Respiratory Health Effects of Wildfire Smoke during Summer of 2018 in the Jämtland Härjedalen Region, Sweden
Abstract
:1. Introduction
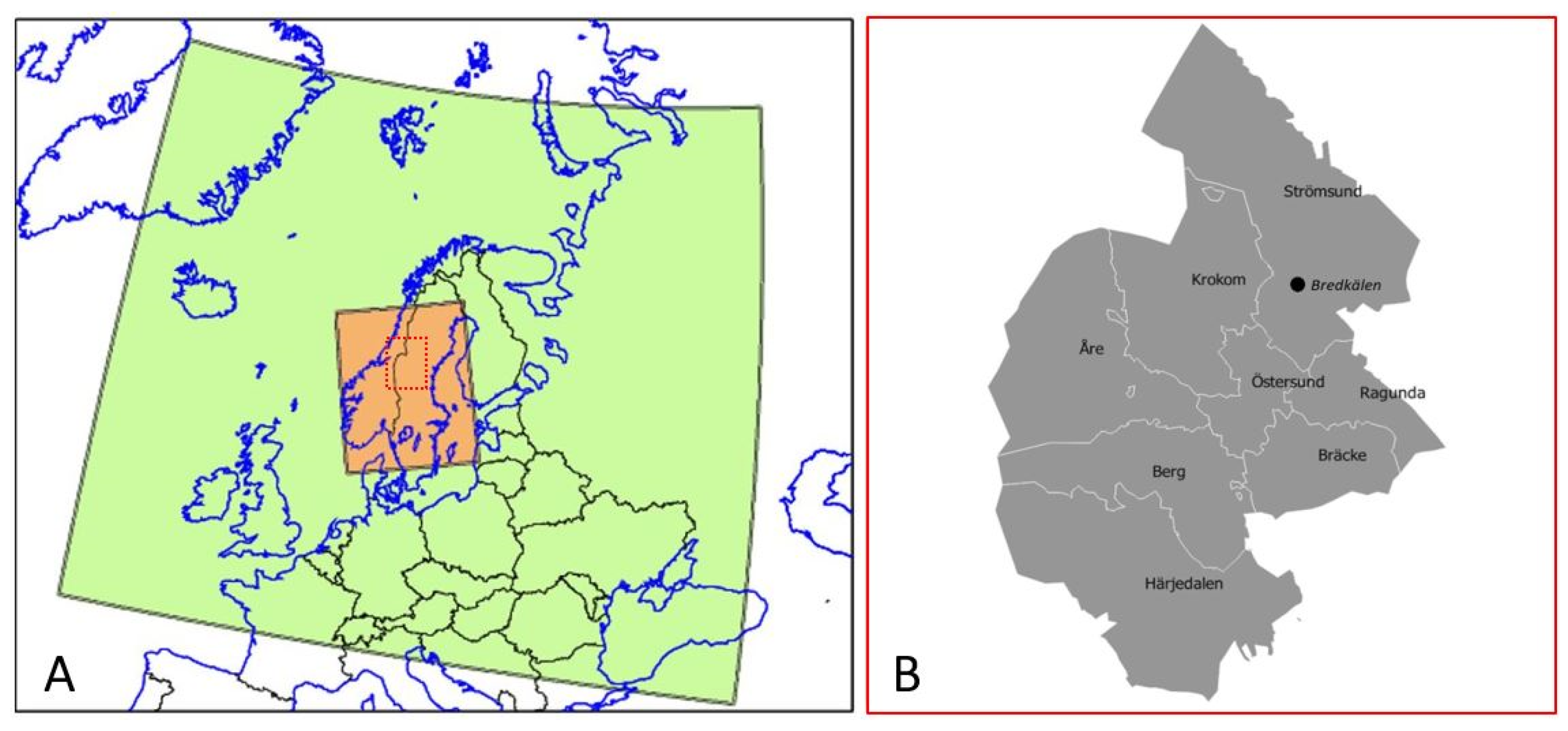
2. Methods
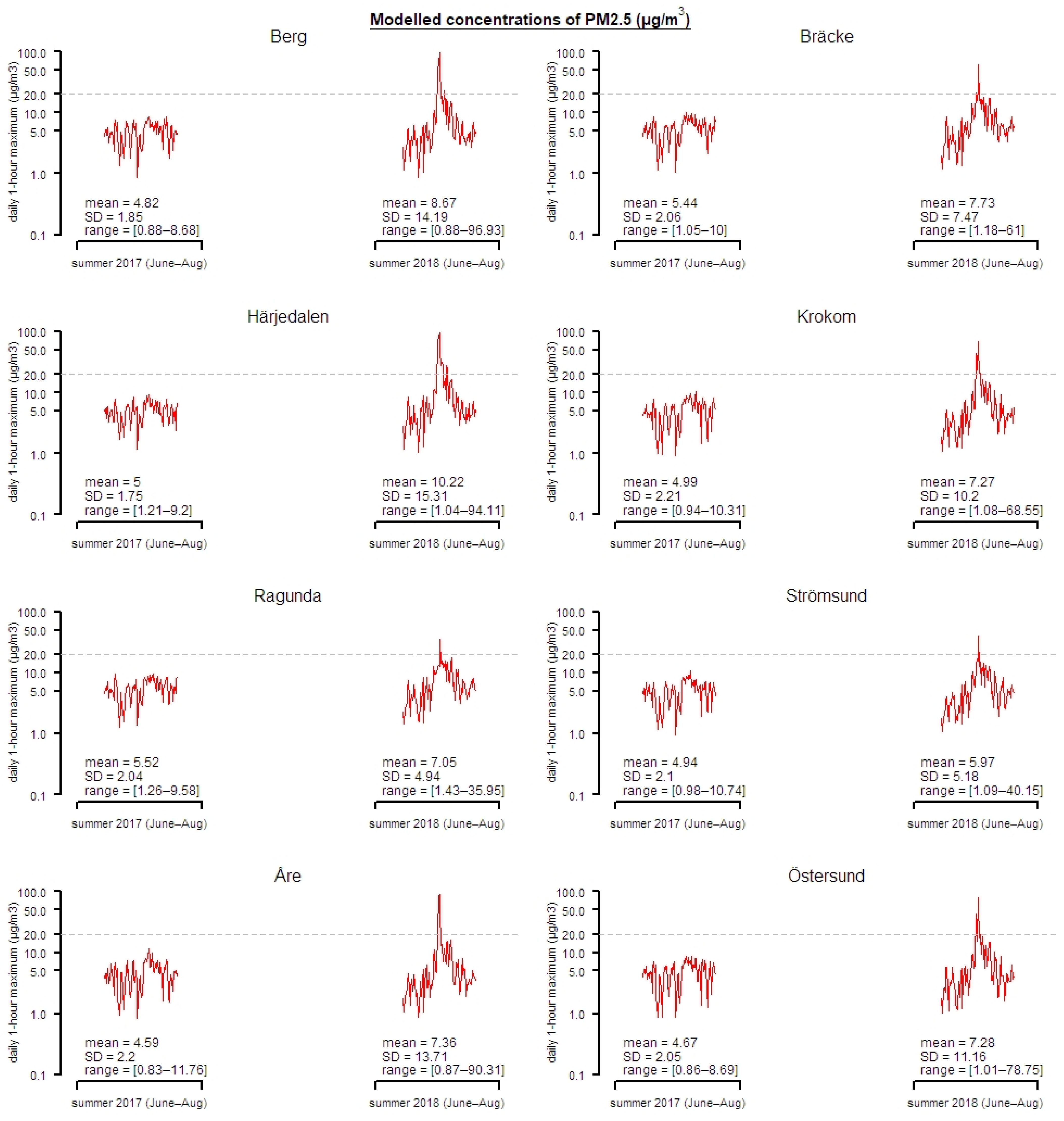
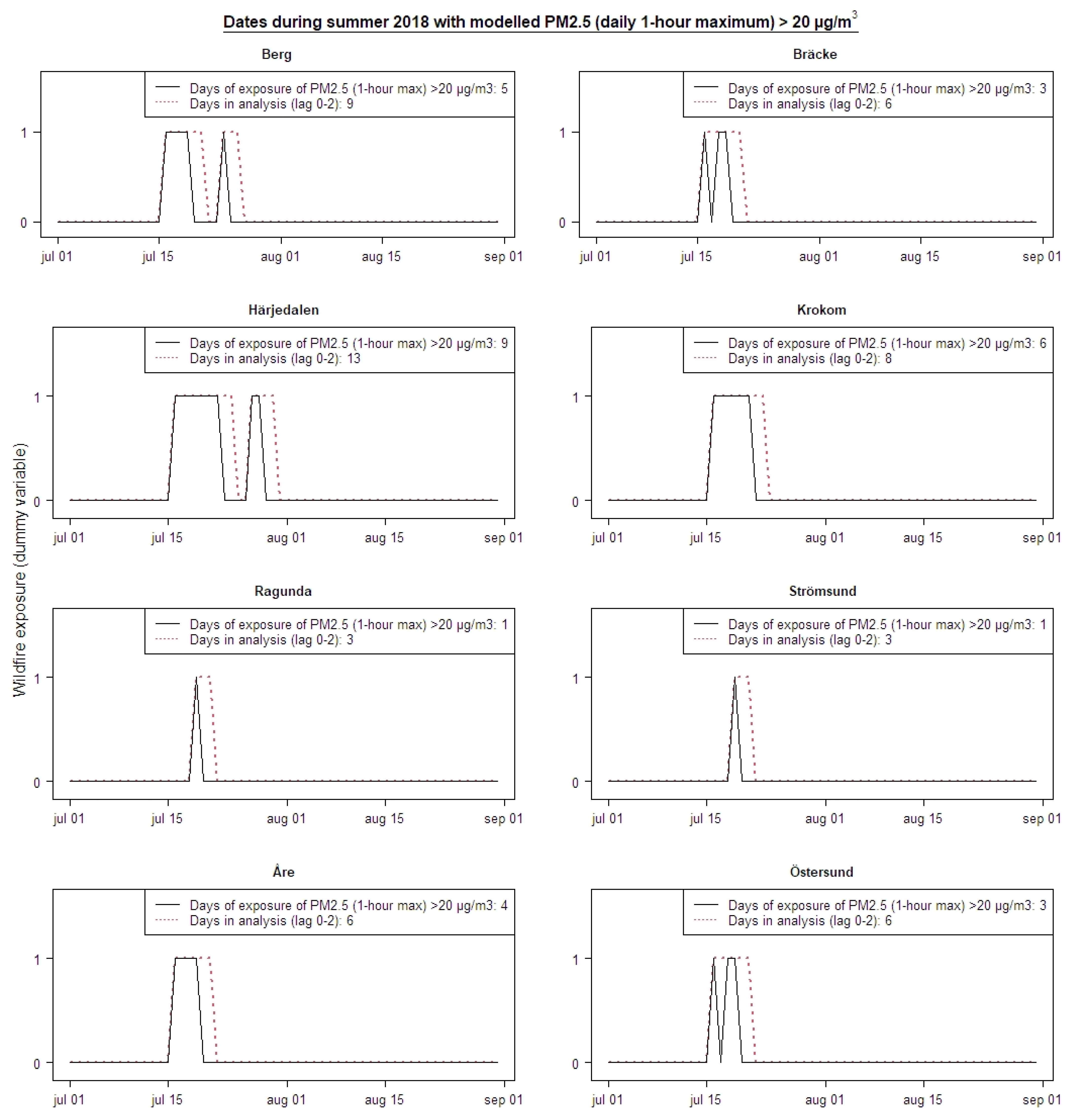
2.1. Exposure Data Modelling
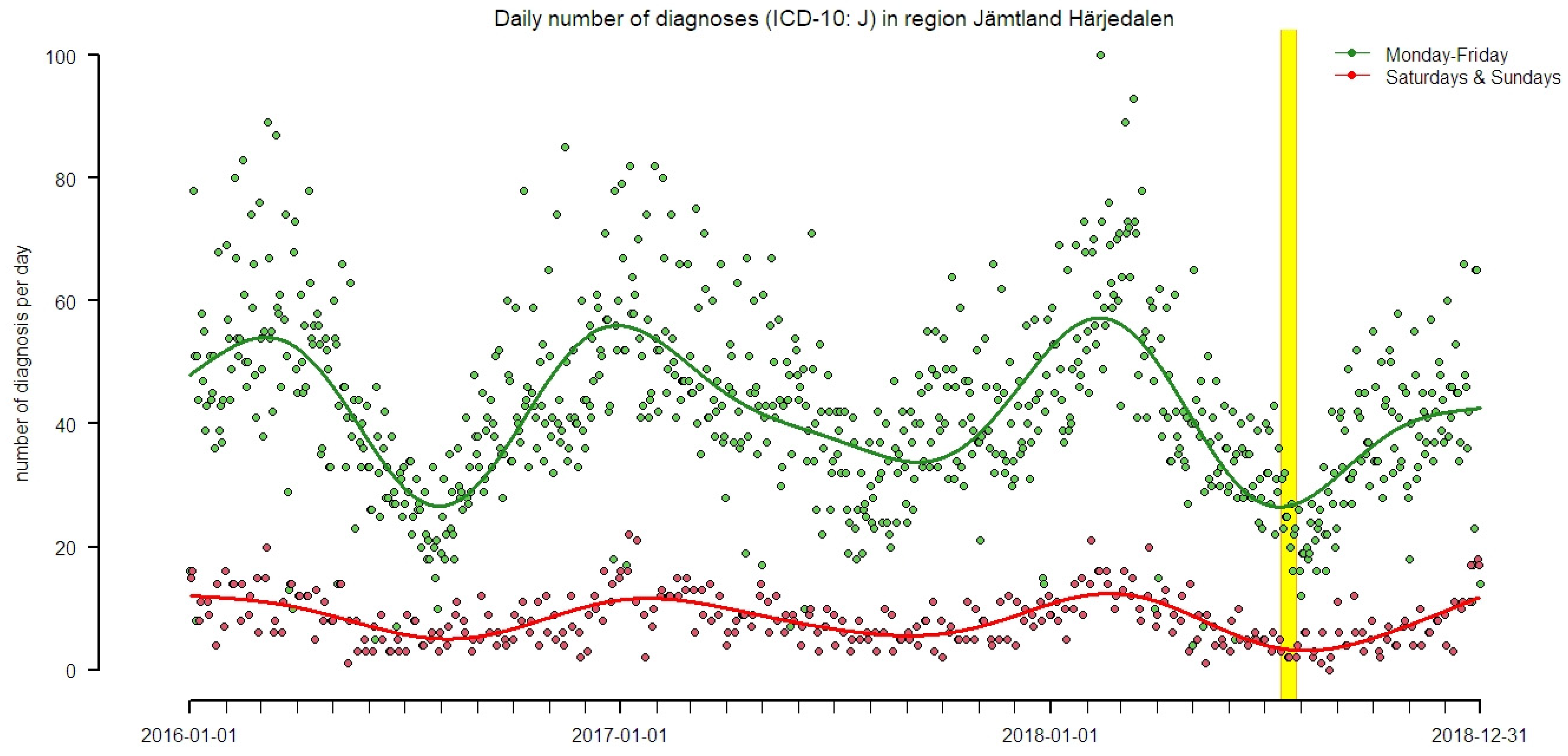
2.2. Health Data
2.3. Epidemiological Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flannigan, M.; Cantin, A.S.; de Groot, W.J.; Wotton, M.; Newbery, A.; Gowman, L.M. Global wildland fire season severity in the 21st century. For. Ecol. Manag. 2013, 294, 54–61. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, O.M.; Salis, M.; Ager, A.A.; Arca, B.; Alcasena, F.J.; Monteiro, A.T.; Finney, M.A.; Del Giudice, L.; Scoccimarro, E.; Spano, D. Assessing Climate Change Impacts on Wildfire Exposure in Mediterranean Areas. Risk Anal. 2017, 37, 1898–1916. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; Brauer, M.; Johnston, F.H.; Jerrett, M.; Balmes, J.R.; Elliott, C.T. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ. Health Persp. 2016, 124, 1334–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.B.; Yu, P.; Abramson, M.J.; Johnston, F.H.; Samet, J.M.; Bell, M.L.; Haines, A.; Ebi, K.L.; Li, S.S.; Guo, Y.M. Wildfires, Global Climate Change, and Human Health. N. Engl. J. Med. 2020, 383, 2173–2181. [Google Scholar] [CrossRef]
- Rittmaster, R.; Adamowicz, W.L.; Amiro, B.; Pelletier, R.T. Economic analysis of health effects from forest fires. Can. J. For. Res. 2006, 36, 868–877. [Google Scholar] [CrossRef]
- Kurt, O.K.; Zhang, J.J.; Pinkerton, K.E. Pulmonary health effects of air pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Brook, R.D.; Biswal, S.; Rajagopalan, S. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol 2020, 17, 656–672. [Google Scholar] [CrossRef]
- Oudin, A. Short review: Air pollution, noise and lack of greenness as risk factors for Alzheimer’s disease- epidemiologic and experimental evidence. Neurochem. Int. 2020, 134. [Google Scholar] [CrossRef] [PubMed]
- Oudin, A.; Forsberg, B.; Adolfsson, A.N.; Lind, N.; Modig, L.; Nordin, M.; Nordin, S.; Adolfsson, R.; Nilsson, L.G. Traffic-Related Air Pollution and Dementia Incidence in Northern Sweden: A Longitudinal Study. Environ. Health Persp. 2016, 124, 306–312. [Google Scholar] [CrossRef]
- Olsson, D.; Ekstrom, M.; Forsberg, B. Temporal Variation in Air Pollution Concentrations and Preterm Birth-A Population Based Epidemiological Study. Int. J. Environ. Res. Public Health 2012, 9, 272–285. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six US cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, F.H.; Henderson, S.B.; Chen, Y.; Randerson, J.T.; Marlier, M.; DeFries, R.S.; Kinney, P.; Bowman, D.M.J.S.; Brauer, M. Estimated Global Mortality Attributable to Smoke from Landscape Fires. Environ. Health Persp. 2012, 120, 695–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.C.; Pereira, G.; Uhl, S.A.; Bravo, M.A.; Bell, M.L. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ. Res. 2015, 136, 120–132. [Google Scholar] [CrossRef] [Green Version]
- San-Miguel-Ayanz, J.; Durrant, T.; Boca, R.; Libertà, G.; Branco, A.; de Rigo, D.; Ferrari, D.; Maianti, P.; Artes, T.; Oom, D.; et al. Forest fires in Europe, Middle East and North Africa 2018; EUR 29856 EN; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-12591-4. [Google Scholar] [CrossRef]
- Robertson, L.; Langner, J.; Engardt, M. An Eulerian limited-area atmospheric transport model. J. Appl. Meteorol. 1999, 38, 190–210. [Google Scholar] [CrossRef]
- Andersson, C.; Langner, J.; Bergstroumm, R. Interannual variation and trends in air pollution over Europe due to climate variability during 1958–2001 simulated with a regional CTM coupled to the ERA40 reanalysis. Tellus B Chem. Phys. Meteorol. 2007, 59, 77–98. [Google Scholar] [CrossRef] [Green Version]
- Andersson, C.; Bergström, R.; Bennet, C.; Robertson, L.; Thomas, M.; Korhonen, H.; Lehtinen, K.; Kokkola, H. MATCH-SALSA–Multi-scale Atmospheric Transport and CHemistry model coupled to the SALSA aerosol microphysics model–Part 1: Model description and evaluation. Geosci. Model Dev. 2015, 8, 171–189. [Google Scholar] [CrossRef] [Green Version]
- Marecal, V.; Peuch, V.H.; Andersson, C.; Andersson, S.; Arteta, J.; Beekmann, M.; Benedictow, A.; Bergstrom, R.; Bessagnet, B.; Cansado, A.; et al. A regional air quality forecasting system over Europe: The MACC-II daily ensemble production. Geosci. Model Dev. 2015, 8, 2777–2813. [Google Scholar] [CrossRef] [Green Version]
- Andersson, C.; Alpfjord, H.; Robertson, L.; Karlsson, P.E.; Engardt, M. Reanalysis of and attribution to near-surface ozone concentrations in Sweden during 1990–2013. Atmos. Chem. Phys. 2017, 17, 13869–13890. [Google Scholar] [CrossRef] [Green Version]
- Geels, C.; Andersson, C.; Hänninen, O.; Lansø, A.S.; Schwarze, P.E.; Skjøth, C.A.; Brandt, J. Future premature mortality due to O3, secondary inorganic aerosols and primary PM in Europe—sensitivity to changes in climate, anthropogenic emissions, population and building stock. Int. J. Environ. Res. Public Health 2015, 12, 2837–2869. [Google Scholar] [CrossRef] [Green Version]
- Orru, H.; Åström, C.; Andersson, C.; Tamm, T.; Ebi, K.L.; Forsberg, B. Ozone and heat-related mortality in Europe in 2050 significantly affected by changes in climate, population and greenhouse gas emission. Environ. Res. Lett. 2019, 14, 074013. [Google Scholar] [CrossRef]
- Simpson, D.; Benedictow, A.; Berge, H.; Bergstrom, R.; Emberson, L.D.; Fagerli, H.; Flechard, C.R.; Hayman, G.D.; Gauss, M.; Jonson, J.E.; et al. The EMEP MSC-W chemical transport model-technical description. Atmos. Chem. Phys. 2012, 12, 7825–7865. [Google Scholar] [CrossRef] [Green Version]
- Carter, W.P. Condensed atmospheric photooxidation mechanisms for isoprene. Atmos. Environ. 1996, 30, 4275–4290. [Google Scholar] [CrossRef]
- Bergström, J.R. Carbonaceous Aerosol in Europe-Out of the Woods and Into the Blue? Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2015. [Google Scholar]
- Hodzic, A.; Kasibhatla, P.S.; Jo, D.S.; Cappa, C.D.; Jimenez, J.L.; Madronich, S.; Park, R.J. Rethinking the global secondary organic aerosol (SOA) budget: Stronger production, faster removal, shorter lifetime. Atmos. Chem. Phys. 2016, 16, 7917–7941. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, J.; Heil, A.; Andreae, M.; Benedetti, A.; Chubarova, N.; Jones, L.; Morcrette, J.-J.; Razinger, M.; Schultz, M.; Suttie, M. Biomass burning emissions estimated with a global fire assimilation system based on observed fire radiative power. Biogeosciences 2012, 9, 527–554. [Google Scholar] [CrossRef] [Green Version]
- Walter, C.; Freitas, S.R.; Kottmeier, C.; Kraut, I.; Rieger, D.; Vogel, H.; Vogel, B. The importance of plume rise on the concentrations and atmospheric impacts of biomass burning aerosol. Atmos. Chem. Phys. 2016, 16, 9201–9219. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, J.; Flemming, J.; Schultz, M.; Suttie, M.; Wooster, M. The MACC Global Fire Assimilation System: First Emission Products (GFASvO); ECMWF: Reading, UK, 2009. [Google Scholar]
- Granier, C.; Darras, S.; van der Gon, H.D.; Jana, D.; Elguindi, N.; Bo, G.; Michael, G.; Marc, G.; Jalkanen, J.-P.; Kuenen, J. The Copernicus Atmosphere Monitoring Service Global and Regional Emissions (April 2019 Version); Copernicus Atmosphere Monitoring Service; ECMWF: Reading, UK, 2019. [Google Scholar]
- Reid, C.E.; Maestas, M.M. Wildfire smoke exposure under climate change: Impact on respiratory health of affected communities. Curr. Opin. Pulm. Med. 2019, 25, 179–187. [Google Scholar] [CrossRef]
- Arriagada, N.B.; Horsley, J.A.; Palmer, A.J.; Morgan, G.G.; Tham, R.; Johnston, F.H. Association between fire smoke fine particulate matter and asthma-related outcomes: Systematic review and meta-analysis. Environ. Res. 2019, 179. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Nguyen, P.-Y.; Trent, M.; Seale, H.; Chughtai, A.A.; Shah, S.; Marks, G.B. Adverse Health Effects in People with and without Pre-Existing Respiratory Conditions During Bushfire Smoke Exposure in the 2019/20 Australian Summer. Am. J. Resp. Crit. Care 2021. [Google Scholar] [CrossRef]
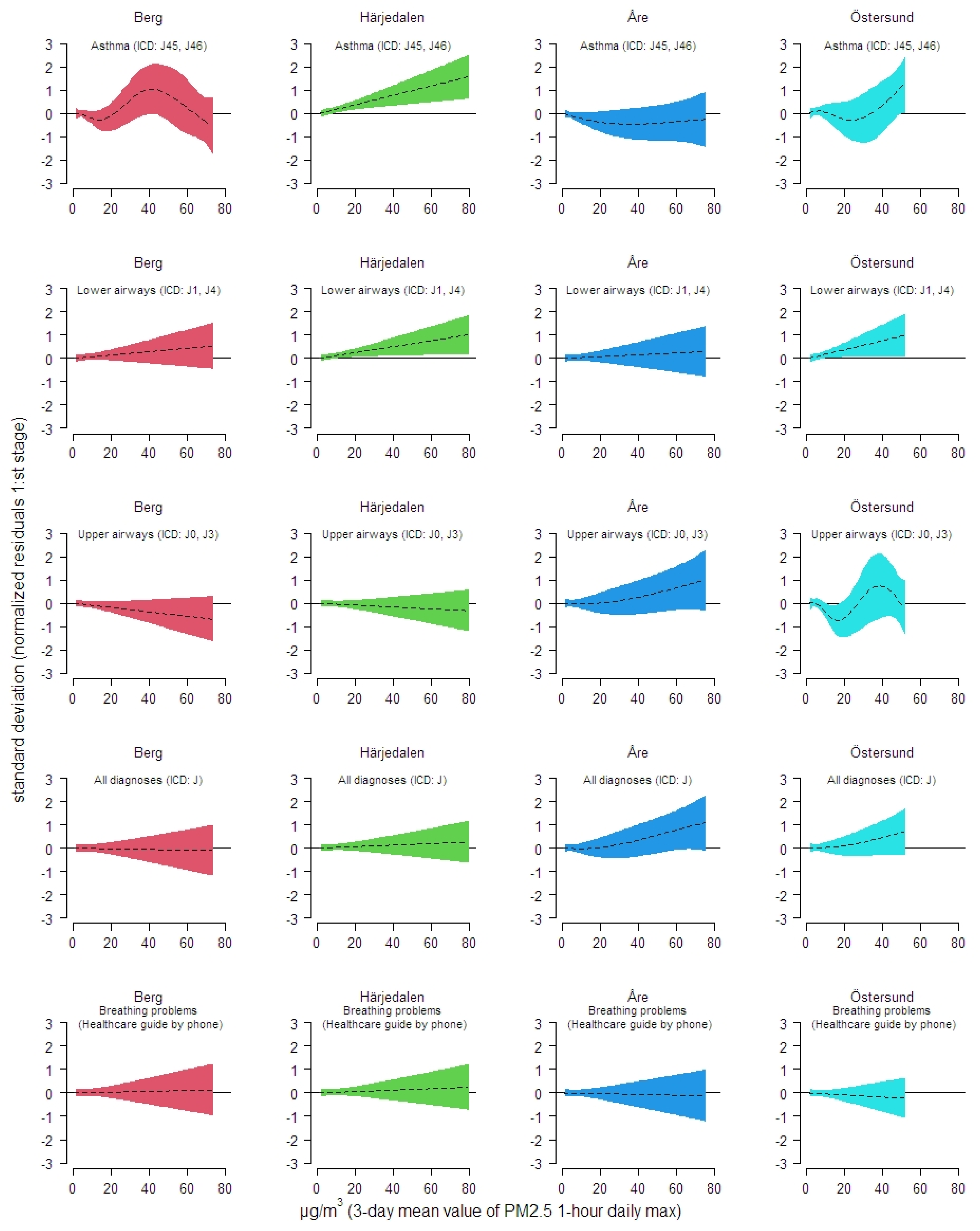
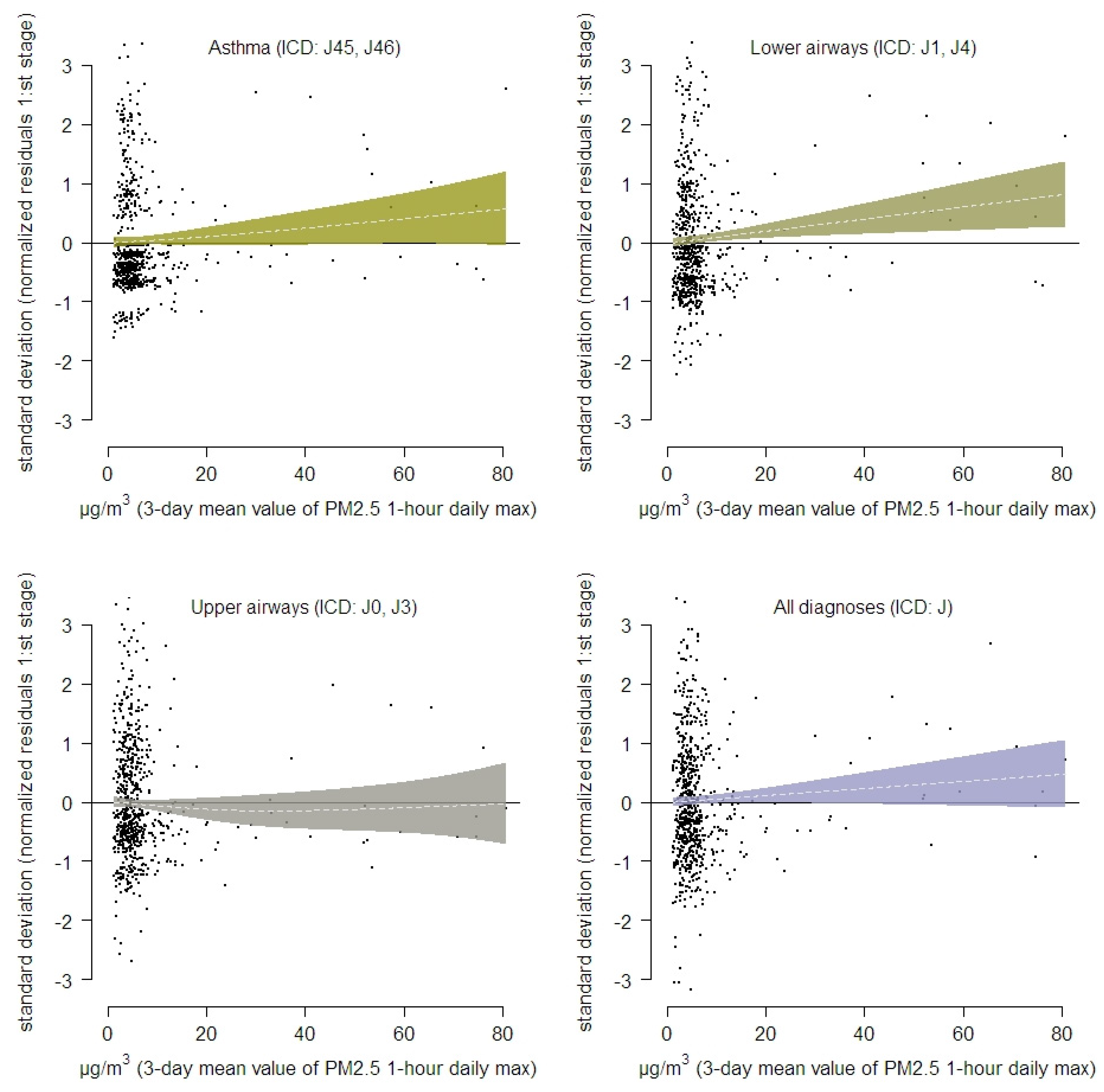
| Municipality/(Population Count) | Asthma (ICD: J45, J46) | Lower Airways (ICD: J1, J4) | Upper Airways (ICD: J0, J3) | All Respiratory (ICD: J00-J99) | Breathing Problems (Healthcare Guide) |
|---|---|---|---|---|---|
| Berg (pop = 7097) | N = 145 | N = 412 | N = 665 | N = 1364 | N = 191 |
| {0.13}/[0–2] | {0.38}/[0–3] | {0.61}/[0–6] | {1.24}/[0–6] | {0.18}/[0–3] | |
| Bräcke (pop = 6376) | N = 162 | N = 522 | N = 889 | N = 1657 | N = 220 |
| {0.15}/[0–2] | {0.48}/[0–5] | {0.81}/[0–6] | {1.51}/[0–8] | {0.21}/[0–3] | |
| Härjedalen (pop = 10147) | N = 383 | N = 985 | N = 2176 | N = 3757 | N = 315 |
| {0.35}/[0–5] | {0.9}/[0–6] | {1.99}/[0–11] | {3.43}/[0–14] | {0.3}/[0–3] | |
| Krokom (pop = 14858) | N = 412 | N = 1002 | N = 2235 | N = 3627 | N = 461 |
| {0.38}/[0–3] | {0.91} /[0–6] | {2.04}/[0–9] | {3.31}/[0–16] | {0.44}/[0–4] | |
| Ragunda (pop = 5343) | N = 121 | N = 351 | N = 637 | N = 1185 | N = 242 |
| {0.11}/[0–3] | {0.32}/[0–4] | {0.58}/[0–5] | {1.08}/[0–9] | {0.23}/[0–6] | |
| Strömsund (pop = 13253) | N = 361 | N = 929 | N = 1739 | N = 3217 | N = 435 |
| {0.33}/[0–3] | {0.85}/[0–6] | {1.59}/[0–12] | {2.94}/[0–17] | {0.42}/[0–4] | |
| Åre (pop = 11529) | N = 475 | N = 859 | N = 2421 | N = 3760 | N = 244 |
| {0.43}/[0–4] | {0.78}/[0–7] | {2.21}/[0–10] | {3.43}/[0–15] | {0.23}/[0–4] | |
| Östersund (pop = 63227) | N = 1459 | N = 3864 | N = 10981 | N = 16876 | N = 2505 |
| {1.33}/[0–8] | {3.53}/[0–16] | {10.02}/[0–32] | {15.4}/[0–51] | {2.4}/[0–12] |
| Municipality | Asthma (ICD: J45, J46) | Lower Airways (ICD: J1, J4) | Upper Airways (ICD: J0, J3) | All Respiratory (ICD: J00-J99) | Breathing Problems (Healthcare Guide by Phone) |
|---|---|---|---|---|---|
| RR [95% CI] | RR [95% CI] | RR [95% CI] | RR [95% CI] | RR [95% CI] | |
| Berg | 0.95 [0.11–7.96] | 1.34 [0.53–3.42] | 0 [0–Inf] | 0.62 [0.26–1.50] | 1.49 [0.19–11.88] |
| Bräcke | 1.92 [0.46–8.04] | 1.05 [0.32–3.41] | 0.88 [0.27–2.86] | 0.91 [0.41–2.06] | 1.18 [0.15–9.32] |
| Härjedalen | 2.64 [1.28–5.47] | 1.48 [0.74–2.99] | 0.69 [0.34–1.36] | 1.01 [0.64–1.58] | 1.28 [0.44–3.70] |
| Krokom | 0.39 [0.05–2.85] | 0.22 [0.03–1.64] | 0.62 [0.29–1.34] | 0.50 [0.25–1.00] | 0.84 [0.19–3.76] |
| Ragunda | 0 [0–Inf] | 0 [0–Inf] | 0.90 [0.12–6.81] | 0.49 [0.06–3.80] | 1.02 [0.12–8.47] |
| Strömsund | 2.59 [0.61–11.07] | 3.02 [1.07–8.54] | 0.96 [0.28–3.28] | 1.53 [0.71–3.34] | 2.70 [0.57–12.72] |
| Åre | 0.37 [0.05–2.91] | 1.15 [0.39–3.35] | 1.75 [0.9–3.38] | 1.56 [0.89–2.73] | 3.31 [0.73–15.04] |
| Östersund | 1.44 [0.70–2.97] | 1.41 [0.84–2.36] | 0.86 [0.59–1.25] | 1.12 [0.82–1.52] | 0.82 [0.39–1.72] |
| Meta–estimate | 1.68 [1.09–2.57] | 1.40 [1.01–1.92] | 0.90 [0.70–1.69] | 1.04 [0.85–1.27] | 1.20 [0.75–1.90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tornevi, A.; Andersson, C.; Carvalho, A.C.; Langner, J.; Stenfors, N.; Forsberg, B. Respiratory Health Effects of Wildfire Smoke during Summer of 2018 in the Jämtland Härjedalen Region, Sweden. Int. J. Environ. Res. Public Health 2021, 18, 6987. https://doi.org/10.3390/ijerph18136987
Tornevi A, Andersson C, Carvalho AC, Langner J, Stenfors N, Forsberg B. Respiratory Health Effects of Wildfire Smoke during Summer of 2018 in the Jämtland Härjedalen Region, Sweden. International Journal of Environmental Research and Public Health. 2021; 18(13):6987. https://doi.org/10.3390/ijerph18136987
Chicago/Turabian StyleTornevi, Andreas, Camilla Andersson, Ana Cristina Carvalho, Joakim Langner, Nikolai Stenfors, and Bertil Forsberg. 2021. "Respiratory Health Effects of Wildfire Smoke during Summer of 2018 in the Jämtland Härjedalen Region, Sweden" International Journal of Environmental Research and Public Health 18, no. 13: 6987. https://doi.org/10.3390/ijerph18136987
APA StyleTornevi, A., Andersson, C., Carvalho, A. C., Langner, J., Stenfors, N., & Forsberg, B. (2021). Respiratory Health Effects of Wildfire Smoke during Summer of 2018 in the Jämtland Härjedalen Region, Sweden. International Journal of Environmental Research and Public Health, 18(13), 6987. https://doi.org/10.3390/ijerph18136987






