In-House Filtration Efficiency Assessment of Vapor Hydrogen Peroxide Decontaminated Filtering Facepiece Respirators (FFRs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Filtering Facepiece Respirators (FFRs)
2.2. Filtration Efficiency (FE) Measurement
2.3. Filtration Efficiency (FE) Following VHP Cycles
2.4. Statistical Analysis
3. Results
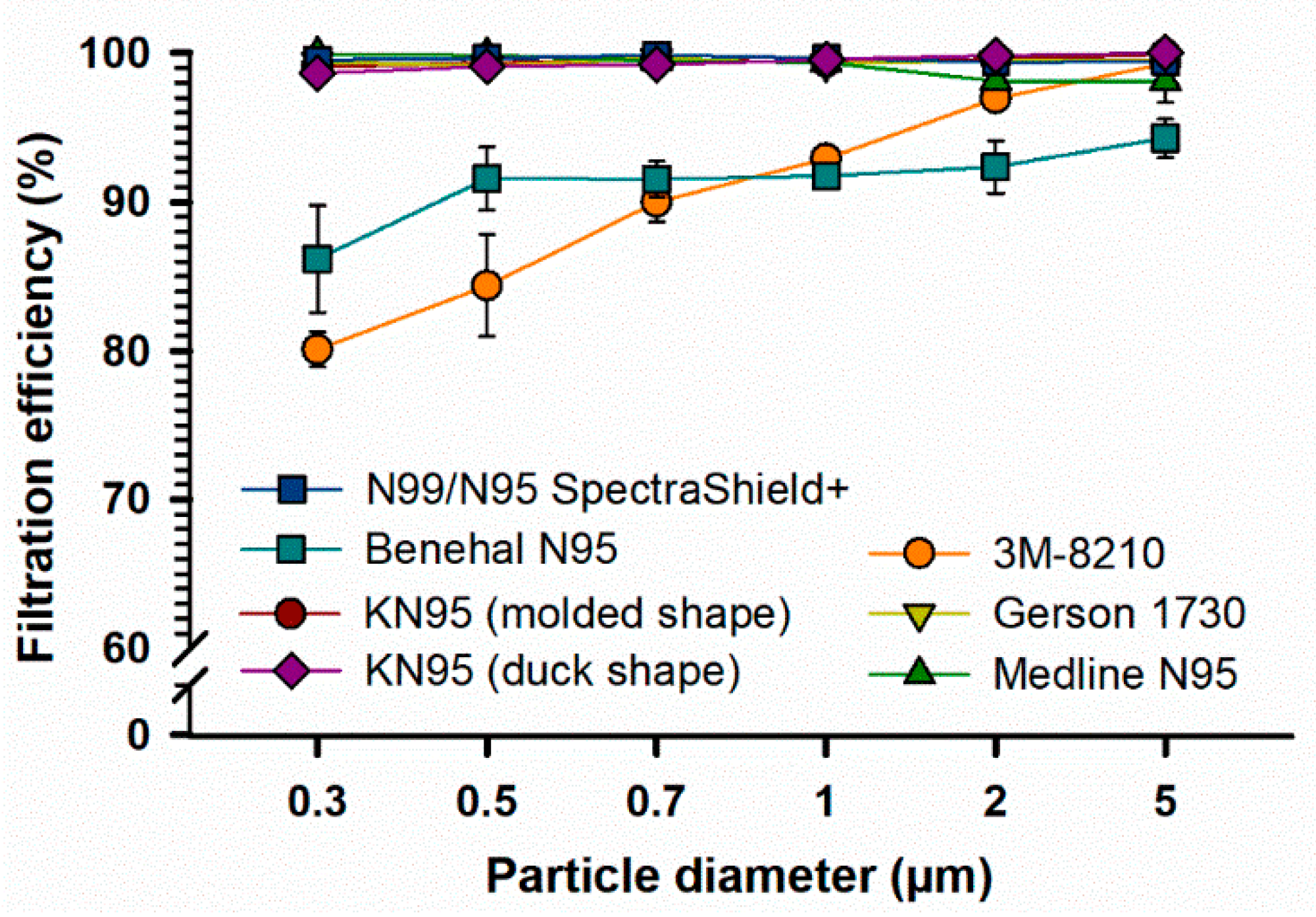
3.1. Filtration Efficiency (FE) of FFRs
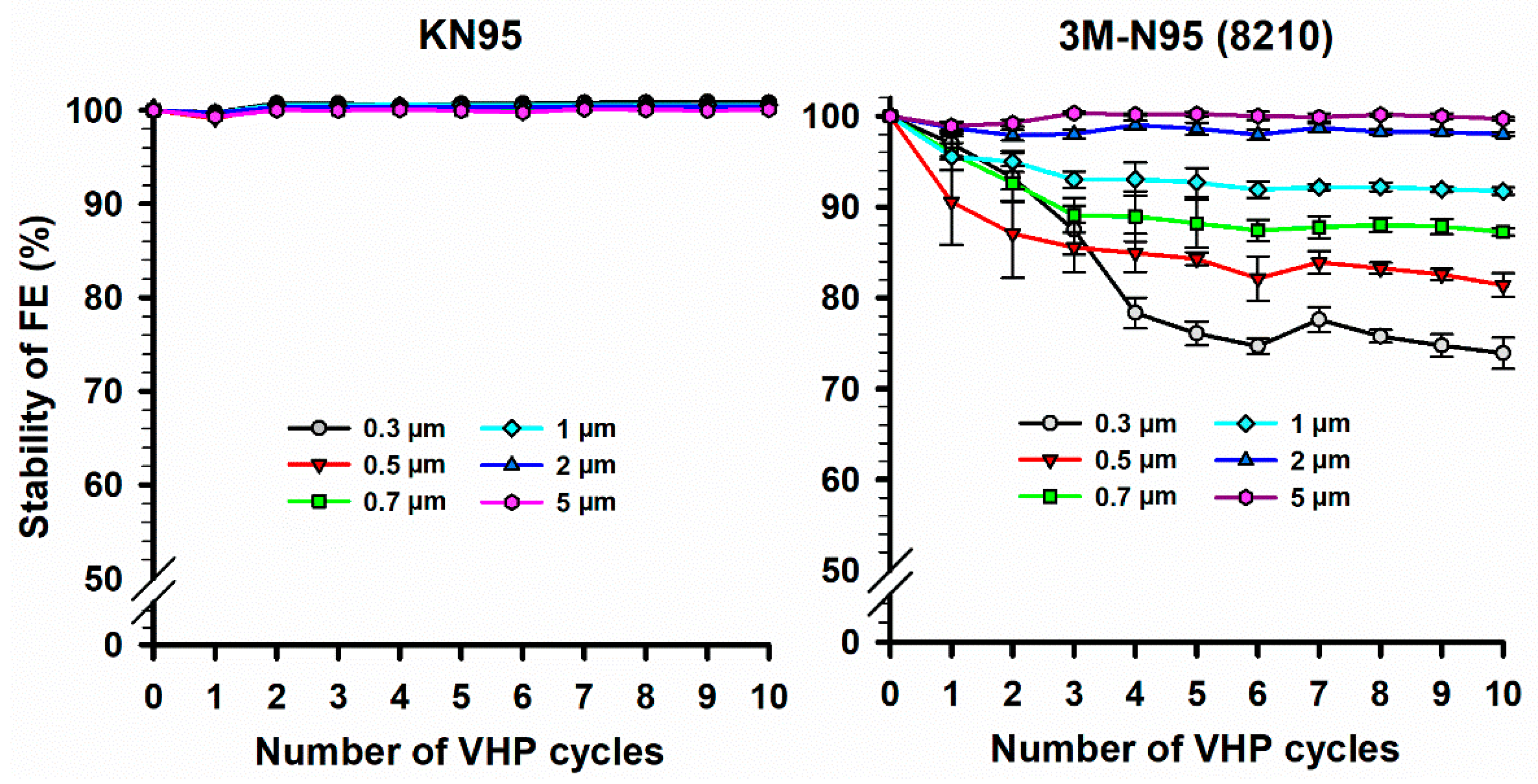
3.2. Filtration Efficiency (FE) Stability of N95 and KN95 Masks Following VHP-STERIS Sterilization Systems
4. Discussion
4.1. Uncertainties and Repeatability of the Experimental Measurements
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | the USA Centers for Disease Control |
| COVID-19 | the novel coronavirus disease |
| EUA | emergency use authorization |
| FDA | the US Food and Drug Administration |
| FFRs | filtering facepiece respirators |
| FE | filtration efficiency |
| NIOSH | the USA National Institute for Occupational Safety and Health |
| PLA | polylactic acid |
| PM | ambient particulate matter |
| STL | StereoLithography |
| UVGI | ultraviolet germicidal irradiation |
| VHP | vapor hydrogen peroxide |
References
- Feng, S.; Shen, C.; Xia, N.; Song, W.; Fan, M.; Cowling, B.J. Rational Use of Face Masks in the COVID-19 Pandemic. Lancet Respir. Med. 2020, 8, 434–436. [Google Scholar] [CrossRef]
- Burki, T. Global Shortage of Personal Protective Equipment. Lancet Infect. Dis. 2020, 20, 785–786. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health (NIOSH). Cfr 84 Respiratory Protective Devices: Final Rules and Notice. Federal Register; Federal Register US Centers for Disease Control and Prevention, National Institute for Occu-Pational Safety and Health: Atlanta, GA, USA, 1997.
- Centers for Disease Control and Prevention (CDC). Niosh-Approved N95 Particulate Filtering Facepiece Respirators. Available online: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.Html (accessed on 21 January 2021).
- Fisher, E.M.; Shaffer, R.E. Considerations for Recommending Extended Use and Limited Reuse of Filtering Facepiece Respirators in Health Care Settings. J. Occup. Environ. Hyg. 2014, 11, D115–D128. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. Available online: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.Html#ref19 (accessed on 13 January 2021).
- Rebmann, T.; Carrico, R.; Wang, J. Physiologic and Other Effects and Compliance With Long-Term Respirator Use Among Medical Intensive Care Unit Nurses. Am. J. Infect. Control. 2013, 41, 1218–1223. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A. Correspondence. Stability of Sars-Cov-2 in Different Environmental Conditions. Lancet Microbe 2020, 1, E10. [Google Scholar] [CrossRef]
- Cramer, A.; Enze, T.; Galanek, M.; Lamere, E.; Li, J.; Gupta, R.; Short, M. Assessment of the Qualitative Fit Test and Quantitative Single-Pass Filtration Efficiency of Disposable N95 Masks Following Gamma Irradiation. JAMA Netw. Open 2020, 3, E209961.e61. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Implementing Filtering Facepiece Respirator (FFR) Reuse, Including Reuse After Decontamination, When There Are Known Shortages of N95 Respirators. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-Reuse-respirators.Html (accessed on 13 January 2021).
- Rodriguez-Martinez, C.E.; Sossa-Briceño, M.P.; Cortes, J. Decontamination and Reuse of N95 Filtering Facemask Respirators: A Systematic Review of the Literature. Am. J. Infect. Control. 2020, 48, 1520–1532. [Google Scholar] [CrossRef]
- Derraik, J.G.B.; Anderson, W.A.; Connelly, E.A.; Anderson, Y.C. Rapid Review of Sars-Cov-1 and Sars-Cov-2 Viability, Susceptibility to Treatment, and the Disinfection and Reuse of Ppe, Particularly Filtering Facepiece Respirators. Int. J. Environ. Res. Public Health 2020, 17, 6117. [Google Scholar] [CrossRef]
- Kierat, W.; Augustyn, W.; Koper, P.; Pawlyta, M.; Chrusciel, A.; Wyrwol, B. The Use of UVC Irradiation to Sterilize Filtering Facepiece Masks Limiting Airborne Cross-Infection. Int. J. Environ. Res. Public Health 2020, 17, 7396. [Google Scholar] [CrossRef]
- O’Hearn, K.; Gertsman, S.; Webster, R.; Tsampalieros, A.; Ng, R.; Gibson, J.; Sampson, M.; Sikora, L.; McNally, J.D. Efficacy and Safety of Disinfectants for Decontamination of N95 and SN95 Filtering Facepiece Respirators: A Systematic Review. J. Hosp. Infect. 2020, 106, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, T.; Filho, N.; Zille, A. Ultraviolet-C As a Viable Reprocessing Method for Disposable Masks and Filtering Facepiece Respirators. Polymers 2021, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Gertsman, S.; Agarwal, A.; O’Hearn, K.; Webster, R.; Tsampalieros, A.; Barrowman, N.; Sampson, M.; Sikora, L.; Staykov, E.; Ng, R.; et al. Microwave- and Heat-Based Decontamination of N95 Filtering Facepiece Respirators: A Systematic Review. J. Hosp. Infect. 2020, 106, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Shaffer, R.E. A Method to Determine the Available UV-C Dose for the Decontamination of Filtering Facepiece Respirators. J. Appl. Microbiol. 2010, 110, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kasloff, S.B.; Leung, A.; Cutts, T.; Strong, J.E.; Hills, K.; Vazquez-Grande, G.; Rush, B.; Lother, S.; Zarychanski, R. N95 Mask Decontamination Using Standard Hospital Sterilization Technologies. MedRxiv 2020. [Google Scholar] [CrossRef]
- The United States Food and Drug Administration (FDA). The U.S. Food and Drug Administration (FDA) Issued an Emergency Use Authorization (EUA) for the Emergency Use of the Steris Sterilization System for Use in Decontaminating Compatible N95 Respirators. Available online: https://www.fda.gov/media/136843/Download (accessed on 21 January 2021).
- The United States Food and Drug Administration (FDA). The U.S. Food and Drug Administration (FDA) Issued an Emergency Use Authorization (EUA) for the Emergency Use of the Stryker Sterilization System for Use in Decontaminating Compatible N95 Respirators. Available online: https://www.fda.gov/media/136976/Download (accessed on 6 June 2021).
- The United States Food and Drug Administration (FDA). The U.S. Food and Drug Administration (FDA) Issued an Emergency Use Authorization (EUA) for the Emergency Use of the Sterrad Sterilization System for Use in Decontaminating Compatible N95 Respirators. Available online: https://www.fda.gov/media/136884/Download (accessed on 6 June 2021).
- STERIS Healthcare. Instructions for Healthcare Facilities: Decontamination of Compatible N95 Respirators Using the Steris Sterilization Systems. Available online: https://www.steris.com/-/media/documents/pdfs/covid19-Landing-page/6-8/steris--Instructions-for-Healthcare-Facilities-6520.Ashx (accessed on 13 January 2021).
- Centers for Disease Control and Prevention (CDC). Assessment of Filter Penetration Performance and Fit for Decontam-Inated N95 Respirators. Available online: https://www.cdc.gov/niosh/npptl/respirators/testing/pdfs/NIOSHApproved_Decon_TestPlan10.Pdf (accessed on 12 April 2021).
- 3M. Decontamination of 3m Filtering Facepiece Respirators, Such As N95 Respirators, in the United States—Considerations. Available online: https://multimedia.3m.com/mws/media/1824869O/decontamination-Methods-for-3m-Filtering-Facepiece-Respirators-Technical-bulletin.Pdf (accessed on 13 January 2021).
- Migliore, M. Testing the Limits of Facemask Filtration." International Filtration News. Available online: https://www.filtnews.com/Testing-the-Limits-of-Facemask-Filtration/ (accessed on 3 July 2021).
- Maher, B.; Chavez, R.; Tomaz, G.C.; Nguyen, T.; Hassan, Y. A Fluid Mechanics Explanation of the Effectiveness of Common Materials for Respiratory Masks. Int. J. Infect. Dis. 2020, 99, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.K.; Plana, D.; Yang, H.; Carmack, M.M.; Tian, E.; Sinha, M.S.; Krikorian, D.; Turner, D.; Mo, J.; Li, J.; et al. Analysis of SteraMist Ionized Hydrogen Peroxide Technology in the Sterilization of N95 Respirators and Other PPE. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Salimifard, P.; Rim, D.; Freihaut, J.D. Evaluation of Low-Cost Optical Particle Counters for Monitoring Individual Indoor Aerosol Sources. Aerosol Sci. Technol. 2019, 54, 217–231. [Google Scholar] [CrossRef]
- STERIS Healthcare. Steris Decontamination Solutions for Compatible N95 Respirators for Healthcare Facilities Outside the United States. Available online: https://www.steris.com/healthcare/Steris-Decontamination-Solutions-for-Compatible-n95-Respirators-Non-United-States (accessed on 13 January 2021).
- Chao, C.; Wan, M.P.; Morawska, L.; Johnson, G.; Ristovski, Z.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Li, Y.; Xie, X.; et al. Characterization of Expiration Air Jets and Droplet Size Distributions Immediately at the Mouth Opening. J. Aerosol Sci. 2009, 40, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Lee, B. Minimum Sizes of Respiratory Particles Carrying Sars-Cov-2 and the Possibility of Aerosol Generation. Int. J. Environ. Res. Public Health 2020, 17, 6960. [Google Scholar] [CrossRef]
- Bikov, A.; Paschalaki, K.; Logan-Sinclair, R.; Horváth, I.; Kharitonov, S.A.; Barnes, P.J.; Usmani, O.S.; Paredi, P. Standardised Exhaled Breath Collection for the Measurement of Exhaled Volatile Organic Compounds by Proton Transfer Reaction Mass Spectrometry. BMC Pulm. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Castell, N.; Dauge, F.R.; Schneider, P.; Vogt, M.; Lerner, U.; Fishbain, B.; Broday, D.; Bartonova, A. Can Commercial Low-Cost Sensor Platforms Contribute to Air Quality Monitoring and Exposure Estimates? Environ. Int. 2017, 99, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Kumar, P.; Pilla, F.; Skouloudis, A.N.; DI Sabatino, S.; Ratti, C.; Yasar, A.-U.-H.; Rickerby, D. End-User Perspective of Low-Cost Sensors for Outdoor Air Pollution Monitoring. Sci. Total Environ. 2017, 607–608, 691–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dlugosz, L. Ambient Airborne Particle Concentrations in Hospital Inpatient Rooms. ASHRAE Trans. 2014, 120. Available online: link.gale.com/apps/doc/A394346839/AONE?u=anon~26a941c3&sid=googleScholar&xid=2e41d8d9 (accessed on 25 April 2021).
- Aganovic, A.; Cao, G.; Stenstad, L.-I.; Skogås, J.G. Impact of Surgical Lights on the Velocity Distribution and Airborne Contamination Level in an Operating Room With Laminar Airflow System. Build. Environ. 2017, 126, 42–53. [Google Scholar] [CrossRef]
- Bopp, N.E.; Bouyer, D.H.; Gibbs, C.M.; Nichols, J.E.; Ntiforo, C.A.; Grimaldo, M.A. Multicycle Autoclave Decontamination of N95 Filtering Facepiece Respirators. Appl. Biosaf. 2020, 25, 150–156. [Google Scholar] [CrossRef]
- Kim, J.-M.; Chung, Y.-S.; Jo, H.J.; Lee, N.-J.; Kim, M.S.; Woo, S.H.; Park, S.; Kim, J.W.; Kim, H.M.; Han, M.-G. Identification of Coronavirus Isolated from a Patient in Korea With COVID-19. Osong Public Health Res. Perspect. 2020, 11, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Kwon, N.-J.; Kang, C.-K.; Choe, P.-G.; Yun, J.; Seong, M.-W.; Seo, J.-S.; Oh, M.-D. Virus Isolation from the First Patient With Sars-Cov-2 in Korea. J. Korean Med. Sci. 2020, 24, e84. [Google Scholar]
- O’Dowd, K.; Keerthi, M.N.; Parnia, F.; Snehamol, M.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight Against the Covid-19 Pandemic: A Review of Current Mate-Rials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Modes of Transmission of Virus Causing Covid-19: Implications for Ipc Precaution Recommendations: Scientific Brief, 27 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Transmission of SARSCoV-2: Implications for Infection Prevention Precautions. Scientific Brief. 9 July 2020. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 9 July 2020).
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2) from a Symptomatic Patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.G.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D. Aerodynamic Characteristics and Rna Concentration of Sars-Cov-2 Aerosol in Wuhan Hospitals During Covid-19 Outbreak. BioRxiv 2020. [Google Scholar] [CrossRef]
- Cai, C.; Floyd, E.L. Effects of Sterilization With Hydrogen Peroxide and Chlorine Dioxide Solution on the Filtration Efficiency of N95, KN95, and Surgical Face Masks. JAMA Netw. Open 2020, 3, e2012099. [Google Scholar] [CrossRef]
- Oral, E.; Wannomae, K.K.; Connolly, R.L.; Gardecki, J.A.; Leung, H.M.; Muratoglu, O.K.; Griffiths, A.; Honko, A.N.; Avena, L.E.; McKay, L.G.A. Vapor H2o2 Sterilization As a Decontamination Method for the Reuse of N95 Respirators in the Covid-19 Emergency. MedRxiv 2020. [Google Scholar] [CrossRef]
- Viscusi, D.J.; King, W.P.; Shaffer, R.E. Effect of Decontamination on the Filtration Efficiency of Two Filtering Facepiece Respirator Models. J. Int. Soc. Respir. Prot. 2007, 24, 93. [Google Scholar]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.D.; Sambol, A.R.; Shaffer, R.E. Evaluation of Multiple (3-Cycle) Decontamination Processing for Filtering Facepiece Respirators. J. Eng. Fibers Fabr. 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bhattacharjee, B.; Sangeetha, D.N.; Subramanian, V.; Venkatraman, B. Evaluation of Filtration Efficacy of Various Types of Face-Masks Using Ambient and Pao Aerosols Following With Different Sterilization Methods. MedRxiv 2020. [Google Scholar] [CrossRef]
- Wigginton, K.R.; Arts, P.J.; Clack, H.L.; Fitzsimmons, W.J.; Gamba, M.; Harrison, K.R.; LeBar, W.; Lauring, A.S.; Li, L.; Roberts, W.W. Validation of N95 Filtering Facepiece Respirator Decon-Tamination Methods Available at a Large University Hospital. Open Forum Infect. Dis. 2021. [Google Scholar] [CrossRef]
- TSI Incorporated. Calibration Certificate Failure Explanation for Aerotrak Particle Counters. Available online: https://tsi.com/getmedia/490d98b8-d8e2-4c2d-9632-2b036ac1657f/Calibration_Cert_Failure_Explanation_AeroTrak_APCs_CC-119-US-web?ext=.Pdf (accessed on 6 June 2020).
- Viscusi, D.J.; Bergman, M.S.; Eimer, B.C.; Shaffer, R.E. Evaluation of Five Decontamination Methods for Filtering Facepiece Respirators. Ann. Occup. Hyg. 2009, 53, 815–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeAngelis, H.E.; Grillet, A.M.; Nemer, M.B.; Wasiolek, M.A.; Hanson, D.J.; Omana, M.A.; Sanchez, A.L.; Vehar, D.W.; Thelen, P.M. Gamma Radiation Sterilization of N95 Respirators Leads to Decreased Respirator Performance. PLoS ONE 2021, 16, e0248859. [Google Scholar] [CrossRef] [PubMed]
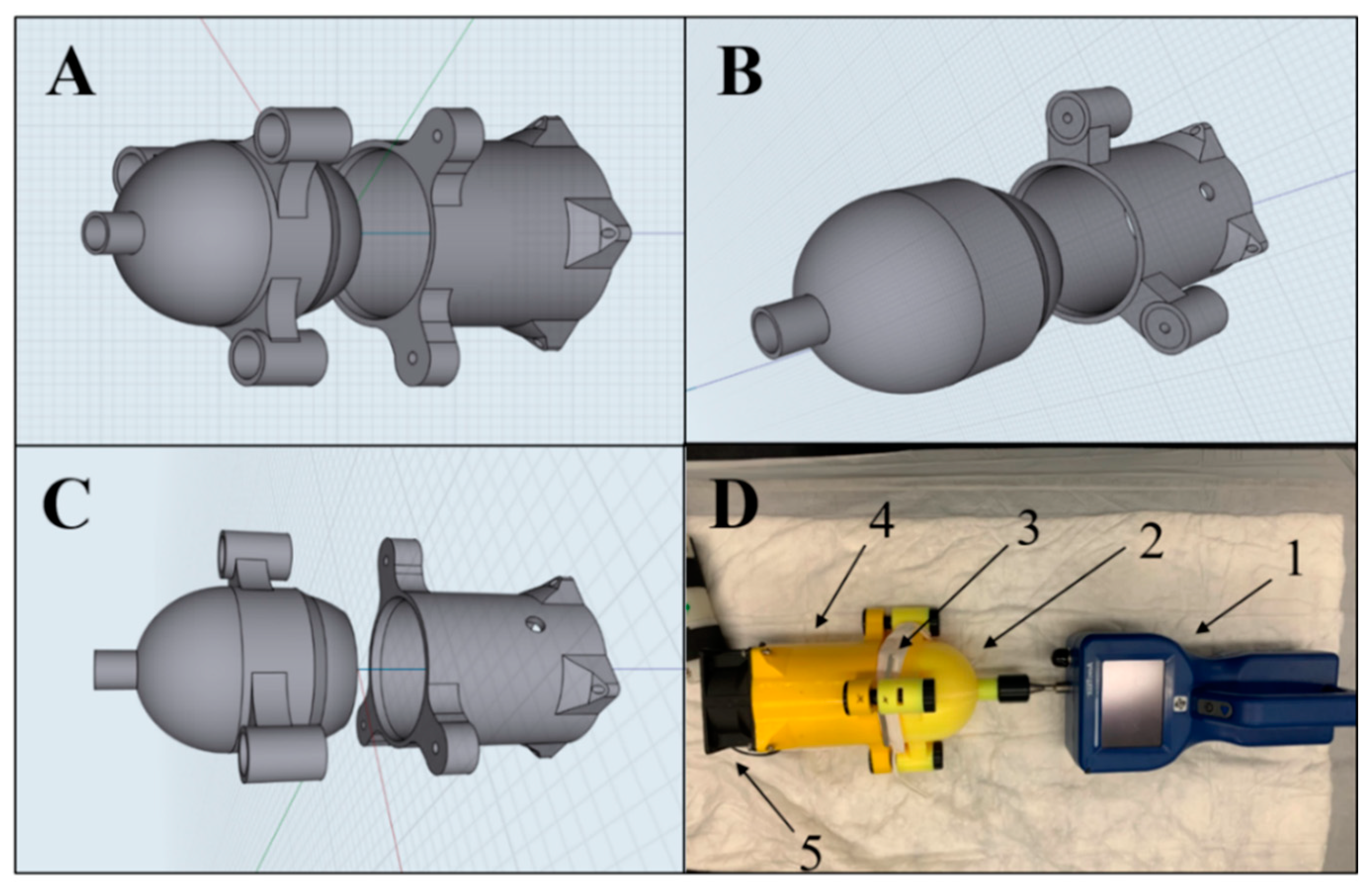
| FFRs | Type | Valve Yes/No | Company | Country | Lot Number | NIOSH Approved |
|---|---|---|---|---|---|---|
| Gerson 1730 Particulate Respirator | N95 | No | Louis M. Gerson | United States | TC-84A-0160 | Yes |
| Medline-Cone Style | N95 | No | Medline | United States | TC-84A-5411 | Yes |
| Benehal Particulate respirator face mask | N95 | Yes | Suzhou Sanical Protective Product | China | 541529 | Yes |
| SpectraShield+ | N99/N95 | No | Nexera Medical | United States | A84740.B1 | Yes |
| KN95 (duck shape) | KN95 | No | Yuyao Yukang Medical Equipment | China | 20200506 | No 1 |
| 3M-8210 2 | N95 | No | 3M | United States 3 | A12199 | Yes |
| KN 95 (molded shape) | KN95 | No | ZhongShan XiaoLan YiShuai Gament Factory | China | 2020042701 | No 1 |
| In-House Method | NIOSH Method | |
|---|---|---|
| Particle size (µm) | ≥0.3 * | ≥0.075 |
| Flow rate (L/min) | 2.8 | 85.0 |
| Aerosol concentration (mg/m3) | Ambient particulate matter air (20–50) | 200 |
| Testing filter | TSI AeroTrak particle counter | TSI 8310 automated filter tester |
| Sampling time/FFR (minute) | 1 | 10 |
| Particle Size (µm) | Percent Uncertainty (%) | Manufacturer’s Percent Uncertainty (%) |
|---|---|---|
| 0.3 | 1.4 | 3.9 |
| 0.5 | 3.0 | 3.9 |
| 0.7 | 4.7 | 3.9 |
| 1 | 3.1 | 3.9 |
| 2 | 4.5 | 3.8 |
| 5 | 4.4 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hadyan, K.; Alsbeih, G.; Nobah, A.; Lindstrom, J.; Falatah, S.; Faran, N.; Al-Ghamdi, S.; Moftah, B.; Alhmaid, R. In-House Filtration Efficiency Assessment of Vapor Hydrogen Peroxide Decontaminated Filtering Facepiece Respirators (FFRs). Int. J. Environ. Res. Public Health 2021, 18, 7169. https://doi.org/10.3390/ijerph18137169
Al-Hadyan K, Alsbeih G, Nobah A, Lindstrom J, Falatah S, Faran N, Al-Ghamdi S, Moftah B, Alhmaid R. In-House Filtration Efficiency Assessment of Vapor Hydrogen Peroxide Decontaminated Filtering Facepiece Respirators (FFRs). International Journal of Environmental Research and Public Health. 2021; 18(13):7169. https://doi.org/10.3390/ijerph18137169
Chicago/Turabian StyleAl-Hadyan, Khaled, Ghazi Alsbeih, Ahmad Nobah, Jeffrey Lindstrom, Sawsan Falatah, Nawarh Faran, Salem Al-Ghamdi, Belal Moftah, and Rashed Alhmaid. 2021. "In-House Filtration Efficiency Assessment of Vapor Hydrogen Peroxide Decontaminated Filtering Facepiece Respirators (FFRs)" International Journal of Environmental Research and Public Health 18, no. 13: 7169. https://doi.org/10.3390/ijerph18137169
APA StyleAl-Hadyan, K., Alsbeih, G., Nobah, A., Lindstrom, J., Falatah, S., Faran, N., Al-Ghamdi, S., Moftah, B., & Alhmaid, R. (2021). In-House Filtration Efficiency Assessment of Vapor Hydrogen Peroxide Decontaminated Filtering Facepiece Respirators (FFRs). International Journal of Environmental Research and Public Health, 18(13), 7169. https://doi.org/10.3390/ijerph18137169








