, P.F.; Poriazova, E.G. Brown Tumor at the Jaw in Patients with Secondary Hyperparathyroidism Due to Chronic Renal Failure. Acta Med. 2013, 56, 83–86. [Google Scholar] [CrossRef]
- Verma, P.; Verma, K.G.; Verma, D.; Patwardhan, N. Craniofacial Brown Tumor as a Result of Secondary Hyperparathyroidism in Chronic Renal Disease Patient: A Rare Entity. J. Oral Maxillofac. Pathol. 2014, 18, 267–270. [Google Scholar] [CrossRef]
- Jafari-Pozve, N.; Ataie-Khorasgani, M.; Jafari-Pozve, S.; Ataie-Khorasgani, M. Maxillofacial Brown Tumors in Secondary Hyperparathyroidism: A Case Report and Literature Review. J. Res. Med. Sci. 2014, 19, 1099–1102. [Google Scholar]
- Qaisi, M.; Loeb, M.; Montague, L.; Caloss, R. Mandibular Brown Tumor of Secondary Hyperparathyroidism Requiring Extensive Resection: A Forgotten Entity in the Developed World? Case Rep. Med. 2015, 2015, 567543. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, I.V.; Queiroz, S.P.; Medeiros, R.; Ribeiro, R.B.; Crusoé-Rebello, I.M.; Leão, J.C. Brown Tumor of Secondary Hyperparathyroidism: Surgical Approach and Clinical Outcome. Oral Maxillofac. Surg. 2016, 20, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Can, Ö.; Boynueğri, B.; Gökçe, A.M.; Özdemir, E.; Ferhatoğlu, F.; Canbakan, M.; Şahin, G.M.; Titiz, M.İ.; Apaydın, S. Brown Tumors: A Case Report and Review of the Literature. Case Rep. Nephrol. Dial. 2016, 6, 46–52. [Google Scholar] [CrossRef]
- Brabyn, P.; Capote, A.; Belloti, M.; Zylberberg, I. Hyperparathyroidism Diagnosed Due to Brown Tumors of the Jaw: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2017, 75, 2162–2169. [Google Scholar] [CrossRef]
- Aerden, T.; Grisar, K.; Nys, M.; Politis, C. Secondary Hyperparathyroidism Causing Increased Jaw Bone Density and Mandibular Pain: A Case Report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, e37–e41. [Google Scholar] [CrossRef]
- Kartini, D.; Siswiandari, M.K.; Wibisana, G.; Yulian, E.D.; Kurnia, A.; Panigoro, S.S.; Albar, A.Z.; Ramli, M. Craniofacial Brown Tumor in Patients with Secondary Hyperparathyroidism to Chronic Renal Failure: Report of Two Cases in Cipto Mangunkusumo Hospital. Case Rep. Oncol. Med. 2018, 2018, 1801652. [Google Scholar] [CrossRef]
- Xu, W.; Qu, Y.; Shi, W.; Ma, B.; Jiang, H.; Wang, Y.; Qu, N.; Zhu, Y. Multiple Bone Brown Tumor Secondary to Primary Hyperparathyroidism: A Case Report and Literature Review. Gland Surg. 2019, 8, 810–816. [Google Scholar] [CrossRef]
- Cunningham, J.; Locatelli, F.; Rodriguez, M. Secondary Hyperparathyroidism: Pathogenesis, Disease Progression, and Therapeutic Options. Clin. J. Am. Soc. Nephrol. 2011, 6, 913–921. [Google Scholar] [CrossRef]
- Galitzer, H.; Ben-Dov, I.Z.; Silver, J.; Naveh-Many, T. Parathyroid Cell Resistance to Fibroblast Growth Factor 23 in Secondary Hyperparathyroidism of Chronic Kidney Disease. Kidney Int. 2010, 77, 211–218. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Ogata, H.; Koiwa, F. Secondary Hyperparathyroidism: Pathogenesis and Latest Treatment. Apher. Dial. 2019, 23, 309–318. [Google Scholar] [CrossRef]
- Maanaoui, M.; Hamroun, A.; Lebas, C.; Lenain, R.; Lionet, A. Osteitis Fibrosa Cystica von Recklinghausen. J. Nephrol. 2021, 34, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular Reconstruction: Overview. J. Maxillofac. Oral Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.N.; Lakshmiah, S.R.; Narayan, B.; Lowe, D.; Brownson, P.; Brown, J.S.; Vaughan, E.D. A Comparison of the Long-Term Morbidity Following Deep Circumflex Iliac and Fibula Free Flaps for Reconstruction Following Head and Neck Cancer. Plast. Reconstr. Surg. 2003, 112, 1517–1525, discussion 1526–1527. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Pre-Operative | Post-Operative | 6-Month Follow-Up | 12-Month Follow-Up | 24-Month Follow-Up | 5-Year Follow-Up | Normal Range |
|---|---|---|---|---|---|---|---|
| Calcium | 2.08 mmol/L | 2.27 mmol/L | 2.3 mmol/L | 2.27 mmol/L | 2.2 mmol/L | 2.32 mmol/L | 2.11–2.59 mmol/L |
| Phosphate | 0.78 mmol/L | 1.4 mmol/L | 1.21 mmol/L | 1.13 mmol/L | 1.6 mmol/L | 1.39 mmol/L | 0.84–1.45 mmol/L |
| PTH | 200.80 pmol/L | 7.74 pmol/L | 12.3 pmol/L | 9.57 pmol/L | 10.8 pmol/L | 13.6 pmol/L | 1.2–4.5 pmol/L |
| Vitamin D | 20 µg/L | 72 µg/L | 65 µg/L | 63 µg/L | 56 µg/L | 82 µg/L | 20–70 µg/L |
| Creatinine | 3 mg/dL | 2.6 mg/dL | 2.76 mg/dL | 2.15 mg/dL | 2.58 mg/dL | 2.96 mg/dL | 0.5–0.9 mg/dL |
| Author and Reference | Year | Country | Sex | Age | Tumor Location | Tumor Size | Local Treatment | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Jović et al. [10] | 2004 | Serbia | M | 25 | Maxilla | - | Surgical resection | - |
| Triantafillidou et al. [11] | 2006 | Greece | F | 21 | Mandible | - | Surgical resection | 7 years |
| F | 70 | Mandible | - | Surgical resection | 7 years | |||
| F | 68 | Mandible | - | Surgical resection | 7 years | |||
| Pérez-Guillermo et al. [12] | 2006 | Spain | M | 61 | Maxilla | 3 × 2 cm | Surgical resection | - |
| Leal et al. [13] | 2006 | Brazil | F | 31 | Maxilla | - | Surgical resection | - |
| Jakubowski [14] | 2011 | USA | M | 49 | Mandible | 8 × 2 × 4 cm | - | - |
| Preeti P. Nair [15] | 2011 | India | F | 35 | Mandible | 2.5 × 2.7 cm | Conservative (Vitamin D Supplementation) | - |
| Thomas et al. [16] | 2011 | India | F | 27 | Mandible | 4 × 6 cm | Surgical resection | 9 months (no recurrence) |
| Praveen and Thriveni [17] | 2012 | India | F | 21 | Maxilla and mandible | 6 × 7 cm; 2 × 2 cm; 4 × 2 cm | Surgical resection | 3 years (no recurrence) |
| Arunkumar et al. [18] | 2012 | India | F | 12 | Mandible | - | Surgical resection | 6 months (no recurrence) |
| Pechalova and Poriazova [19] | 2013 | Bulgaria | M | 19 | Mandible | - | Surgical resection | - |
| F | 49 | Maxilla | - | Surgical resection | - | |||
| Verma Pradhuman [20] | 2014 | India | F | 31 | Mandible | 6 × 5 cm | - | - |
| Jafari-Pozve Nasim [21] | 2014 | Iran | M | 28 | Maxilla | - | Parathyroidectomy | - |
| Mohammed Qaisi [22] | 2015 | USA | M | 43 | Mandible | 5 × 7 cm | Surgical resection | - |
| Queiroz et al. [23] | 2016 | Brazil | F | 53 | Mandible | - | Surgical resection | 8 months (no recurrence) |
| Özgür Can et al. [24] | 2016 | Turkey | F | 30 | Mandible/maxilla | - | Surgical resection | 6 months (no recurrence) |
| Brabyn Philip [25] | 2017 | Spain | F | 42 | Maxilla | - | - | - |
| Aerden Thomas [26] | 2018 | Belgium | M | 32 | Mandible | - | - | - |
| Kartini Diani [27] | 2018 | Indonesia | F | 26 | Mandible | 3 × 4 cm | Surgical resection | 7 months (no recurrence) |
| F | 31 | Maxilla | 4 × 3 cm | Subtotal parathyroidectomy | 5 months (no recurrence) | |||
| Xu Weibo [28] | 2019 | China | F | 30 | Mandible | - | Surgical resection | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shavlokhova, V.; Goeppert, B.; Gaida, M.M.; Saravi, B.; Weichel, F.; Vollmer, A.; Vollmer, M.; Freudlsperger, C.; Mertens, C.; Hoffmann, J. Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism: A Case Report with 5 Years Follow-Up and Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 7370. https://doi.org/10.3390/ijerph18147370
Shavlokhova V, Goeppert B, Gaida MM, Saravi B, Weichel F, Vollmer A, Vollmer M, Freudlsperger C, Mertens C, Hoffmann J. Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism: A Case Report with 5 Years Follow-Up and Review of the Literature. International Journal of Environmental Research and Public Health. 2021; 18(14):7370. https://doi.org/10.3390/ijerph18147370
Chicago/Turabian StyleShavlokhova, Veronika, Benjamin Goeppert, Matthias M. Gaida, Babak Saravi, Frederic Weichel, Andreas Vollmer, Michael Vollmer, Christian Freudlsperger, Christian Mertens, and Jürgen Hoffmann. 2021. "Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism: A Case Report with 5 Years Follow-Up and Review of the Literature" International Journal of Environmental Research and Public Health 18, no. 14: 7370. https://doi.org/10.3390/ijerph18147370
APA StyleShavlokhova, V., Goeppert, B., Gaida, M. M., Saravi, B., Weichel, F., Vollmer, A., Vollmer, M., Freudlsperger, C., Mertens, C., & Hoffmann, J. (2021). Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism: A Case Report with 5 Years Follow-Up and Review of the Literature. International Journal of Environmental Research and Public Health, 18(14), 7370. https://doi.org/10.3390/ijerph18147370








