Decoding the Role of Gut-Microbiome in the Food Addiction Paradigm
Abstract
:“All disease begins in the gut.”Hippocrates
1. Introduction
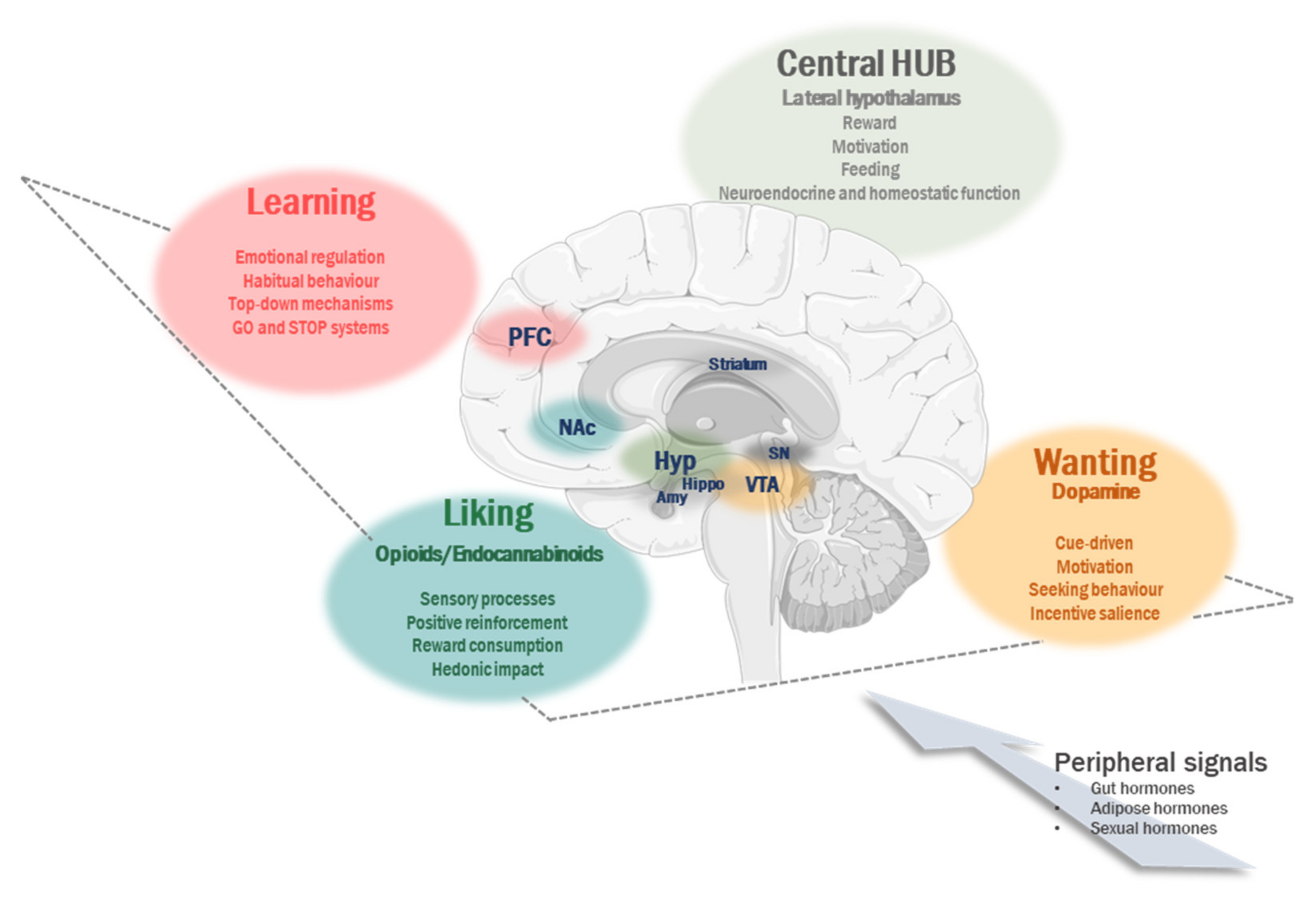
2. Non-Homeostatic Contribution to Regulation of Food Intake
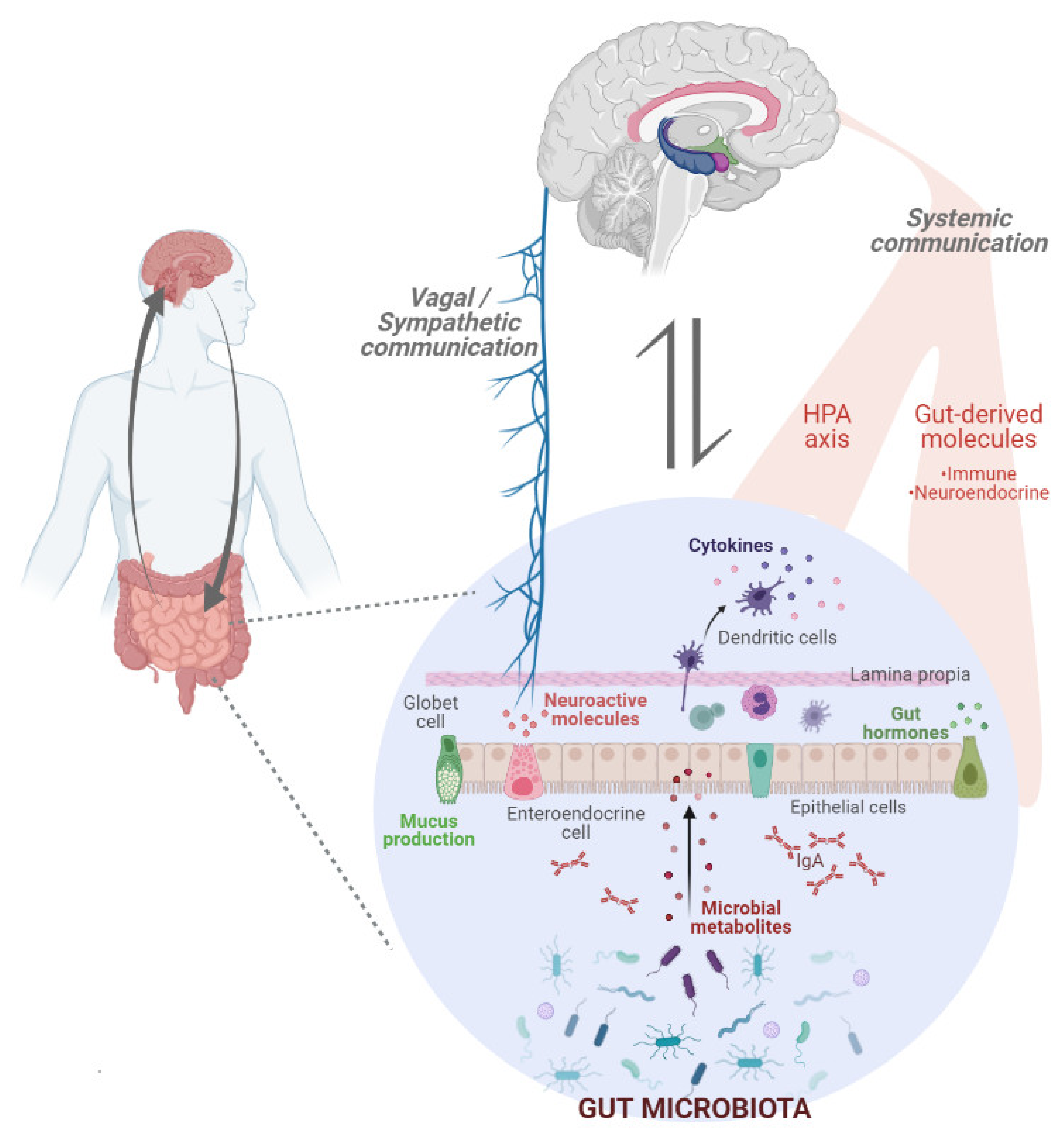
3. Gut Microbiota: A Key Player in the Regulation of Eating Behaviour
4. Food Addiction: A New Mental Disorder?
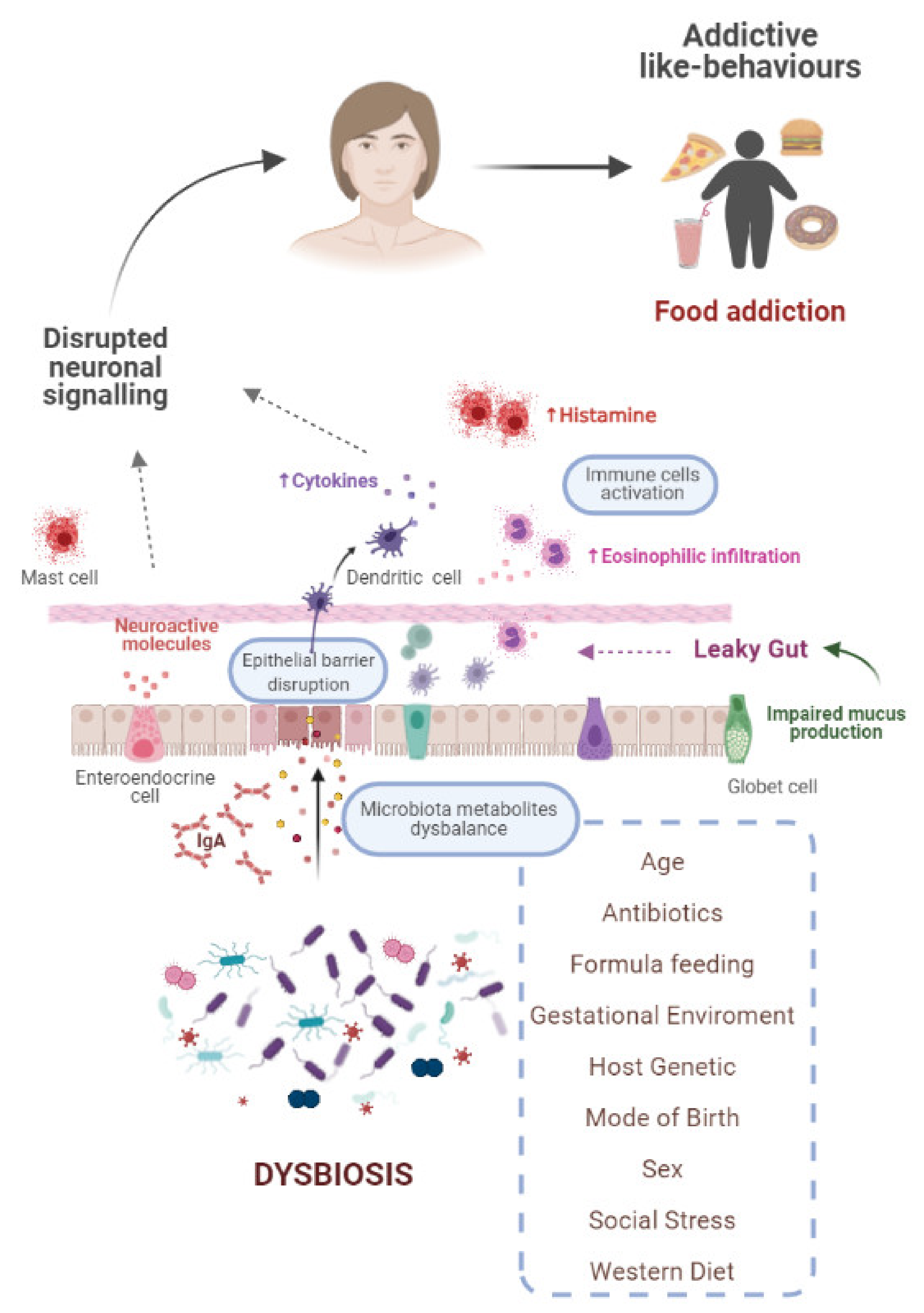
5. Interrelationship between Gut Microbiota and Food Addiction
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yeo, G.S.H.; Heisler, L. Unraveling the brain regulation of appetite: Lessons from genetics. Nat. Neurosci. 2012, 15, 1343–1349. [Google Scholar] [CrossRef]
- Higgs, S. Cognitive processing of food rewards. Appetite 2016, 104, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovich, G.D. Learning and the motivation to eat: Forebrain circuitry. Physiol. Behav. 2011, 104, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Reichelt, A.C.; Westbrook, R.F.; Morris, M.J. Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br. J. Pharmacol. 2015, 172, 5225–5238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novelle, M.G.; Diéguez, C. Food Addiction and Binge Eating: Lessons Learned from Animal Models. Nutrients 2018, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Hebebrand, J.; Albayrak, Ö.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menzies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocr. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.T.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain dis-orders. Nat. Rev. Microbiol. 2020, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. The role of the brain–gut–microbiota axis in psychology: The importance of considering gut microbiota in the development, perpetuation, and treatment of psychological disorders. Brain Behav. 2019, 9, e01408. [Google Scholar] [CrossRef]
- Münger, E.; Montiel-Castro, A.J.; Langhans, W.; Pacheco-López, G. Reciprocal Interactions Between Gut Microbiota and Host Social Behavior. Front. Integr. Neurosci. 2018, 12, 21. [Google Scholar] [CrossRef]
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Ebravo-Ruiseco, G.; Epacheco-Lopez, G. The microbiota-gut-brain axis: Neurobehavioral correlates, health and sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018, 27, 42–56. [Google Scholar] [CrossRef]
- Novelle, M.G.; Diéguez, C. Unravelling the role and mechanism of adipokine and gastrointestinal signals in animal models in the nonhomeostatic control of energy homeostasis: Implications for binge eating disorder. Eur. Eat. Disord. Rev. 2018, 26, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Bonnavion, P.; Mickelsen, L.E.; Fujita, A.; De Lecea, L.; Jackson, A.C. Hubs and spokes of the lateral hypothalamus: Cell types, circuits and behaviour. J. Physiol. 2016, 594, 6443–6462. [Google Scholar] [CrossRef]
- Petrovich, G.D. Lateral Hypothalamus as a Motivation-Cognition Interface in the Control of Feeding Behavior. Front. Syst. Neurosci. 2018, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Qualls-Creekmore, E.; Münzberg, H. Modulation of Feeding and Associated Behaviors by Lateral Hypothalamic Circuits. Endocrinology 2018, 159, 3631–3642. [Google Scholar] [CrossRef] [Green Version]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; E Robinson, T.; Aldridge, J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009, 9, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Benoit, S.C.; Davis, J.F.; Davidson, T. Learned and cognitive controls of food intake. Brain Res. 2010, 1350, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, D.S.; Woods, S.C. Physiological Regulation: How It Really Works. Cell Metab. 2016, 24, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Grigson, P.S.; Hajnal, A. Once is too much: Conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behav. Neurosci. 2007, 121, 1234–1242. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.; Woods, S.C.; Pelchat, M.; Grigson, P.; Stice, E.; Farooqi, S.; Khoo, C.S.; Mattes, R.D.; Beauchamp, G.K. Food reward system: Current perspectives and future research needs. Nutr. Rev. 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Kosheleff, A.R.; Araki, J.; Hsueh, J.; Le, A.; Quizon, K.; Ostlund, S.B.; Maidment, N.T.; Murphy, N.P. Pattern of access determines influence of junk food diet on cue sensitivity and palatability. Appetite 2018, 123, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Furlong, T.; Jayaweera, H.K.; Balleine, B.W.; Corbit, L.H. Binge-Like Consumption of a Palatable Food Accelerates Habitual Control of Behavior and Is Dependent on Activation of the Dorsolateral Striatum. J. Neurosci. 2014, 34, 5012–5022. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, A.; Dietrich, A.; Mathar, D.; Pössel, M.; Villringer, A.; Neumann, J. Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite 2015, 87, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Graybiel, A.M. Habits, Rituals, and the Evaluative Brain. Annu. Rev. Neurosci. 2008, 31, 359–387. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Chapter 4—Habitual overeating. In Compulsive Eating Behavior and Food Addiction; Cottone, P., Sabino, V., Moore, C.F., Koob, G.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 83–95. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu. Rev. Psychol. 2016, 67, 23–50. [Google Scholar] [CrossRef]
- Gillan, C.M.; Robbins, T.W.; Sahakian, B.J.; van den Heuvel, O.A.; van Wingen, G. The role of habit in compulsivity. Eur. Neuropsychopharmacol. 2016, 26, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Wirz, L.; Bogdanov, M.; Schwabe, L. Habits under stress: Mechanistic insights across different types of learning. Curr. Opin. Behav. Sci. 2018, 20, 9–16. [Google Scholar] [CrossRef]
- Schwabe, L.; Wolf, O.T. Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behav. Brain Res. 2011, 219, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Pivarunas, B.; Conner, B. Impulsivity and emotion dysregulation as predictors of food addiction. Eat. Behav. 2015, 19, 9–14. [Google Scholar] [CrossRef]
- Bourdier, L.; Orri, M.; Carre, A.; Gearhardt, A.; Romo, L.; Dantzer, C.; Berthoz, S. Are emotionally driven and addictive-like eating behaviors the missing links between psychological distress and greater body weight? Appetite 2018, 120, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.J.; Jenkins, S.M. Food Addiction Is Associated with Irrational Beliefs via Trait Anxiety and Emotional Eating. Nutrients 2019, 11, 1711. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, E.; Gray, K.; Miller, G.; Tyler, R.; Wiers, C.E.; Volkow, N.D.; Wang, G.-J. Food addiction A common neurobiological mechanism with drug abuse. Front. Biosci. 2018, 23, 811–836. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Dalley, J.W.; Everitt, B.; Robbins, T.W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology 2017, 42, 1375–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourley, S.L.; Taylor, J.R. Going and stopping: Dichotomies in behavioral control by the prefrontal cortex. Nat. Neurosci. 2016, 19, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, D.; Volkow, N.D. Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit. Rev. Biochem. Mol. Biol. 2012, 48, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Leinninger, G.M.; Jo, Y.-H.; Leshan, R.L.; Louis, G.W.; Yang, H.; Barrera, J.G.; Wilson, H.; Opland, D.M.; Faouzi, M.A.; Gong, Y.; et al. Leptin Acts via Leptin Receptor-Expressing Lateral Hypothalamic Neurons to Modulate the Mesolimbic Dopamine System and Suppress Feeding. Cell Metab. 2009, 10, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omrani, A.; de Vrind, V.A.J.; Lodder, B.; Stoltenborg, I.; Kooij, K.; Wolterink-Donselaar, I.G.; Luijendijk-Berg, M.C.M.; Garner, K.M.; van ‘t Sant, L.J.; Rozeboom, A.; et al. Identification of novel neurocircuitry through which leptin targets multiple inputs to the dopamine system to reduce food reward seeking. Biol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Fulton, S.; Woodside, B.; Shizgal, P. Modulation of Brain Reward Circuitry by Leptin. Science 2000, 287, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Al Massadi, O.; Nogueiras, R.; Dieguez, C.; Girault, J.-A. Ghrelin and food reward. Neuropharmacology 2019, 148, 131–138. [Google Scholar] [CrossRef]
- Skibicka, K.P.; Dickson, S. Enteroendocrine hormones—Central effects on behavior. Curr. Opin. Pharmacol. 2013, 13, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Klump, K.L.; Culbert, K.M.; Sisk, C.L. Sex Differences in Binge Eating: Gonadal Hormone Effects Across Development. Annu. Rev. Clin. Psychol. 2017, 13, 183–207. [Google Scholar] [CrossRef]
- Novelle, M.G.; Diéguez, C. Updating gender differences in the control of homeostatic and hedonic food intake: Implications for binge eating disorder. Mol. Cell. Endocrinol. 2019, 497, 110508. [Google Scholar] [CrossRef]
- Ferrario, C.R.; Labouèbe, G.; Liu, S.; Nieh, H.-A.E.; Routh, V.H.; Xu, S.; O’Connor, E.C. Homeostasis Meets Motivation in the Battle to Control Food Intake. J. Neurosci. 2016, 36, 11469–11481. [Google Scholar] [CrossRef]
- Morales, I.; Berridge, K.C. ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.M.; Surette, M.G.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- García-Cabrerizo, R.; Carbia, C.; O´riordan, K.J.; Schellekens, H.; Cryan, J.F. Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 2020. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8,. [Google Scholar] [CrossRef] [PubMed]
- Covasa, M.; Stephens, R.W.; Toderean, R.; Cobuz, C. Intestinal Sensing by Gut Microbiota: Targeting Gut Peptides. Front. Endocrinol. 2019, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; El Aidy, S.; Ross, P.; Roy, B.L.; Stanton, C.; et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalla, M.A.; Stengel, A. Effects of microbiome changes on endocrine ghrelin signaling—A systematic review. Peptides 2020, 133, 170388. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol. Dis. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Moieni, M.; Inagaki, T.K.; Muscatell, K.A.; Irwin, M.R. In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology 2017, 42, 242–253. [Google Scholar] [CrossRef]
- Nusslock, R.; Miller, G.E. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol. Psychiatry 2016, 80, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Haq, R.; Schlachetzki, J.C.; Glass, C.K.; Mazmanian, S.K. Microbiome–microglia connections via the gut–brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef] [Green Version]
- Erny, D.; De Angelis, A.L.H.; Jaitin, D.A.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Lacagnina, M.; Rivera, P.D.; Bilbo, S.D. Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology 2017, 42, 156–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Martos, M.; Girard, B.; Mendonça-Netto, S.; Perroy, J.; Valjent, E.; Maldonado, R.; Martin, M. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addict. Biol. 2017, 23, 735–749. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.M.; Morris, L.S.; Marchesi, J. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J. Endocrinol. 2013, 218, R37–R47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef]
- Sinha, R.; Gu, P.; Hart, R.; Guarnaccia, J. Food craving, cortisol and ghrelin responses in modeling highly palatable snack intake in the laboratory. Physiol. Behav. 2019, 208, 112563. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric micro-biota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Seitz, J.; Trinh, S.; Herpertz-Dahlmann, B. The Microbiome and Eating Disorders. Psychiatr. Clin. N. Am. 2019, 42, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Battistini, L.; Caltagirone, C.; Borsellino, G. Gut Microbiota and Disorders of the Central Nervous System. Neuroscientist 2020, 26, 487–502. [Google Scholar] [CrossRef]
- Person, H.; Keefer, L. Psychological comorbidity in gastrointestinal diseases: Update on the brain-gut-microbiome axis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110209. [Google Scholar] [CrossRef]
- Noonan, S.; Zaveri, M.; Macaninch, E.; Martyn, K. Food & mood: A review of supplementary prebiotic and probiotic interventions in the treatment of anxiety and depression in adults. BMJ Nutr. Prev. Health 2020, 3, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, A.C.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: A systematic review. BMC Psychiatry 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Cani, P.; Neyrinck, A.; Maton, N.; Delzenne, N.M. Oligofructose Promotes Satiety in Rats Fed a High-Fat Diet: Involvement of Glucagon-Like Peptide-1. Obes. Res. 2005, 13, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2005, 60, 567–572. [Google Scholar] [CrossRef]
- Canfora, E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.; Chambers, E.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [Green Version]
- Arora, T.; Sharma, R.; Frost, G. Propionate. Anti-obesity and satiety enhancing factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2017, 67, 1269–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluitman, K.S.; Wijdeveld, M.; Nieuwdorp, M.; Ijzerman, R.G. Potential of butyrate to influence food intake in mice and men. Gut 2018, 67, 1203–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16 (Suppl. S1), S21–S26. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Jankiewicz, A.; Guzmán-Quevedo, O.; Fénelon, V.S.; Zizzari, P.; Quarta, C.; Bellocchio, L.; Tailleux, A.; Charton, J.; Fernandois, D.; Henricsson, M.; et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021. [Google Scholar] [CrossRef]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Huang, W.; Young, R.; Jones, K.; Horowitz, M.; Rayner, C.; Wu, T. Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease. Nutrients 2021, 13, 1104. [Google Scholar] [CrossRef] [PubMed]
- Meckel, K.; Kiraly, D.D. A potential role for the gut microbiome in substance use disorders. Psychopharmacology 2019, 236, 1513–1530. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Heisler, L.K.; Jobst, E.E.; Sutton, G.M.; Zhou, L.; Borok, E.; Thornton-Jones, Z.; Liu, H.Y.; Zigman, J.M.; Balthasar, N.; Kishi, T.; et al. Serotonin Reciprocally Regulates Melanocortin Neurons to Modulate Food Intake. Neuron 2006, 51, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Mahony, S.O.; Clarke, G.; Borre, Y.; Dinan, T.; Cryan, J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.-T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, R.; Pessione, E. The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef] [Green Version]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.D.A.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [Green Version]
- Suyama, S.; Yada, T. New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 2018, 68, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Ye, C.-P.; Jones, J.E.; Elmquist, J.K.; Lowell, B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008, 11, 998–1000. [Google Scholar] [CrossRef] [Green Version]
- Farrar, A.; Font, L.; Pereira, M.; Mingote, S.; Bunce, J.; Chrobak, J.; Salamone, J. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience 2008, 152, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Root, D.H.; Melendez, R.I.; Zaborszky, L.; Napier, T.C. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog. Neurobiol. 2015, 130, 29–70. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, S.; Hare, B.; Duman, R.S. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr. Opin. Behav. Sci. 2017, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sandoval-Salazar, C.; Ramírez-Emiliano, J.; Trejo-Bahena, A.; Oviedo-Solís, C.I.; Solís-Ortiz, M.S. A high-fat diet decreases GABA concentration in the frontal cortex and hippocampus of rats. Biol. Res. 2016, 49, 15. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.; Mancano, G.; Kashofer, K.; Fröhlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.; Delzenne, N.M.; et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2019, 22, 877–893. [Google Scholar] [CrossRef] [Green Version]
- González-Arancibia, C.; Urrutia-Piñones, J.; Illanes-González, J.; Martinez-Pinto, J.; Sotomayor-Zárate, R.; Julio-Pieper, M.; Bravo, J.A. Do your gut microbes affect your brain dopamine? Psychopharmacology 2019, 236, 1611–1622. [Google Scholar] [CrossRef]
- Pennisi, E. Meet the psychobiome. Science 2020, 368, 570–573. [Google Scholar] [CrossRef]
- Matarazzo, I.; Toniato, E.; Robuffo, I. Psychobiome Feeding Mind: Polyphenolics in Depression and Anxiety. Curr. Top. Med. Chem. 2019, 18, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- NIDA. The Science of Drug Use and Addiction. Available online: https://www.drugabuse.gov/publications/media-guide/science-drug-use-addiction-basics (accessed on 3 May 2021).
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Uhl, G.R.; Koob, G.F.; Cable, J. The neurobiology of addiction. Ann. N. Y. Acad. Sci. 2019, 1451, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Feltenstein, M.W.; E See, R. The neurocircuitry of addiction: An overview. Br. J. Pharmacol. 2008, 154, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Naish, K.R.; MacKillop, J.; Balodis, I.M. The Concept of Food Addiction: A Review of the Current Evidence. Curr. Behav. Neurosci. Rep. 2018, 5, 281–294. [Google Scholar] [CrossRef]
- Fletcher, P.; Kenny, P.J. Food addiction: A valid concept? Neuropsychopharmacology 2018, 43, 2506–2513. [Google Scholar] [CrossRef] [Green Version]
- Meule, A. A Critical Examination of the Practical Implications Derived from the Food Addiction Concept. Curr. Obes. Rep. 2019, 8, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Gearhardt, A.N.; Hebebrand, J. The concept of “food addiction” helps inform the understanding of overeating and obesity: YES. Am. J. Clin. Nutr. 2021, 113, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.; Cook, B.; Ellrott, T. Food addiction, eating addiction and eating disorders. Proc. Nutr. Soc. 2020, 79, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Meule, A. Chapter 1—A history of “food addiction”. In Compulsive Eating Behavior and Food Addiction; Cottone, P., Sabino, V., Moore, C.F., Koob, G.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Rogers, P.J. Food and drug addictions: Similarities and differences. Pharmacol. Biochem. Behav. 2017, 153, 182–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, S.-L.; Pai, N. What is in a name? Is food addiction a misnomer? Asian J. Psychiatry 2017, 25, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Bach, G.; Jiménez-Murcia, S.; Fernández-Aranda, F.; Potenza, M.N. Chapter 14—Addressing controversies surrounding food addiction. In Compulsive Eating Behavior and Food Addiction; Cottone, P., Sabino, V., Moore, C.F., Koob, G.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 419–448. [Google Scholar] [CrossRef]
- Sarkar, S.; Kochhar, K.P.; Khan, N.A. Fat Addiction: Psychological and Physiological Trajectory. Nutrients 2019, 11, 2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onaolapo, A.; Onaolapo, O. Food additives, food and the concept of ‘food addiction’: Is stimulation of the brain reward circuit by food sufficient to trigger addiction? Pathophysiology 2018, 25, 263–276. [Google Scholar] [CrossRef]
- Cocores, J.A.; Gold, M.S. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med. Hypotheses 2009, 73, 892–899. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, H.K.; Christiansen, P.; Halford, J.C.G.; A Hardman, C. The development and validation of the Addiction-like Eating Behaviour Scale. Int. J. Obes. 2017, 41, 1710–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finlayson, G. Food addiction and obesity: Unnecessary medicalization of hedonic overeating. Nat. Rev. Endocrinol. 2017, 13, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziauddeen, H.; Fletcher, P.C. Is food addiction a valid and useful concept? Obes. Rev. 2012, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.J. Is it time to consider the “food use disorder”? Appetite 2017, 115, 16–18. [Google Scholar] [CrossRef]
- Ziauddeen, H.; Farooqi, S.; Fletcher, P. Obesity and the brain: How convincing is the addiction model? Nat. Rev. Neurosci. 2012, 13, 279–286. [Google Scholar] [CrossRef]
- Delormier, T.; Frohlich, K.L.; Potvin, L. Food and eating as social practice—Understanding eating patterns as social phenomena and implications for public health. Sociol. Health Illn. 2009, 31, 215–228. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Leshner, A.I. Addiction Is a Brain Disease, and It Matters. Science 1997, 278, 45–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eblum, K.; Thanos, P.K.; Gold, M.S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 2014, 5, 919. [Google Scholar] [CrossRef] [Green Version]
- Diana, M. The Dopamine Hypothesis of Drug Addiction and Its Potential Therapeutic Value. Front. Psychiatry 2011, 2, 64. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, M. Brain Change in Addiction as Learning, Not Disease. N. Engl. J. Med. 2018, 379, 1551–1560. [Google Scholar] [CrossRef] [Green Version]
- Woods, S.C. The eating paradox: How we tolerate food. Psychol. Rev. 1991, 98, 488–505. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain–gut–microbiome interactions in obesity and food addiction. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Santana, A.; Heijtz, R.D. Bacterial Peptidoglycans from Microbiota in Neurodevelopment and Behavior. Trends Mol. Med. 2020, 26, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Chattipakorn, S.C.; Sriwichaiin, S.; Luca, A. Cognitive-Behavioural Correlates of Dysbiosis: A Review. Int. J. Mol. Sci. 2020, 21, 4834. [Google Scholar] [CrossRef] [PubMed]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psychiatry Rep. 2017, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lam, Y.Y.; Maguire, S.; Palacios, T.; Caterson, I.D. Are the Gut Bacteria Telling Us to Eat or Not to Eat? Reviewing the Role of Gut Microbiota in the Etiology, Disease Progression and Treatment of Eating Disorders. Nutrients 2017, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; I Riezu-Boj, J.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [Green Version]
- Cohen, L.J.; Cho, J.H.; Gevers, D.; Chu, H. Genetic Factors and the Intestinal Microbiome Guide Development of Microbe-Based Therapies for Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 2174–2189. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex Interactions Among Diet, Gastrointestinal Transit, and Gut Microbiota in Humanized Mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Salas Garcia, M.C.; Yee, A.; Gilbert, J.A.; Dsouza, M. Dysbiosis in Children Born by Caesarean Section. Ann. Nutr. Metab. 2018, 73 (Suppl. S3), S24–S32. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuman, H.; Forsythe, P.; Uzan, A.; Avni, O.; Koren, O. Antibiotics in early life: Dysbiosis and the damage done. FEMS Microbiol. Rev. 2018, 42, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, T.S.; Mayer, E.A.; Osadchiy, V.; Chang, C.; Katzka, W.; Lagishetty, V.; Gonzalez, K.; Kalani, A.; Stains, J.; Jacobs, J.P.; et al. A Distinct Brain-Gut-Microbiome Profile Exists for Females with Obesity and Food Addiction. Obesity 2020, 28, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Novelle, M.G.; Leis, R.; Diéguez, C.; Skrede, S.; Lopez, M. Effects of Neonatal Programming on Hypothalamic Mechanisms Controlling Energy Balance. Horm. Metab. Res. 2013, 45, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molle, R.D.; Bischoff, A.R.; Portella, A.K.; Silveira, P.P. The fetal programming of food preferences: Current clinical and experimental evidence. J. Dev. Orig. Health Dis. 2016, 7, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Mirpuri, J. Evidence for maternal diet-mediated effects on the offspring microbiome and immunity: Implications for public health initiatives. Pediatr. Res. 2021, 89, 301–306. [Google Scholar] [CrossRef]
- Al Rubaye, H.; Adamson, C.C.; Jadavji, N.M. The role of maternal diet on offspring gut microbiota development: A review. J. Neurosci. Res. 2021, 99, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Chen, C.; Fang, H.; Zhao, Y.; Fei, W.; Chen, F.; Zheng, C. The Role of Microbiomes in Pregnant Women and Offspring: Research Progress of Recent Years. Front. Pharmacol. 2020, 11, 643. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Morrison, K.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Bordoni, L.; Morano, S.; Calleja-Agius, J.; Lalor, J.G. Nutri-Epigenetics and Gut Microbiota: How Birth Care, Bonding and Breastfeeding Can Influence and Be Influenced? Int. J. Mol. Sci. 2020, 21, 5032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Neuringer, M.; Erdman, J.W., Jr.; Kuchan, M.J.; Renner, L.; Johnson, E.E.; Wang, X.; Kroenke, C.D. The effects of breastfeeding versus formula-feeding on cerebral cortex maturation in infant rhesus macaques. NeuroImage 2019, 184, 372–385. [Google Scholar] [CrossRef]
- Forestell, C. Flavor Perception and Preference Development in Human Infants. Ann. Nutr. Metab. 2017, 70 (Suppl. S3), S17–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.; Leung, J.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.; Shanahan, F.; Dinan, T.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, S.B.; Burrows, T.L.; Hayes, T.; Hsia, C.Y.; Watkins, A.; Curtis, J.; Ward, P.B. Dietary intake, food addiction and nutrition knowledge in young people with mental illness. Nutr. Diet. 2020, 77, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.; Chaaya, N.; Beecher, K.; Ali, S.A.; Belmer, A.; Bartlett, S. The impact of sugar consumption on stress driven, emotional and addictive behaviors. Neurosci. Biobehav. Rev. 2019, 103, 178–199. [Google Scholar] [CrossRef]
- Pursey, K.M.; Collins, C.E.; Stanwell, P.; Burrows, T.L. Foods and dietary profiles associated with ‘food addiction’ in young adults. Addict. Behav. Rep. 2015, 2, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Ayton, A.; Ibrahim, A.; Dugan, J.; Galvin, E.; Wright, O.W. Ultra-processed foods and binge eating: A retrospective observational study. Nutrition 2021, 84, 111023. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochowska, M.; Laskus, T.; Radkowski, M. Gut Microbiota in Neurological Disorders. Arch. Immunol. Ther. Exp. 2019, 67, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Jaggar, M.; Rea, K.; Spichak, S.; Dinan, T.G.; Cryan, J.F. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front. Neuroendocrinol. 2020, 56, 100815. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef]
- Osadchiy, V.; Mayer, E.A.; Bhatt, R.; Labus, J.S.; Gao, L.; Kilpatrick, L.A.; Liu, C.; Tillisch, K.; Naliboff, B.; Chang, L.; et al. History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obes. Sci. Pract. 2019, 5, 416–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loxton, N.J.; Tipman, R.J. Reward sensitivity and food addiction in women. Appetite 2017, 115, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Peterson, V.L.; Richards, J.B.; Meyer, P.J.; Cabrera-Rubio, R.; Tripi, J.A.; King, C.P.; Polesskaya, O.; Baud, A.; Chitre, A.S.; Bastiaanssen, T.F.S.; et al. Sex-dependent associations between addiction-related behaviors and the microbiome in outbred rats. EBioMedicine 2020, 55, 102769. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novelle, M.G. Decoding the Role of Gut-Microbiome in the Food Addiction Paradigm. Int. J. Environ. Res. Public Health 2021, 18, 6825. https://doi.org/10.3390/ijerph18136825
Novelle MG. Decoding the Role of Gut-Microbiome in the Food Addiction Paradigm. International Journal of Environmental Research and Public Health. 2021; 18(13):6825. https://doi.org/10.3390/ijerph18136825
Chicago/Turabian StyleNovelle, Marta G. 2021. "Decoding the Role of Gut-Microbiome in the Food Addiction Paradigm" International Journal of Environmental Research and Public Health 18, no. 13: 6825. https://doi.org/10.3390/ijerph18136825
APA StyleNovelle, M. G. (2021). Decoding the Role of Gut-Microbiome in the Food Addiction Paradigm. International Journal of Environmental Research and Public Health, 18(13), 6825. https://doi.org/10.3390/ijerph18136825





