Evaluation of Fluoride Adsorption Mechanism and Capacity of Different Types of Bone Char
Abstract
1. Introduction
2. Materials and Methods
2.1. BC Synthesis
2.2. Characterization of BC
2.3. Adsorption Kinetics and Isotherm of BC
2.4. Fluoride Adsorption at Different pH
2.5. Desorption of Fluoride Ions
2.6. Adsorption Kinetics and Isotherms
2.6.1. The Pseudo-First-Order Equation
2.6.2. Pseudo-Second-Order Equation
2.6.3. Adsorption Isotherms
3. Results
3.1. BC Adsorbent Characteristics
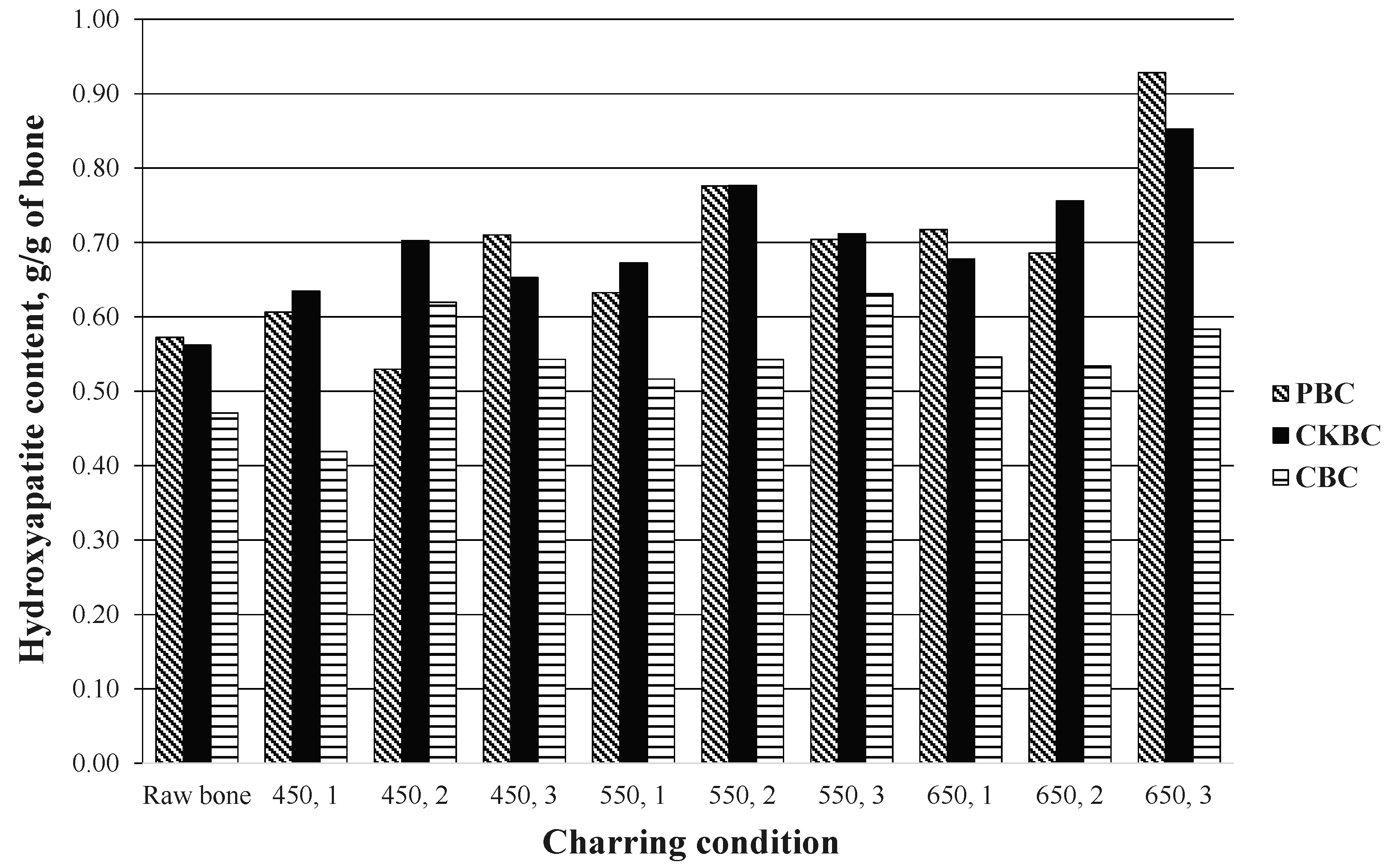
3.1.1. HAP Content of BCs
3.1.2. Textural Properties of BCs
3.1.3. Points of Zero Charge of BCs
3.2. Effect of Solution pH
3.3. Kinetic Adsorption of BC Adsorbents
3.4. Adsorption Isotherm of BC Adsorbents
3.5. Desorption of Fluoride Adsorbed on BC Adsorbents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mohebbi, M.R.; Saeedi, R.; Montazeri, A.; Vaghefi, K.A.; Labbafi, S.; Oktaie, S.; Abtahi, M.; Mohagheghian, A. Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI). Ecol. Indic. 2013, 30, 28–34. [Google Scholar] [CrossRef]
- Hybel, A.-M.; Godskesen, B.; Rygaard, M. Selection of spatial scale for assessing impacts of groundwater-based water supply on freshwater resources. J. Environ. Manag. 2015, 160, 90–97. [Google Scholar] [CrossRef]
- Abbasnia, A.; Alimohammadi, M.; Mahvi, A.H.; Nabizadeh, R.; Yousefi, M.; Mohammadi, A.A.; Pasalari, H.; Mirzabeigi, M. Assessment of groundwater quality and evaluation of scaling and corrosiveness potential of drinking water samples in villages of Chabahr city, Sistan and Baluchistan province in Iran. Data Brief 2018, 16, 182–192. [Google Scholar] [CrossRef]
- Banerjee, A. Groundwater fluoride contamination: A reappraisal. Geosci. Front. 2015, 6, 277–284. [Google Scholar] [CrossRef]
- Navarro, O.; González, J.; Júnez-Ferreira, H.E.; Bautista, C.-F.; Cardona, A. Correlation of Arsenic and Fluoride in the groundwater for human consumption in a semiarid region of Mexico. Procedia Eng. 2017, 186, 333–340. [Google Scholar] [CrossRef]
- Dehbandi, R.; Moore, F.; Keshavarzi, B. Geochemical sources, hydrogeochemical behavior, and health risk assessment of fluoride in an endemic fluorosis area, central Iran. Chemosphere 2018, 193, 763–776. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, A.; Verma, K.; Paliwal, S.; Sharma, S.; Dwivedi, J. Fluoride: A review of pre-clinical and clinical studies. Environ. Toxicol. Pharmacol. 2017, 56, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.A.; Jason, M.; Tanzer, J.M.; Milgrom, P.M. Fluorides and Other Preventive Strategies for Tooth Decay. Dent. Clin. North Am. 2018, 62, 207–234. [Google Scholar] [CrossRef]
- McGrady, M.; Ellwood, R.; Srisilapanan, P.; Korwanich, N.; Taylor, A.; Goodwin, M.; Pretty, I. Dental fluorosis in populations from Chiang Mai, Thailand with different fluoride exposures—Paper 2: The ability of fluorescence imaging to detect differences in fluorosis prevalence and severity for different fluoride intakes from water. BMC Oral Health 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Death, C.; Coulson, G.; Kierdorf, U.; Kierdorf, H.; Morris, W.K.; Hufschmid, J. Dental fluorosis and skeletal fluoride content as biomarkers of excess fluoride exposure in marsupials. Sci. Total Environ. 2015, 533, 528–541. [Google Scholar] [CrossRef]
- Pramanik, S.; Saha, D. The genetic influence in fluorosis. Environ. Toxicol. Pharmacol. 2017, 56, 157–162. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Chemical Fact Sheet; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Shen, J.; Richards, B.; Schäfer, A.I. Renewable energy powered membrane technology: Case study of St. Dorcas borehole in Tanzania demonstrating fluoride removal via nanofiltration/reverse osmosis. Sep. Purif. Technol. 2016, 170, 445–452. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.S.; He, J.Y.; Xu, W.H.; Huang, X.J.; Liu, J.H. Enhanced fluoride removal from water by sulfate-doped hydroxyapatite hierarchical hollow microspheres. Chem. Eng. J. 2016, 285, 616–624. [Google Scholar] [CrossRef]
- Zhang, J.; Brutus, T.E.; Cheng, J.; Meng, X. Fluoride removal by Al, Ti, and Fe hydroxides and coexisting ion effect. J. Environ. Sci. 2017, 57, 190–195. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Defluoridation of drinking water using adsorption processes. J. Hazard. Mater. 2013, 248–249, 1–19. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Silvestre-Albero, J.; Aguayo-Villarreal, I.A.; Mendoza-Castillo, D.I. Physico-chemical characterization of metal-doped bone chars andtheir adsorption behavior for water defluoridation. Appl. Surf. Sci. 2015, 355, 748–760. [Google Scholar] [CrossRef]
- Wongrueng, A.; Sookwong, B.; Rakruam, P.; Wattanachira, S. Kinetic Adsorption of Fluoride from an Aqueous Solution onto a Dolomite Sorbent. Eng. J. 2016, 20, 1–9. [Google Scholar] [CrossRef][Green Version]
- Phillips, D.H.; Gupta, B.S.; Mukhopadhyay, S.; Gupta, A.K.S. Arsenic and fluoride removal from contaminated drinking water with Haix-Fe-Zr and Haix-Zr resin beads. J. Environ. Manag. 2018, 215, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Sani, T.; Gómez-Hortigüela, L.; Perez-Pariente, J.; Chebude, Y.; Díaz, I. Defluoridation performance of nano-hydroxyapatite/stilbite composite compared with bone char. Sep. Purif. Technol. 2016, 157, 241–248. [Google Scholar] [CrossRef]
- Zúñiga-Muro, N.M.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Tapia-Picazoa, J.C. Fluoride adsorption properties of cerium-containing bone char. J. Fluor. Chem. 2017, 197, 63–73. [Google Scholar] [CrossRef]
- Patel, S.; Han, J.; Qiu, W.; Gao, W. Synthesis and characterisation of mesoporous bone char obtained by pyrolysis of animal bones, for environmental application. J. Environ. Chem. Eng. 2015, 3, 2368–2377. [Google Scholar] [CrossRef]
- Yami, T.L.; Chamberlain, J.F.; Butler, E.C.; Sabatini, D.A. Using a High-Capacity Chemically Activated Cow Bone to Remove Fluoride: Field-Scale Column Tests and Laboratory Regeneration Studies. J. Environ. Eng. 2016, 143, 04016083. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Rivera-Utrilla, J.; Medellin-Castillo, N.; Sanchez-Polo, M. Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chem. Eng. J. 2010, 158, 458–467. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Hernández-Montoya, V.; Moreno-Virgen, M.R.; Tovar-Gómez, R.; Montes-Morán, M.A. Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water. J. Anal. Appl. Pyrol. 2013, 104, 10–18. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Padilla-Ortega, E.; Tovar-García, L.D.; Leyva-Ramos, R.; Ocampo-Pérez, R.; Carrasco-Marín, F.; Berber-Mendoza, M.S. Removal of fluoride from aqueous solution using acid and thermally treated bone char. Adsorption 2016, 22, 951–961. [Google Scholar] [CrossRef]
- Gatabi, M.P.; Moghaddam, H.M.; Ghorbani, M. Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J. Mol. Liq. 2016, 216, 117–125. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Hui, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar]
- Naushad, M.; Alothman, Z.A. Equilibrium and Kinetic Studies in Adsorption of Toxic Metal Ions for Wastewater Treatment. In A Book on Ion Exchange, Adsorption and Solvent Extraction; Nova Science Pub: New York, NY, USA, 2013. [Google Scholar]
- Brunson, L.R.; Sabatini, D.A. An Evaluation of Fish Bone Char as an Appropriate Arsenic and Fluoride Removal Technology for Emerging Regions. Environ. Eng. Sci. 2009, 26, 1777–1783. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Leyva-Ramos, R.; Padilla-Ortega, E.; Ocampo Perez, R.; Flores-Cano, J.V.; Berber-Mendoza, M.S. Adsorption capacity of bone char for removing fluoride from water solution. Role of hydroxyapatite content, adsorption mechanism and competing anions. J. Ind. Eng. Chem. 2014, 20, 4014–4021. [Google Scholar] [CrossRef]
- Chuah, C.J.; Lye, H.R.; Ziegler, A.D.; Wood, S.H.; Kongpun, C.; Rajchagool, S. Fluoride: A naturally-occurring health hazard in drinking-water resources of Northern Thailand. Sci. Total. Environ. 2016, 545–546, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Medellin-Castillo, N.A.; Leyva-Ramos, R.; Ocampo-Perez, R.; Garcia de la Cruz, R.F.; Aragón-Piña, A.; Martinez-Rosales, J.M.; Guerrero-Coronado, R.M.; Fuentes-Rubio, L. Adsorption of Fluoride from Water Solution on Bone Char. Ind. Eng. Chem. Res. 2007, 46, 9205–9212. [Google Scholar] [CrossRef]
- Alkurdi, S.S.A.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; McKnight, S. Effect of pyrolysis conditions on bone char characterization and its ability foe arsenic and fluoride removal. Environ. Polltion 2020, 262, 114221. [Google Scholar] [CrossRef] [PubMed]
- Alkurdi, S.S.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; Marchuk, A. Inorganic arsenic species removal from water using bone char: A detailed study on adsorption kinetic and isotherm models using error functions analysis. J. Hazard. Mater. 2021, 405, 124112. [Google Scholar] [CrossRef] [PubMed]
- Nigri, E.M.; Santos, A.L.A.; Bhatnagar, A.; Rocha, S.D.F. Chemical regeneration of bone char association with a continuous system for defluoridation of water. Braz. J. Chem. Eng. 2019, 36, 1631–1643. [Google Scholar] [CrossRef]
- Madhu. Different between Calcination and Pyrolysis. Available online: https://www.differencebetween.com/difference-between-calcination-and-pyrolysis/ (accessed on 11 August 2020).





| Parameters | PBC | CKBC | CBC |
|---|---|---|---|
| Specific surface area (m2/g) | 83.79 | 62.80 | 103.11 |
| Total pore volume (cc/g) | 0.3490 | 0.3288 | 0.3353 |
| Average pore size (Å) | 83.31 | 104.70 | 65.05 |
| BC Type. | HAP Content (g/g of BC) | Surface Area (m2/g of BC) | Specific Surface Area of HAP (m2/g of HAP) | qe of Fluoride, (mg/g of HAP) |
|---|---|---|---|---|
| PBC | 0.928 | 83.79 | 90.29 | 0.438 (n=3) |
| CKBC | 0.853 | 62.80 | 73.62 | 0.407 (n=3) |
| CBC | 0.631 | 103.11 | 163.41 | 0.788 (n=3) |
| BC Type | qe,exp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg/g) | Kp1, (min−1) | R2 | qe,cal (mg/g) | Kp2, g/(mg·min) | R2 | ||
| PBC | 0.366 | 0.192 | 0.003 | 0.300 | 0.361 | 0.516 | 0.992 |
| CKBC | 0.347 | 0.173 | 0.007 | 0.913 | 0.349 | 0.107 | 0.995 |
| CBC | 0.497 | 1.000 | 0.005 | 0.401 | 0.502 | 0.053 | 0.992 |
| BC Type | Langmuir | Freundlich | |||
|---|---|---|---|---|---|
| KL, (L/mg) | R2 | n | KF, (L/g) | R2 | |
| PBC | 1.99 × 10−3 | 0.792 | 0.532 | 0.051 | 0.640 |
| CKBC | 1.22 × 10−3 | 0.771 | 0.207 | 0.001 | 0.413 |
| CBC | 1.96 × 10−3 | 0.938 | 0.569 | 0.059 | 0.877 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawangjang, B.; Induvesa, P.; Wongrueng, A.; Pumas, C.; Wattanachira, S.; Rakruam, P.; Punyapalakul, P.; Takizawa, S.; Khan, E. Evaluation of Fluoride Adsorption Mechanism and Capacity of Different Types of Bone Char. Int. J. Environ. Res. Public Health 2021, 18, 6878. https://doi.org/10.3390/ijerph18136878
Sawangjang B, Induvesa P, Wongrueng A, Pumas C, Wattanachira S, Rakruam P, Punyapalakul P, Takizawa S, Khan E. Evaluation of Fluoride Adsorption Mechanism and Capacity of Different Types of Bone Char. International Journal of Environmental Research and Public Health. 2021; 18(13):6878. https://doi.org/10.3390/ijerph18136878
Chicago/Turabian StyleSawangjang, Benyapa, Phacharapol Induvesa, Aunnop Wongrueng, Chayakorn Pumas, Suraphong Wattanachira, Pharkphum Rakruam, Patiparn Punyapalakul, Satoshi Takizawa, and Eakalak Khan. 2021. "Evaluation of Fluoride Adsorption Mechanism and Capacity of Different Types of Bone Char" International Journal of Environmental Research and Public Health 18, no. 13: 6878. https://doi.org/10.3390/ijerph18136878
APA StyleSawangjang, B., Induvesa, P., Wongrueng, A., Pumas, C., Wattanachira, S., Rakruam, P., Punyapalakul, P., Takizawa, S., & Khan, E. (2021). Evaluation of Fluoride Adsorption Mechanism and Capacity of Different Types of Bone Char. International Journal of Environmental Research and Public Health, 18(13), 6878. https://doi.org/10.3390/ijerph18136878








