The Effect of Periodontitis on Dementia and Cognitive Impairment: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Screening and Data Extraction
2.4. Quality Assessment
2.5. Reporting Bias
2.6. Data Pre-Processing
2.7. Statistical Analysis
3. Results
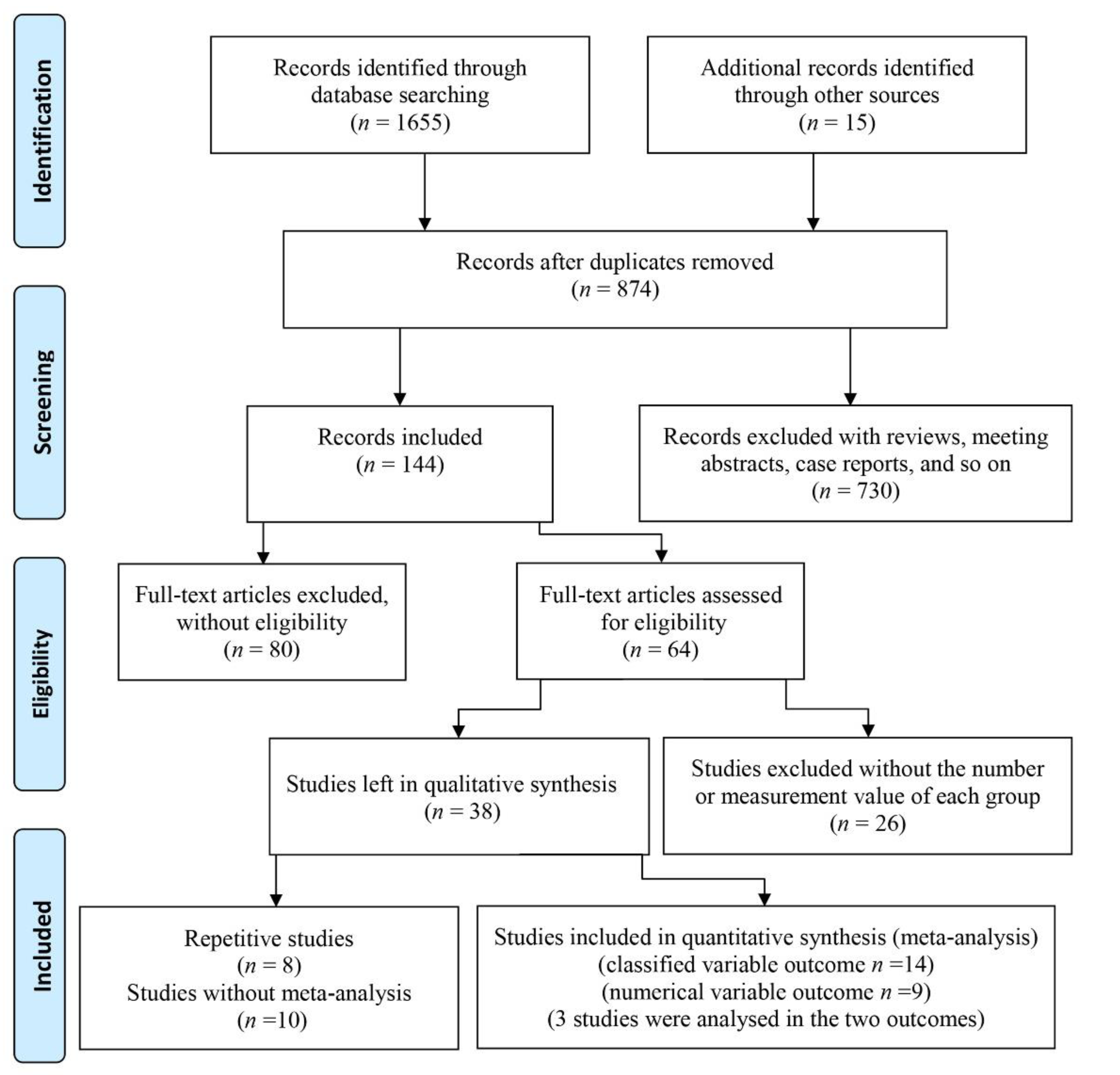
3.1. Included Studies
3.2. Main Characteristics of Included Studies
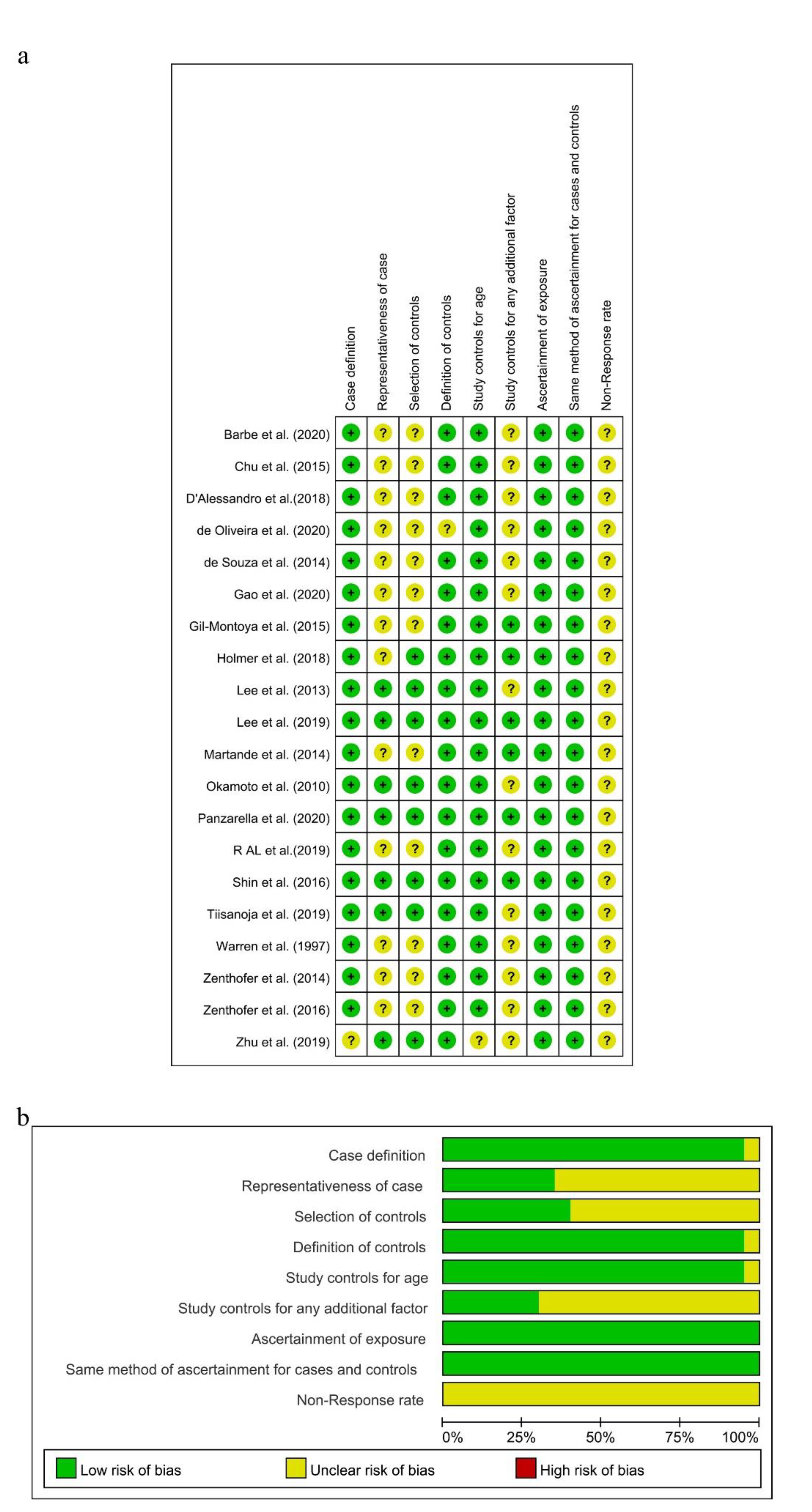
3.3. Quality Assessment of Eligible Studies
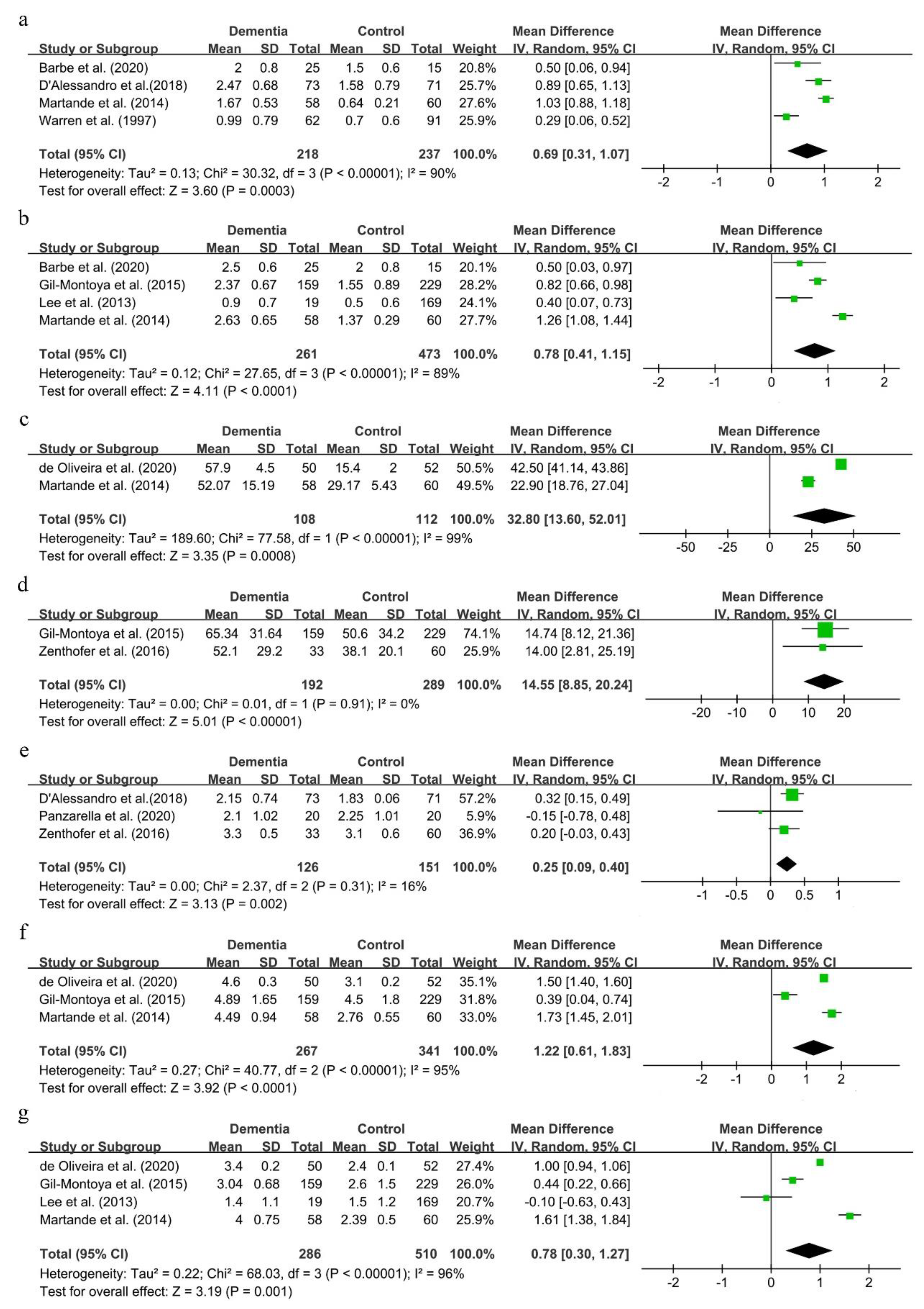
3.4. Association between Periodontitis and Cognitive Impairment
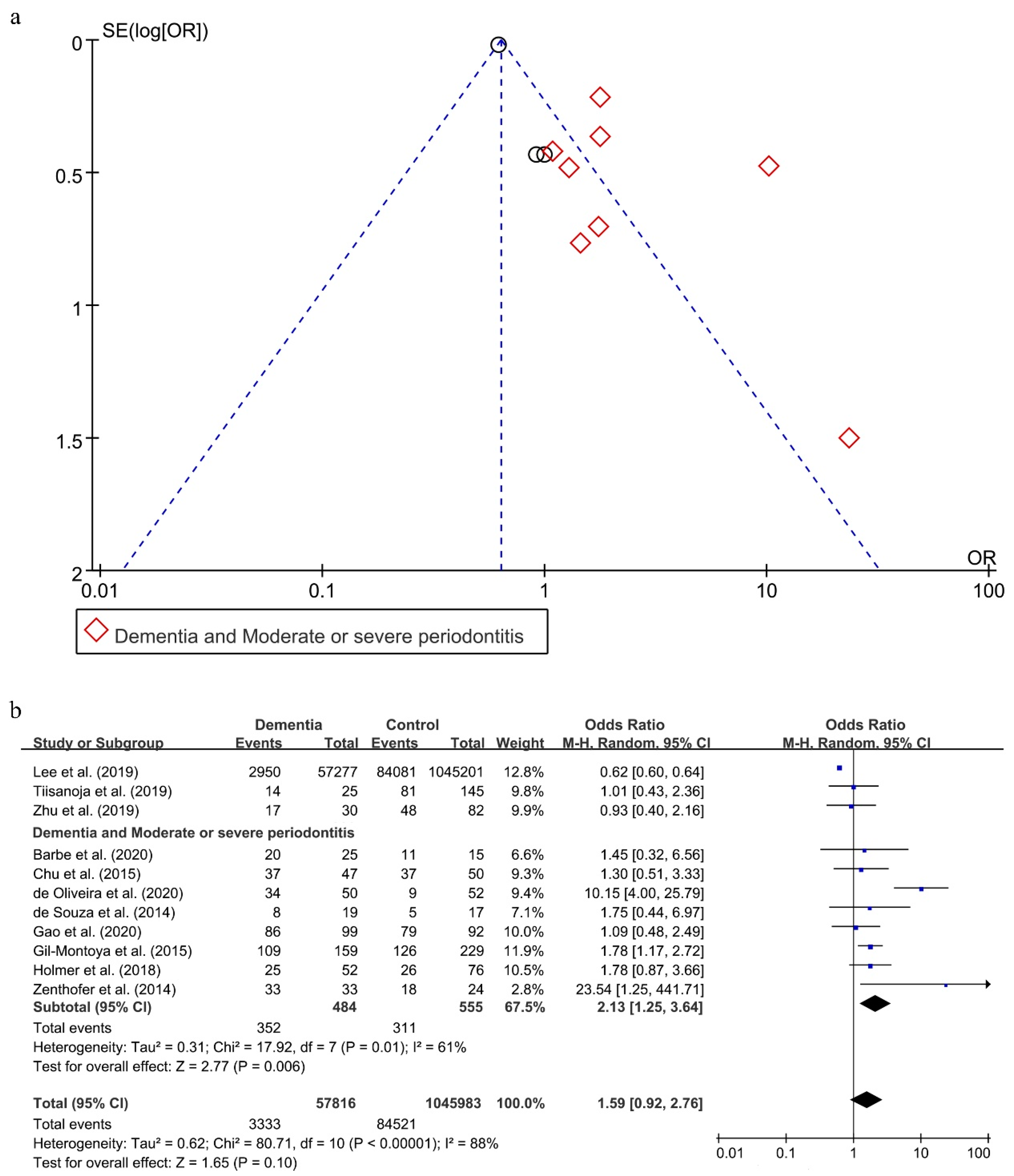
3.5. Relationship between Periodontitis and Dementia
3.6. Periodontal Status in Dementia Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef] [Green Version]
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat. Med. 2006, 12, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, V.; Schurr, J.; Ball, M.J.; Pelaez, R.P.; Bazan, N.G.; Lukiw, W.J. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: Transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res. 2002, 70, 462–473. [Google Scholar] [CrossRef]
- Yaffe, K.; Lindquist, K.; Penninx, B.W.; Simonsick, E.M.; Pahor, M.; Kritchevsky, S.; Launer, L.; Kuller, L.; Rubin, S.; Harris, T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 2003, 61, 76–80. [Google Scholar] [CrossRef]
- McGeer, P.L.; Guo, J.P.; Lee, M.; Kennedy, K.; McGeer, E.G. Alzheimer’s disease can be spared by nonsteroidal anti-inflammatory drugs. J. Alzheimer’s Dis. 2018, 62, 1219–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balin, B.J.; Gérard, H.C.; Arking, E.J.; Appelt, D.M.; Branigan, P.J.; Abrams, J.T.; Whittum-Hudson, J.A.; Hudson, A.P. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol. Immunol. 1998, 187, 23–42. [Google Scholar] [CrossRef]
- Miklossy, J.; Kis, A.; Radenovic, A.; Miller, L.; Forro, L.; Martins, R.; Reiss, K.; Darbinian, N.; Darekar, P.; Mihaly, L.; et al. Beta-amyloid deposition and Alzheimer’s type changes induced by Borrelia spirochetes. Neurobiol. Aging 2006, 27, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, A.B.; Crean, S.; Olsen, I.; Singhrao, S.K. Periodontitis, microbiomes and their role in Alzheimer’s disease. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Dunn, N.; Mullee, M.; Perry, V.H.; Holmes, C. Association between dementia and infectious disease: Evidence from a case-control study. Alzheimer Dis. Assoc. Disord. 2005, 19, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lee, H.C.; Hu, C.J.; Huang, L.K.; Chao, S.P.; Lin, C.P.; Su, E.C.Y.; Lee, Y.C.; Chen, C.C. Periodontitis as a modifiable risk factor for dementia: A nationwide population-based cohort study. J. Am. Geriatr. Soc. 2017, 65, 301–305. [Google Scholar] [CrossRef]
- Wu, B.; Plassman, B.L.; Crout, R.J.; Liang, J. Cognitive function and oral health among community-dwelling older adults. J. Gerontol. A 2008, 63, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Naorungroj, S.; Schoenbach, V.J.; Wruck, L.; Mosley, T.H.; Gottesman, R.F.; Alonso, A.; Heiss, G.; Beck, J.; Slade, G.D. Tooth loss, periodontal disease, and cognitive decline in the Atherosclerosis Risk in Communities (ARIC) study. Community Dent. Oral Epidemiol. 2015, 43, 47–57. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leira, Y.; Dominguez, C.; Seoane, J.; Seoane-Romero, J.; Pias-Peleteiro, J.M.; Takkouche, B.; Blanco, J.; Aldrey, J.M. Is periodontal disease associated with Alzheimer’s disease? A systematic review with meta-analysis. Neuroepidemiology 2017, 48, 21–31. [Google Scholar] [CrossRef]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef]
- Zenthofer, A.; Schroder, J.; Cabrera, T.; Rammelsberg, P.; Hassel, A.J. Comparison of oral health among older people with and without dementia. Community Dent. Health 2014, 31, 27–31. [Google Scholar] [CrossRef]
- Okamoto, N.; Morikawa, M.; Amano, N.; Yanagi, M.; Takasawa, S.; Kurumatani, N. Effects of tooth loss and the apolipoprotein E epsilon 4 allele on mild memory impairment in the Fujiwara-kyo study of Japan: A nested case-control study. J. Alzheimers Dis. 2017, 55, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Brodaty, H.; Connors, M.H.; Loy, C.; Teixeira-Pinto, A.; Stocks, N.; Gunn, J.; Mate, K.E.; Pond, C.D. Screening for dementia in primary care: A comparison of the GPCOG and the MMSE. Dement. Geriatr. Cogn. Disord. 2016, 42, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Alfotawi, R.; Alzahrani, S.; Alhefdhi, R.; Altamimi, A.; Alfadhel, A.; Alshareef, A.; Aldawsari, B.; Sonbol, S.; Alsubaie, F.; Alwahibi, A.; et al. The relation between teeth loss and cognitive decline among Saudi population in the city of Riyadh: A pilot study. Saudi Dent. J. 2020, 32, 232–241. [Google Scholar] [CrossRef]
- Okamoto, N.; Morikawa, M.; Okamoto, K.; Habu, N.; Iwamoto, J.; Tomioka, K.; Saeki, K.; Yanagi, M.; Amano, N.; Kurumatani, N. Relationship of tooth loss to mild memory impairment and cognitive impairment: Findings from the fujiwara-kyo study. Behav. Brain Funct. 2010, 6, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, N.; Morikawa, M.; Okamoto, K.; Habu, N.; Hazaki, K.; Harano, A.; Iwamoto, J.; Tomioka, K.; Saeki, K.; Kurumatani, N. Tooth loss is associated with mild memory impairment in the elderly: The Fujiwara-kyo study. Brain Res. 2010, 1349, 68–75. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; Sanchez-Lara, I.; Carnero-Pardo, C.; Fornieles, F.; Montes, J.; Vilchez, R.; Burgos, J.S.; Gonzalez-Moles, M.A.; Barrios, R.; Bravo, M. Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J. Periodontol. 2015, 86, 244–253. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; Sanchez-Lara, I.; Carnero-Pardo, C.; Fornieles-Rubio, F.; Montes, J.; Barrios, R.; Gonzalez-Moles, M.A.; Bravo, M. Oral hygiene in the elderly with different degrees of cognitive impairment and dementia. J. Am. Geriatr. Soc. 2016, 65, 642–647. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; Barrios, R.; Santana, S.; Sanchez-Lara, I.; Pardo, C.C.; Fornieles-Rubio, F.; Montes, J.; Ramirez, C.; Gonzalez-Moles, M.A.; Burgos, J.S. Association between periodontitis and amyloid beta peptide in elderly people with and without cognitive impairment. J. Periodontol. 2017, 88, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Gil Montoya, J.A.; Barrios, R.; Sanchez-Lara, I.; Ramos, P.; Carnero, C.; Fornieles, F.; Montes, J.; Santana, S.; Luna, J.D.D.; Gonzalez-Moles, M.A. Systemic inflammatory impact of periodontitis on cognitive impairment. Gerodontology 2020, 37, 11–18. [Google Scholar] [CrossRef]
- Cestari, J.A.F.; Fabri, G.M.C.; Kalil, J.; Nitrini, R.; Jacob, W.; de Siqueira, J.T.T.; Siqueira, S. Oral infections and cytokine levels in patients with Alzheimer’s disease and mild cognitive impairment compared with controls. J. Alzheimers Dis. 2016, 52, 1479–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza Rolim, T.; Fabri, G.M.; Nitrini, R.; Anghinah, R.; Teixeira, M.J.; de Siqueira, J.T.; Cestari, J.A.; de Siqueira, S.R. Oral infections and orofacial pain in Alzheimer’s disease: A case-control study. J. Alzheimer’s Dis. 2013, 38, 823–829. [Google Scholar] [CrossRef]
- Chen, C.K.; Wu, Y.T.; Chang, Y.C. Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 2017, 9, 1–7. [Google Scholar] [CrossRef]
- Tzeng, N.S.; Chung, C.H.; Yeh, C.B.; Huang, R.Y.; Yuh, D.Y.; Huang, S.Y.; Lu, R.B.; Chang, H.A.; Kao, Y.C.; Chiang, W.S.; et al. Are chronic periodontitis and gingivitis associated with dementia? A nationwide, retrospective, matched-cohort study in Taiwan. Neuroepidemiology 2016, 47, 82–93. [Google Scholar] [CrossRef]
- Sorensen, C.E.; Hansen, N.L.; Mortensen, E.L.; Lauritzen, M.; Osler, M.; Pedersen, A.M.L. Hyposalivation and poor dental health status are potential correlates of age-related cognitive decline in late midlife in Danish men. Front. Aging Neurosci. 2018, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Aragón, F.; Zea-Sevilla, M.A.; Montero, J.; Sancho, P.; Corral, R.; Tejedor, C.; Frades-Payo, B.; Paredes-Gallardo, V.; Albaladejo, A. Oral health in Alzheimer’s disease: A multicenter case-control study. Clin. Oral Investig. 2018, 22, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Syrjala, A.M.; Ylostalo, P.; Ruoppi, P.; Komulainen, K.; Hartikainen, S.; Sulkava, R.; Knuuttila, M. Dementia and oral health among subjects aged 75 years or older. Gerodontology 2012, 29, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Demmer, R.T.; Norby, F.L. Periodontal disease and incident dementia: The Atherosclerosis Risk in Communities study (ARIC). Neurology 2020, 95, e1660–e1671. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.R.; Costa, J.L.R.; Ambrosano, G.M.B.; Garcia, R. Oral health of the elderly with Alzheimer’s disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Clark, J.J.; Naorungroj, S. Oral health in nursing home residents with different cognitive statuses. Gerodontology 2013, 30, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Morse, D.E.; Holm-Pedersen, P.; Mortensen, E.L.; Avlund, K. Periodontal inflammation in relation to cognitive function in an older adult Danish population. J. Alzheimer’s Dis. 2012, 28, 613–624. [Google Scholar] [CrossRef]
- Nilsson, H.; Sanmartin Berglund, J.; Renvert, S. Longitudinal evaluation of periodontitis and development of cognitive decline among older adults. J. Clin. Periodontol. 2018, 45, 1142–1149. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kuo, H.K. Association between cognitive function and periodontal disease in older adults. J. Am. Geriatr. Soc. 2008, 56, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.E.; Huang, R.Y.; Cheng, W.C.; Kao, T.W.; Chen, W.L. Association between periodontitis and cognitive impairment: Analysis of national health and nutrition examination survey (NHANES) III. J. Clin. Periodontol. 2019, 46, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Holmer, J.; Eriksdotter, M.; Schultzberg, M.; Pussinen, P.J.; Buhlin, K. Association between periodontitis and risk of Alzheimer’s disease, mild cognitive impairment and subjective cognitive decline: A case-control study. J. Clin. Periodontol. 2018, 45, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Shin, M.S.; Ahn, Y.B.; Choi, B.Y.; Nam, J.H.; Kim, H.D. Periodontitis is associated with cognitive impairment in elderly Koreans: Results from the yangpyeong cohort study. J. Am. Geriatr. Soc. 2016, 64, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Barbe, A.G.; Kupeli, L.S.; Hamacher, S.; Noack, M.J. Impact of regular professional toothbrushing on oral health, related quality of life, and nutritional and cognitive status in nursing home residents. Int. J. Dent. Hyg. 2020, 18, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Panzarella, V.; Mauceri, R.; Baschi, R.; Maniscalco, L.; Campisi, G.; Monastero, R. Oral health status in subjects with amnestic mild cognitive impairment and Alzheimer’s disease: Data from the Zabút aging project. J. Alzheimer’s Dis. 2020. [Google Scholar] [CrossRef]
- de Oliveira Araújo, R.; Villoria, G.E.M.; Luiz, R.R.; Esteves, J.C.; Leão, A.T.T.; Feres-Filho, E.J. Association between periodontitis and Alzheimer’s disease and its impact on the self-perceived oral health status: A case-control study. Clin. Oral Investig. 2021, 5, 555–562. [Google Scholar] [CrossRef]
- Chu, C.H.; Ng, A.; Chau, A.M.; Lo, E.C. Oral health status of elderly chinese with dementia in Hong Kong. Oral Health Prev. Dent. 2015, 13, 51–57. [Google Scholar] [CrossRef]
- Lee, K.H.; Wu, B.; Plassman, B.L. Cognitive function and oral health-related quality of life in older adults. J. Am. Geriatr. Soc. 2013, 61, 1602–1607. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.S.; Chen, K.J.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. The oral health status of Chinese elderly people with and without dementia: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 1913. [Google Scholar] [CrossRef] [Green Version]
- Warren, J.J.; Chalmers, J.M.; Levy, S.M.; Blanco, V.L.; Ettinger, R.L. Oral health of persons with and without dementia attending a geriatric clinic. Spec. Care Dent. 1997, 17, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Martande, S.S.; Pradeep, A.R.; Singh, S.P.; Kumari, M.; Suke, D.K.; Raju, A.P.; Naik, S.B.; Singh, P.; Guruprasad, C.N.; Chatterji, A. Periodontal health condition in patients with Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2014, 29, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Zenthofer, A.; Cabrera, T.; Rammelsberg, P.; Hassel, A.J. Improving oral health of institutionalized older people with diagnosed dementia. Aging Ment. Health 2016, 20, 303–308. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Costi, T.; Alkhamis, N.; Bagattoni, S.; Sadotti, A.; Piana, G. Oral health status in Alzheimer’s disease patients: A descriptive study in an Italian population. J. Contemp. Dent. Pract. 2018, 19, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Choi, Y.Y. Association between oral health and dementia in the elderly: A population-based study in Korea. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Tiisanoja, A.; Syrjälä, A.M.; Tertsonen, M.; Komulainen, K.; Pesonen, P.; Knuuttila, M.; Hartikainen, S.; Ylöstalo, P. Oral diseases and inflammatory burden and Alzheimer’s disease among subjects aged 75 years or older. Spec. Care Dent. 2019, 39, 158–165. [Google Scholar] [CrossRef]
- Zhu, A. The correlation between mild cognitive impairment (MCI) and alzheimer’s disease (AD) and chronic periodontal disease in elderly of tibetan at high altitude. Alzheimer’s Dement. 2019, 15, P1157. [Google Scholar] [CrossRef]
- Burke, S.L.; Maramaldi, P.; Cadet, T.; Kukull, W. Associations between depression, sleep disturbance, and apolipoprotein E in the development of Alzheimer’s disease: Dementia. Int. Psychogeriatr. 2016, 28, 1409–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, R.; Sabbah, W.; Tsakos, G.; D’Aiuto, F.; Watt, R.G. Oral health and cognitive function in the third national health and nutrition examination survey (NHANES III). Psychosom. Med. 2008, 70, 936–941. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kimura, Y.; Yoshihara, A.; Ogawa, H.; Yamaga, T.; Sato, M.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fukutomi, E.; et al. Oral health status in relation to cognitive function among older Japanese. Clin. Exp. Dent. Res. 2015, 1, 3–9. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yoshihara, A.; Kimura, Y.; Sato, M.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fukutomi, E.; Chen, W.; Imai, H.; et al. Longitudinal relationship of severe periodontitis with cognitive decline in older Japanese. J. Periodontal Res. 2016, 51, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, K.; Chang, J.; Kim, S.M.; Kim, S.J.; Cho, H.; Park, S.M. Association of chronic periodontitis on Alzheimer’s disease or vascular dementia. J. Am. Geriatr. Soc. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.L.; Hu, H.Y.; Huang, L.Y.; Chou, P.; Chu, D. Periodontal disease associated with higher risk of dementia: Population-based cohort study in Taiwan. J. Am. Geriatr. Soc. 2017, 65, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Sparks Stein, P.; Steffen, M.J.; Smith, C.; Jicha, G.; Ebersole, J.L.; Abner, E.; Dawson, D. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Mcgeer, P.L.; Mcgeer, E.G. Inflammation, autotoxicity and Alzheimer disease. Neurobiol. Aging 2001, 22, 799–809. [Google Scholar] [CrossRef]
- Maldonado, A.; Laugisch, O.; Burgin, W.; Sculean, A.; Eick, S. Clinical periodontal variables in patients with and without dementia-a systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimer’s Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef]
- Wu, Z.; Ni, J.J.; Liu, Y.C.; Teeling, J.L.; Takayama, F.; Collcutt, A.; Ibbett, P.; Nakanishi, H. Cathepsin B plays a critical role in inducing Alzheimer’s disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav. Immun. 2017, 65, 350–361. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Hayashi, Y.; Nakanishi, H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience 2012, 216, 133–142. [Google Scholar] [CrossRef]
- Wu, Z.; Nakanishi, H. Lessons from microglia aging for the link between inflammatory bone disorders and Alzheimer’s disease. J. Immunol. Res. 2015, 2015, 471342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A. Accuracy of NHANES periodontal examination protocols. J. Dent. Res. 2010, 89, 1208. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. ICD-11. Lancet 2019, 393, 2275. [Google Scholar] [CrossRef] [Green Version]

| First Author-Year | Age (Years) | Sample Size | Cognition Status Criteria | PD Criteria | Conclusion |
|---|---|---|---|---|---|
| Articles included in the quantitative analysis | |||||
| Barbe et al. (2020) [47] | 82 | 40 | medical records | CPITN | There was no relationship between periodontitis and dementia (p = 0.705). |
| Chu et al. (2015) [50] | ≥60 | 97 | medical records | CPI | There was no significant difference in the prevalence of advanced periodontal disease (CPI ≥ 3) between the dementia and control group (p = 0.64). |
| D’Alessandro et al. (2018) [56] | >65 | 144 | medical records | CPI, GI | AD patients presented numbers of CPI, and GI was significantly higher (p ≤ 0.005). |
| de Oliveira et al. (2020) [49] | 71.17 | 102 | CDR and MMSE | PPD, CAL | AD patients had greater CAL than controls. Periodontitis was a variable most likely associated with AD (p < 0.001). |
| de Souza et al. (2014) [32] | ≥59 | 36 | NINCDS-ADRDA | PPD, CAL | A higher prevalence of periodontal infections (p = 0.002) was observed in the AD group compared to the control group. |
| Gao et al. (2020) [52] | ≥65 | 187 | medical records | PPD, LoA | There was no significant difference of periodontal status observed in the dementia group compared to the control group. |
| Gil-Montoya et al. (2015) [27] | >50 | 388 | DSM-IV and NINCDS-ADRDA | CAL | A statistically significant association was observed between CAL and cognitive impairment after controlling for confounding factors (p = 0.049). |
| Holmer et al. (2018) [45] | ≥50 | 128 | medical records | MABL | Marginal periodontitis was associated with early cognitive impairment and AD. |
| Lee et al. (2013) [51] | ≥70 | 188 | DSM- IV | PPD, PI | There was no significant difference of pocket depth and plaque index observed. |
| Lee et al. (2019) [57] | ≥65 | 1102478 | medical records | medical records | There was a significant relationship between periodontitis and dementia, except for the group of men aged ≥81 years. |
| Martande et al. (2014) [54] | ≥50 | 118 | NINCDS-ADRDA | PPD, CAL | The periodontal health status of individuals with AD deteriorated with disease progression and was closely related to their cognitive function. |
| Okamoto et al. (2010) [25] | ≥65 | 3456 | DSM-III R | CPI | There was a significant relationship between periodontitis and MMI (p = 0.043). |
| Panzarella et al. (2020) [48] | 81.15 | 60 | DSM-IV | CPI | The scores of the CPI did not statistically differ between AD patients and control group. |
| R et al. (2019) [24] | ≥ 60 | 83 | MoCA | CPI | No statistical significant correlation with regard to periodontal disease and MoCA test scores (p = 0.319). |
| Shin et al. (2016) [46] | 69.04 | 189 | MMSE-KC | RABL | Periodontitis was independently associated with cognitive impairment after controlling for various confounders. |
| Tiisanoja et al. (2019) [58] | 80.9 | 170 | DSM-IV | PPD | Periodontal disease and stomatitis were associated, although non-statistically, with AD and dementia. |
| Warren et al. (1997) [53] | 80.9 | 118 | medical records | GI | Those with severe dementia had poorer gingival health and oral hygiene. |
| Zenthofer et al. (2014) [20] | 80.9 ≥ 54 | 57 | MMSE | CPITN | Mean CPITN of participants in the dementia group was significantly worse than those of participants in the non-dementia group (p < 0.001). |
| Zenthofer et al. (2016) [55] | ≥54 | 93 | medical records | GBI, CPITN | In bivariate testing, participants with dementia had a significantly lower GBI (p < 0.05), and a lower CPITN (p < 0.01) at follow-up. |
| Zhu et al. (2019) [59] | 64.06 | 112 | unclear | unclear | Executive function, language and short-term memory of early cognitive decline were associated with periodontal disease. |
| Articles excluded in the quantitative analysis | |||||
| Aragon et al. (2018) [36] | 72.38 | 106 | McKhann et al. diagnosed criteria | CPI | After taking into account the influence of age, Alzheimer’s patients had worse oral health (caries and periodontal disease). |
| Cestari et al. (2016) [31] | ≥56 | 65 | NINCDS-ADRDA | PPD, CAL | There were no differences in periodontal indexes among groups. |
| Chen et al. (2013) [40] | ≥50 | 700 | MMSE | Calculus/PI/GBI (%) | Demented participants presented with heavy plaque/calculus or severe gingival bleeding, significantly more than that in non-impaired group (p< 0.01). |
| Chen et al. (2017) [33] | ≥50 | 27963 | ICD-9-CM | ICD-9-CM | 10-year chronic periodontitis exposure was associated with a 1.707-fold increase in the risk of developing AD. |
| Demmer et al. (2020) [38] | 63 | 8275 | DSM-V | Periodontal Profile Class | Periodontal disease was modestly associated with incident MCI and dementia in a community-based cohort of black and white participants. |
| Gil-Montoya et al. (2020) [30] | 76.8 | 309 | DSM-IV and NINCDS-ADRDA | CAL | Systemic inflammation derived from periodontal disease plays a relevant role in the aetiology of cognitive impairment. |
| Gil-Montoya et al. (2017) [28] | ≥51 | 564 | DSM-IV and NINCDS-ADRDA | BI, PI | Gingival inflammation is independently associated with cognitive impairment, even at its earliest stage. |
| Gil-Montoya et al. (2017) [29] | ≥51 | 288 | DSM-IV and NINCDS-ADRDA | CAL | Periodontitis may be a modulating variable of the association between Aβ and cognitive impairment. |
| Kamer et al. (2012) [41] | 70 | 152 | DST | PI | Subjects with PI had significantly lower adjusted mean DST scores compared to subjects without PI. |
| Nilsson et al. (2018) [42] | ≥60 | 566 | MMSE | MABL | A statistically significant association between prevalence of periodontitis and cognitive decline after adjustments of confounding factors. |
| Okamoto et al. (2010) [26] | ≥65 | 2646 | MMSE | CPI | No significant differences were found in CPI code between the two groups. |
| Okamoto et al. (2017) [21] | ≥65 | 471 | MMSE | CPI | No significant differences were found in CPI code between the two groups. |
| Ribeiro et al. (2012) [39] | ≥59 | 60 | DSM-IV | OHI | Elderly subjects with AD had poorer oral health than those without the disease. |
| Sorensen et al. (2018) [35] | 56 | 193 | Intelligence-Struktur-Test | PPD | The two groups did not differ significantly with respect to the presence of periodontitis. |
| Sung et al. (2019) [44] | ≥20 | 4663 | SRTT, SDST, SDLT | PPD, CAL | Periodontal status was associated with cognitive impairment in a nationally representative sample of US adults. |
| Syrjala et al. (2012) [37] | 82 | 180 | DSM-IV | PPD | Dementia patients had an increased likelihood of having teeth with deep periodontal pockets, compared with non-demented persons. |
| Tzeng et al. (2016) [34] | ≥20 | 8828 | DSM-IV | ICD-9-CM | Patients with chronic periodontitis and gingivitis have a higher risk of developing dementia. |
| Yu et al. (2008) [43] | 70.4 | 803 | DSST | BOP | Higher cognitive function was associated with lower odds of periodontal disease. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Chang, S.; Pi, X.; Hua, F.; Jiang, H.; Liu, C.; Du, M. The Effect of Periodontitis on Dementia and Cognitive Impairment: A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6823. https://doi.org/10.3390/ijerph18136823
Guo H, Chang S, Pi X, Hua F, Jiang H, Liu C, Du M. The Effect of Periodontitis on Dementia and Cognitive Impairment: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(13):6823. https://doi.org/10.3390/ijerph18136823
Chicago/Turabian StyleGuo, Haiying, Shuli Chang, Xiaoqin Pi, Fang Hua, Han Jiang, Chang Liu, and Minquan Du. 2021. "The Effect of Periodontitis on Dementia and Cognitive Impairment: A Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 13: 6823. https://doi.org/10.3390/ijerph18136823
APA StyleGuo, H., Chang, S., Pi, X., Hua, F., Jiang, H., Liu, C., & Du, M. (2021). The Effect of Periodontitis on Dementia and Cognitive Impairment: A Meta-Analysis. International Journal of Environmental Research and Public Health, 18(13), 6823. https://doi.org/10.3390/ijerph18136823







