Gestational Diabetes Mellitus (GDM) Risk for Declared Family History of Diabetes, in Combination with BMI Categories
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Inclusion Criteria
2.3. Methods
2.4. Sample Size
2.5. Definitions of Dependent Variables
2.6. Independent Variables
2.7. Covariates
2.8. Statistical Analyses
3. Results
3.1. Basic Characteristics of the Partcipants
3.2. Risk of GDM (-1 and -2) for Diabetes in the Parents and Grandparents
3.3. Risk of GDM (-1 and -2) after Cohort Dissection into Pre-pregnancy BMI Categories
4. Discussion
Advantages and Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOR | Adjusted odds ratio |
| BMI | Body mass index |
| CI | Confidence intervals |
| DOHaD | Developmental Origins of Health and Disease |
| GDM | Gestational diabetes mellitus |
| GWG | Gestational weight gain |
| IOM | Institute of Medicine |
| OGTT | Oral glucose tolerance test |
| OR | Odds ratio |
| T2D | Type 2 diabetes |
| PE | Preeclampsia |
| PIH | Pregnancy-induced hypertension |
| WHO | World Health Organization |
References
- American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Begum, S.; Vieira, M.C.; Seed, P.; Lawlor, D.L.; Sattar, N.; Nelson, S.M.; Welsh, P.; Pasupathy, D.; Poston, L.; et al. Metabolic phenotyping by treatment modality in obese women with gestational diabetes suggests diverse pathophysiology: An exploratory study. PLoS ONE 2020, 15, e0230658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational diabetes mellitus. Endocrinol. Metab. Clin. N. Am. 2019, 48, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational diabetes mellitus: A harbinger of the vicious cycle of diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef] [PubMed]
- Mecacci, F.; Lisi, F.; Vannuccini, S.; Ottanelli, S.; Rambaldi, M.P.; Serena, C.; Simeone, S.; Petraglia, F. Different gestational diabetes phenotypes: Which insulin regimen fits better? Front. Endocrinol. (Lausanne) 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Behboudi-Gandevani, S.; Parajuli, R.; Vaismoradi, M. A systematic review of the prevalence of gestational diabetes in norway. Int. J. Environ. Res. Public Health 2021, 18, 1423. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during pregnancy: A maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. a clinical review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef]
- Kampmann, U.; Madsen, L.R.; Skajaa, G.O.; Iversen, D.S.; Moeller, N.; Ovesen, P. Gestational diabetes: A clinical update. World J. Diabetes 2015, 6, 1065–1072. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S.; Chong, Y.S. Risk factors for gestational diabetes: Is prevention possible? Diabetologia 2016, 59, 1385–1390. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, H.D.; Kapur, A.; Divakar, H.; Hod, M. Gestational diabetes mellitus-innovative approach to prediction, diagnosis, management, and prevention of future NCD-mother and offspring. Front. Endocrinol. (Lausanne) 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Farpour-Lambert, N.J.; Ells, L.J.; Martinez de Tejada, B.; Scott, C. Obesity and weight gain in pregnancy and postpartum: An evidence review of lifestyle interventions to inform maternal and child health policies. Front. Endocrinol. (Lausanne) 2018, 9, 546. [Google Scholar] [CrossRef] [Green Version]
- Clarke, E.; Cade, T.J.; Brennecke, S. Early pregnancy screening for women at high-risk of GDM results in reduced neonatal morbidity and similar maternal outcomes to routine screening. J. Pregnancy 2020, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska, M.; Sajdak, S.; Więckowska, B.; Manevska, N.; Lubiński, J. The influence of maternal BMI on adverse pregnancy outcomes in older women. Nutrients 2020, 12, 2838. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, K.; Evangelou, E.; Yiallouros, P.; Christophi, C.A.; Middleton, N.; Papatheodorou, E.; Papatheodorou, S.I. Risk factors for gestational diabetes: An umbrella review of meta-analyses of observational studies. PLoS ONE 2019, 14, e0215372. [Google Scholar] [CrossRef]
- Gou, B.-H.; Guan, H.-M.; Bi, Y.-X.; Ding, B.-J. Gestational diabetes: Weight gain during pregnancy and its relationship to pregnancy outcomes. Chin. Med. J. (Engl.) 2019, 132, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, S.K.; Kim, Y.L. Gestational diabetes mellitus diagnosed at 24 to 28 weeks of gestation in older and obese women: Is it too late? PLoS ONE 2019, 14, e0225955. [Google Scholar] [CrossRef] [Green Version]
- Natamba, B.K.; Namara, A.A.; Nyirenda, M.J. Burden, risk factors and maternal and offspring outcomes of gestational diabetes mellitus (GDM) in sub-saharan africa (SSA): A systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 450. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Chang, Q.; Wu, Q.-J.; Gao, S.-Y.; Zhao, H.; Liu, Y.-S.; Jiang, Y.-T.; Zhao, Y.-H. Relationship between maternal central obesity and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of cohort studies. J. Diabetes Res. 2020, 2020. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; You, S.Y.; Kwon, H.-S.; Yoon, K.-H.; Lee, S.-H. Prepregnancy smoking and the risk of gestational diabetes requiring insulin therapy. Sci. Rep. 2020, 10, 13901. [Google Scholar] [CrossRef]
- Carroll, X.; Liang, X.; Zhang, W.; Zhang, W.; Liu, G.; Turner, N.; Leeper-Woodford, S. Socioeconomic, environmental and lifestyle factors associated with gestational diabetes mellitus: A matched case-control study in Beijing, China. Sci. Rep. 2018, 8, 8103. [Google Scholar] [CrossRef]
- Londero, A.P.; Rossetti, E.; Pittini, C.; Cagnacci, A.; Driul, L. Maternal age and the risk of adverse pregnancy outcomes: A retrospective cohort study. BMC Pregnancy Childbirth 2019, 19, 261. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Ching, S.M.; Ramachandran, V.; Yee, A.; Hoo, F.K.; Chia, Y.C.; Wan Sulaiman, W.A.; Suppiah, S.; Mohamed, M.H.; Veettil, S.K. Prevalence and risk factors of gestational diabetes mellitus in asia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018, 18, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkaabi, J.; Almazrouei, R.; Zoubeidi, T.; Alkaabi, F.M.; Alkendi, F.R.; Almiri, A.E.; Sharma, C.; Souid, A.-K.; Ali, N.; Ahmed, L.A. Burden, associated risk factors and adverse outcomes of gestational diabetes mellitus in twin pregnancies in Al Ain, UAE. BMC Pregnancy Childbirth 2020, 20, 612. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, H.R.; Sharp, G.C.; Relton, C.L.; Lawlor, D.A. Epigenetics and Gestational diabetes: A review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia 2019, 62, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Zhang, L.; Chen, T.; Shi, A.; Xie, K.; Li, Z.; Xu, J.; Chen, Z.; Ji, C.; Wen, J. Genetic susceptibility to gestational diabetes mellitus in a chinese population. Front. Endocrinol. (Lausanne) 2020, 11, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahaya, T.O.; Anyebe, D.A. Genes predisposing to neonatal diabetes mellitus and pathophysiology: Current findings. J. Neonatal Perinat. Med. 2020, 13, 543–553. [Google Scholar] [CrossRef]
- Wu, L.; Cui, L.; Tam, W.H.; Ma, R.C.W.; Wang, C.C. Genetic variants associated with gestational diabetes mellitus: A meta-analysis and subgroup analysis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Konig, M.; Shuldiner, A.R. The genetic interface between gestational diabetes and type 2 diabetes. J. Matern. Fetal. Neonatal Med. 2012, 25, 36–40. [Google Scholar] [CrossRef]
- Tabák, A.G.; Tamás, G.; Péterfalvi, A.; Bosnyák, Z.; Madarász, E.; Rákóczi, I.; Kerényi, Z. The effect of paternal and maternal history of diabetes mellitus on the development of gestational diabetes mellitus. J. Endocrinol. Investig. 2009, 32, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-A.; Yoon, K.-H. The effect of parental transmission of diabetes on the development of gestational diabetes mellitus. Korean J. Intern. Med. 2010, 25, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Kim, J.Y.; Woo, J.-T.; Kim, Y.S.; Kim, S.-H. Familial clustering of type 2 diabetes in korean women with gestational diabetes mellitus. Korean J. Intern. Med. 2010, 25, 269–272. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Asemi, Z.; Lankarani, K.B.; Tabrizi, R.; Maharlouei, N.; Naghibzadeh-Tahami, A.; Yousefzadeh, G.; Sadeghi, R.; Khatibi, S.R.; Afshari, M.; et al. Family history of diabetes and the risk of gestational diabetes mellitus in iran: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11 (Suppl. 1) (Suppl. 1), S99–S104. [Google Scholar] [CrossRef]
- Yen, I.-W.; Lee, C.-N.; Lin, M.-W.; Fan, K.-C.; Wei, J.-N.; Chen, K.-Y.; Chen, S.-C.; Tai, Y.-Y.; Kuo, C.-H.; Lin, C.-H.; et al. Overweight and obesity are associated with clustering of metabolic risk factors in early pregnancy and the risk of GDM. PLoS ONE 2019, 14, e0225978. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.-P. Cardiovascular and metabolic heterogeneity of obesity: Clinical challenges and implications for management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yang, Z.; Zhang, B.; Chen, X.; Yu, L.; Zhu, H.; Xing, X.; Yang, W. Maternal and paternal histories differentially influence risks for diabetes, insulin secretion and insulin resistance in a chinese population. J. Diabetes Investig. 2021, 12, 434–445. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Zhao, G.; Meng, T. Lack of association between IGF2BP2 Rs4402960 polymorphism and gestational diabetes mellitus: A case-control study, meta-analysis and trial sequential analysis. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. Pre-pregnancy obesity vs. other risk factors in probability models of preeclampsia and gestational hypertension. Nutrients 2020, 12, 2681. [Google Scholar] [CrossRef]
- Melero, V.; García de la Torre, N.; Assaf-Balut, C.; Jiménez, I.; Del Valle, L.; Durán, A.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; Izquierdo, N.; et al. Effect of a mediterranean diet-based nutritional intervention on the risk of developing gestational diabetes mellitus and other maternal-fetal adverse events in hispanic women residents in Spain. Nutrients 2020, 12, 3505. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Phelan, S.; Jelalian, E.; Coustan, D.; Caughey, A.B.; Castorino, K.; Hagobian, T.; Muñoz-Christian, K.; Schaffner, A.; Shields, L.; Heaney, C.; et al. Protocol for a randomized controlled trial of pre-pregnancy lifestyle intervention to reduce recurrence of gestational diabetes: Gestational diabetes prevention/prevención de la diabetes gestacional. Trials 2021, 22, 256. [Google Scholar] [CrossRef]
- Onaade, O.; Maples, J.M.; Rand, B.; Fortner, K.B.; Zite, N.B.; Ehrlich, S.F. Physical activity for blood glucose control in gestational diabetes mellitus: Rationale and recommendations for translational behavioral interventions. Clin. Diabetes Endocrinol. 2021, 7, 7. [Google Scholar] [CrossRef]
- Martis, R.; Crowther, C.A.; Shepherd, E.; Alsweiler, J.; Downie, M.R.; Brown, J. Treatments for women with gestational diabetes mellitus: An overview of cochrane systematic reviews. Cochrane Database Syst. Rev. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
| Covariates | Definitions and Categories |
|---|---|
| Maternal age | Maternal age was taken from medical reports. This variable was defined as completed maternal age at conception; in years. Maternal age as a continuous variable was a covariate. |
| Pre-pregnancy BMI (body mass index) | Pre-pregnancy BMI was self-reported in the Questionnaire This variable was defined as the quotient of pre-pregnancy weight (kg) and height (meters) squared. BMI as a continuous variable was a covariate. BMI was assessed in the five following categories as subgroups: (1) normal BMI (18.5–24.9); (2) underweight (<18.5); (3) overweight (25.0–29.9); (4) obesity (≥30); and (5) BMI ≥ 25 kg/m2. |
| GWG (gestational weight gain) | GWG was based on maternal weight before childbirth (from medical reports) and pre-pregnancy weight (self-reported). This variable was defined as the difference between the maternal weight before childbirth and the weight before pregnancy. GWG was assessed in the three following categories regardless of pre-pregnancy BMI categories (according to the Institute of Medicine (IOM) recommendations from 2009): (1) GWG in the range; (2) GWG above the range; and (3) GWG below the range. GWG out of the range was a covariate. |
| Multiparity | Parity was taken from medical records. The two following categories of parity were taken into consideration: (1) multiparity (≥1 prior childbirths) and (2) primiparity (zero prior childbirth). Multiparity was a covariate. |
| Smoking | Smoking status was self-reported in the Questionnaire. Smoking was assessed in the three following categories as subgroups: (1) women who had never smoked; (2) women who smoked before pregnancy); and (3) women who smoked in the first trimester. The category of ‘smokers in the first trimester’ was a covariate. |
| Prior GDM | The maternal history was taken from medical reports. This variable was defined as gestational diabetes mellitus (GDM) in previous pregnancies. The two following categories were taken into consideration: (1) prior GDM; (2) no prior GDM. Prior GDM was a covariate. |
| Maternal Characteristics | Non-Diabetic Group (n = 766) | GDM Group (n = 146) | p * |
|---|---|---|---|
| Median (IQR), n (%) | Median (IQR), n (%) | ||
| Basic characteristics | |||
| Maternal age (years) | 34.0 (30.0–37.0) | 36.0 (33.0–38.0) | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 22.6 (20.4–25.7) | 24.0 (21.5–29.1) | <0.001 |
| Categories: | <0.001 | ||
| Obesity | 66 (8.6%) | 32 (21.9%) | |
| Overweight | 147 (19.2%) | 26 (17.8%) | |
| Normal BMI | 515 (67.2%) | 79 (54.1%) | |
| Underweight | 38 (5.0%) | 9 (6.2%) | |
| GWG (kg) | 14.0 (11.0–17.0) | 10.0 (7.0–15.0) | <0.001 |
| Primiparity | 318 (41.5%) | 64 (43.8%) | 0.602 |
| Smokers in the 1st tr. | 47 (6.1%) | 10 (6.8%) | 0.744 |
| Prior GDM | 3 (0.4%) | 8 (5.5%) | <0.001 |
| Pregnancy outcomes | |||
| Fetal sex—son | 391 (51.0%) | 82 (56.2%) | 0.256 |
| Gestational age (weeks) | 39.0 (38.0–40.0) | 39.0 (38.0–39.8) | 0.032 |
| Birth weight (g) | 3400.0 (3082.5–3707.5) | 3400.0 (3065.0–3780.0) | 0.577 |
| Categories: | 0.047 | ||
| Birth weight < 2500 g | 54 (7.0%) | 6 (4.1%) | |
| Birth weight 2500–4000 g | 636 (83.0%) | 119 (81.5%) | |
| Birth weight > 4000 g | 76 (9.9%) | 21 (14.4%) | |
| PIH | 112 (14.6%) | 25 (17.1%) | 0.438 |
| Basic Risk Factors | GDM-1 Risk | GDM-2 Risk |
|---|---|---|
| AOR-A (95% CI); p * | AOR-A (95% CI); p * | |
| Pre-pregnancy BMI (kg/m2): | ||
| Obesity (≥30) | 2.27 (1.32–3.91); 0.003 | 6.91 (2.38–20.05); <0.001 |
| Overweight (25–29.9) | 0.95 (0.56–1.61); 0.845 | 2.01 (0.64–6.38); 0.234 |
| Underweight (<18.5) | 1.74 (0.76–3.96); 0.187 | 2.33 (0.28–19.67); 0.436 |
| Normal BMI (18.5–24.9) | 1 | |
| Smoking in the 1st tr. | 1.34 (0.64–2.81); 0.434 | - |
| Smoking before pregnancy | 0.63 (0.32–1.23); 0.175 | 0.61 (0.14–2.76); 0.525 |
| Never smoked | 1 | 1 |
| GWG above the range | 0.68 (0.41–1.13); 0.137 | 0.51 (0.16–1.67); 0.267 |
| GWG below the range | 2.39 (1.5–3.82); <0.001 | 2.99 (1.03–8.74); 0.045 |
| GWG in the range | 1 | 1 |
| Maternal age (years): | ||
| ≥40 | 2.31 (0.99–5.34); 0.052 | 1.20 (0.22–6.75); 0.832 |
| 18–24 | 0.43 (0.09–2.00); 0.283 | - |
| 25–29 | 1 | 1 |
| Prior GDM | 9.88 (1.87–52.29); 0.007 | 138.7 (23.7–812.8); <0.001 |
| No prior GDM | 1 | 1 |
| Multiparity | 0.57 (0.38–0.86); 0.007 | 1.91 (0.66–5.53); 0.230 |
| Primiparity | 1 | 1 |
| Preeclampsia | 0.21 (0.03–1.64); 0.138 | 2.21 (0.40–12.08); 0.360 |
| No PIH | 1 | 1 |
| Maternal Characteristics | Non-Diabetic Group (n = 766) | GDM Group (n = 146) | p * |
|---|---|---|---|
| n (%) | n (%) | ||
| Diabetes in Family ** | |||
| -in the mother | 52 (6.8%) | 18 (12.5%) | 0.019 |
| -in the father | 74 (9.7%) | 36 (25.0%) | <0.001 |
| -in both parents simultaneously | 14 (1.8%) | 4 (2.8%) | 0.510 |
| -in the grandmother(s) | 70 (9.2%) | 22 (15.3%) | 0.030 |
| -in the grandfather(s) | 37 (4.8%) | 6 (4.2%) | 0.724 |
| -in both grandparents simultaneously | 8 (1.0%) | 1 (0.7%) | 1 |
| -in both mother and grandmother(s) simultaneously | 2 (0.3%) | 2 (1.4%) | 0.122 |
| -in both father and grandfather(s) simultaneously | 6 (0.8%) | 1 (0.7%) | 1 |
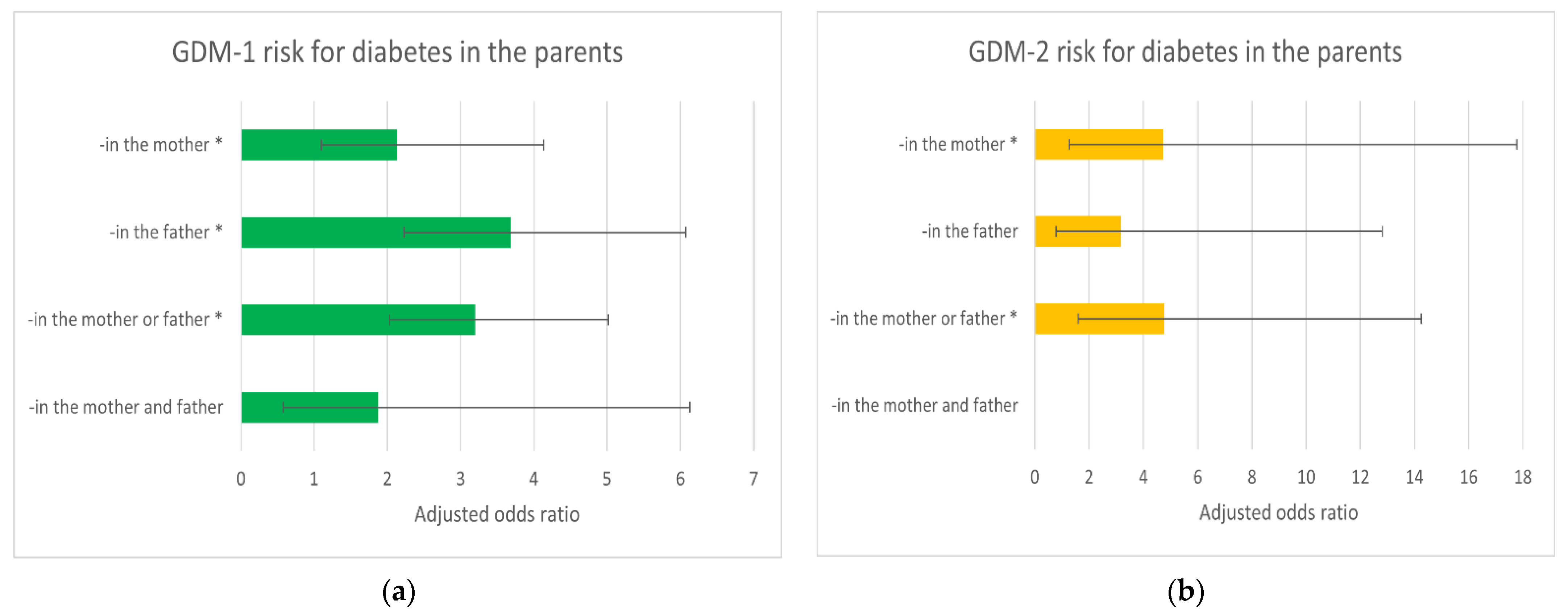
| Risk Factors/ Diabetes in the Parents | Cases/ Controls | OR (95% CI); p | AOR-A (95% CI); p * | AOR-B (95% CI); p * |
|---|---|---|---|---|
| GDM-1 risk | ||||
| -in the father | 32/74 | 3.90 (2.39–6.37); <0.001 | 3.71 (2.25–6.12); <0.001 | 3.68 (2.23–6.07); <0.001 |
| -in the mother | 14/52 | 2.43 (1.27–4.63); 0.007 | 2.13 (1.1–4.14); 0.026 | 2.13 (1.1–4.14); 0.026 |
| -in the mother or father | 42/112 | 3.38 (2.18–5.26); <0.001 | 3.21 (2.05–5.04); <0.001 | 3.20 (2.03–5.02); <0.001 |
| -in both parents simultaneously | 4/14 | 2.58 (0.82–8.07); 0.104 | 1.88 (0.58–6.14); 0.293 | 1.88 (0.58–6.13); 0.294 |
| Ref ** | 62/559 | 1 | 1 | 1 |
| GDM-2 risk | ||||
| -in the father | 4/74 | 2.75 (0.85–8.85); 0.09 | 2.93 (0.87–9.88); 0.083 | 3.16 (0.78–12.81); 0.108 |
| -in the mother | 4/52 | 3.91 (1.2–12.71); 0.023 | 3.36 (0.97–11.62); 0.056 | 4.73 (1.26–17.77); 0.021 |
| -in the mother or father | 8/112 | 3.63 (1.43–9.23); 0.007 | 3.76 (1.42–9.99); 0.008 | 4.77 (1.59–14.25); 0.005 |
| -in both parents simultaneously | 0/14 | - | - | - |
| Ref ** | 11/559 | 1 | 1 | 1 |
| GDM (all cases) risk | ||||
| -in the father | 36/74 | 3.73 (2.34–5.94); <0.001 | 3.51 (2.18–5.66); <0.001 | 3.49 (2.15–5.67); <0.001 |
| -in the mother | 18/52 | 2.65 (1.47–4.78); 0.001 | 2.24 (1.22–4.12); 0.009 | 2.32 (1.26–4.28); 0.007 |
| -in the mother or father | 50/112 | 3.42 (2.26–5.17); <0.001 | 3.2 (2.09–4.89); <0.001 | 3.23 (2.1–4.97); <0.001 |
| -in both parents simultaneously | 4/14 | 2.19 (0.7–6.82); 0.177 | 1.49 (0.46–4.82); 0.509 | 1.51 (0.46–4.91); 0.494 |
| Ref ** | 73/559 | 1 | 1 | 1 |
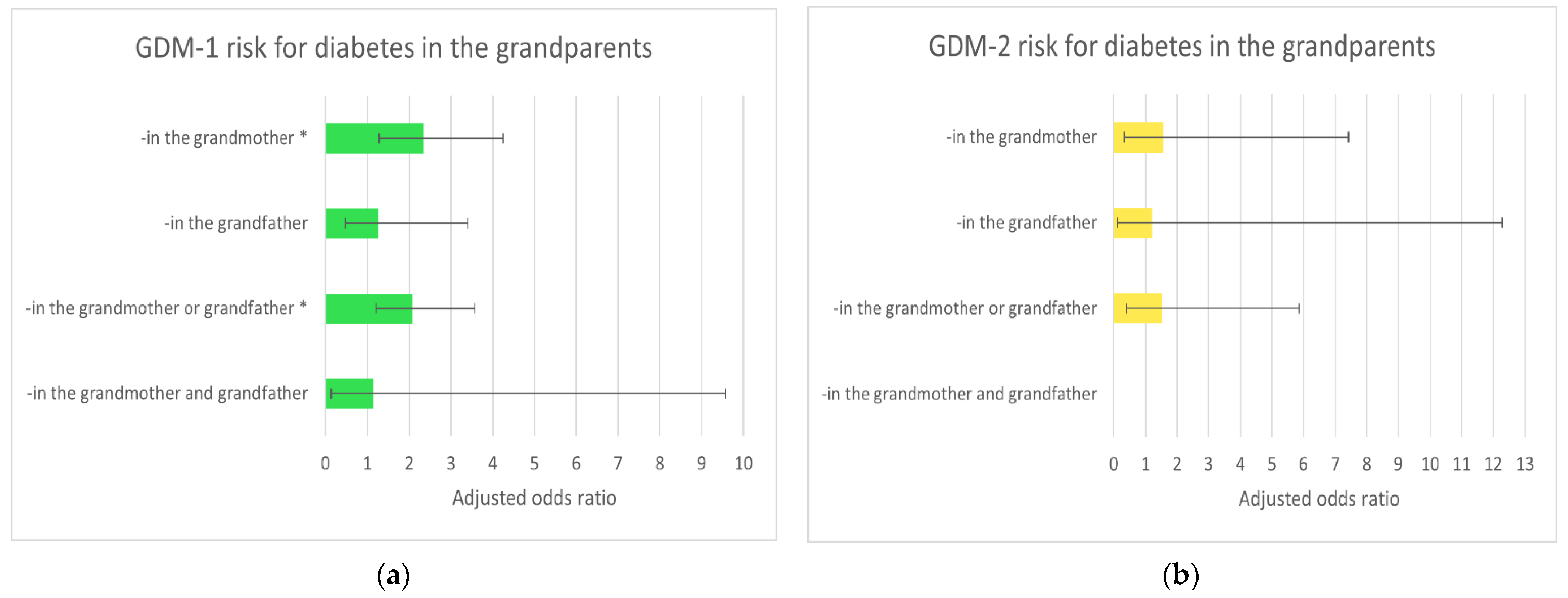
| Risk Factors/ Diabetes in the Grandparents | Cases/ Controls | OR (95% CI); p | AOR-A (95% CI); p * | AOR-B (95% CI); p * |
|---|---|---|---|---|
| GDM-1 risk | ||||
| -in the grandfather(s) | 5/37 | 1.22 (0.46–3.21); 0.690 | 1.26 (0.47–3.36); 0.647 | 1.28 (0.48–3.41); 0.626 |
| -in the grandmother(s) | 19/70 | 2.45 (1.38–4.33); 0.002 | 2.48 (1.38–4.47); 0.002 | 2.34 (1.29–4.24); 0.005 |
| -in the grandfathers or grandmothers | 23/99 | 2.09 (1.24–3.54); 0.006 | 2.18 (1.28–3.73); 0.004 | 2.07 (1.21–3.57); 0.008 |
| -in both simultaneously | 1/8 | 1.13 (0.14–9.16); 0.911 | 1.15 (0.14–9.59); 0.896 | 1.15 (0.14–9.56); 0.898 |
| Ref ** | 62/559 | 1 | 1 | 1 |
| GDM-2 risk | ||||
| -in the grandfather(s) | 1/37 | 1.37 (0.17–10.93); 0.764 | 1.59 (0.18–13.62); 0.674 | 1.21 (0.12–12.29); 0.870 |
| -in the grandmother(s) | 3/70 | 2.18 (0.59–8.00); 0.241 | 1.71 (0.44–6.67); 0.440 | 1.55 (0.33–7.42); 0.581 |
| -in the grandfathers or grandmothers | 4/99 | 2.05 (0.64–6.58); 0.226 | 1.82 (0.54–6.07); 0.332 | 1.53 (0.4–5.87); 0.539 |
| -in both simultaneously | 0/8 | - | - | - |
| Ref ** | 11/559 | 1 | 1 | 1 |
| Risk Factors/ Diabetes in Family | Cases/ Controls | OR (95% CI); p | AOR-A (95% CI); p * | AOR-B (95% CI); p * |
|---|---|---|---|---|
| GDM-1 risk | ||||
| -in both mother and grandmothers simultaneously | 2/2 | 9.02 (1.25–65.13); 0.029 | 8.85 (1.17–66.9); 0.035 | 8.80 (1.16–66.57); 0.035 |
| -in the mother or grandmothers | 31/120 | 2.33 (1.45–3.74); <0.001 | 2.22 (1.36–3.61); 0.001 | 2.14 (1.31–3.50); 0.002 |
| Ref ** | 62/559 | 1 | 1 | 1 |
| -in both father and grandfathers simultaneously | 1/6 | 1.5 (0.18–12.69); 0.708 | 1.41 (0.16–12.14); 0.753 | 1.41 (0.16–12.12); 0.754 |
| -in the father or grandfathers | 36/105 | 3.09 (1.95–4.9); <0.001 | 3.08 (1.92–4.92); <0.001 | 3.05 (1.9–4.88); <0.001 |
| Ref ** | 62/559 | 1 | 1 | 1 |
| GDM-2 risk | ||||
| -in both mother and grandmothers simultaneously | 0/2 | - | - | - |
| -in the mother or grandmothers | 7/120 | 2.96 (1.13–7.8); 0.028 | 2.46 (0.9–6.72); 0.080 | 2.88 (0.95–8.76); 0.062 |
| Ref ** | 11/559 | 1 | 1 | 1 |
| -in both father and grandfathers simultaneously | 0/6 | - | - | - |
| -in the father or grandfathers | 5/105 | 2.42 (0.82–7.11); 0.108 | 2.54 (0.84–7.7); 0.099 | 2.29 (0.67–7.89); 0.188 |
| Ref ** | 11/559 | 1 | 1 | 1 |
| Risk Factors/ Diabetes in the Parents | GDM Risk | GDM-1 Risk | GDM-2 Risk |
|---|---|---|---|
| AOR-A (95% CI); p * | AOR-A (95% CI); p * | AOR-A (95% CI); p * | |
| Whole cohort | |||
| -in the father | 3.51 (2.18–5.66); <0.001 | 3.71 (2.25–6.12); <0.001 | 2.93 (0.87–9.88); 0.083 |
| -in the mother | 2.24 (1.22–4.12); 0.009 | 2.13 (1.1–4.14); 0.026 | 3.36 (0.97–11.62); 0.056 |
| Ref ** | 1 | 1 | 1 |
| Normal BMI | |||
| -in the father | 3.34 (1.75–6.39); <0.001 | 3.24 (1.65–6.38); 0.001 | 3.82 (0.67–21.77); 0.131 |
| -in the mother | 1.67 (0.67–4.19); 0.270 | 1.63 (0.62–4.29); 0.324 | 1.64 (0.15–17.8); 0.684 |
| Ref ** | 1 | 1 | 1 |
| Underweight | |||
| -in the father | 2.23 (0.26–18.95); 0.462 | 2.46 (0.29–20.89); 0.41 | - |
| -in the mother | - | - | - |
| Ref ** | 1 | 1 | 1 |
| Overweight | |||
| -in the father | 6.24 (1.87–20.79); 0.003 | 6.23 (1.7–22.84); 0.006 | 5.11 (0.39–66.95); 0.214 |
| -in the mother | 3.7 (0.86–15.93); 0.079 | 2.94 (0.55–15.6); 0.206 | 6.91 (0.48–100.53); 0.157 |
| Ref ** | 1 | 1 | 1 |
| Obesity | |||
| -in the father | 4.57 (1.29–16.2); 0.019 | 6.66 (1.57–28.36); 0.01 | 2.16 (0.17–27.47); 0.554 |
| -in the mother | 3.6 (0.99–12.99); 0.051 | 3.86 (0.89–16.72); 0.071 | 6.49 (0.6–70.53); 0.124 |
| Ref ** | 1 | 1 | 1 |
| BMI ≥ 25 kg/m2 | |||
| -in the father | 4.36 (1.97–9.64); <0.001 | 4.89 (2.08–11.48); <0.001 | 2.73 (0.48–15.37); 0.256 |
| -in the mother | 3.84 (1.58–9.35); 0.003 | 3.38 (1.25–9.2); 0.017 | 5.56 (1.18–26.24); 0.03 |
| Ref ** | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, M. Gestational Diabetes Mellitus (GDM) Risk for Declared Family History of Diabetes, in Combination with BMI Categories. Int. J. Environ. Res. Public Health 2021, 18, 6936. https://doi.org/10.3390/ijerph18136936
Lewandowska M. Gestational Diabetes Mellitus (GDM) Risk for Declared Family History of Diabetes, in Combination with BMI Categories. International Journal of Environmental Research and Public Health. 2021; 18(13):6936. https://doi.org/10.3390/ijerph18136936
Chicago/Turabian StyleLewandowska, Małgorzata. 2021. "Gestational Diabetes Mellitus (GDM) Risk for Declared Family History of Diabetes, in Combination with BMI Categories" International Journal of Environmental Research and Public Health 18, no. 13: 6936. https://doi.org/10.3390/ijerph18136936
APA StyleLewandowska, M. (2021). Gestational Diabetes Mellitus (GDM) Risk for Declared Family History of Diabetes, in Combination with BMI Categories. International Journal of Environmental Research and Public Health, 18(13), 6936. https://doi.org/10.3390/ijerph18136936






