Ten Thousand-Fold Higher than Acceptable Bacterial Loads Detected in Kenyan Hospital Environments: Targeted Approaches to Reduce Contamination Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Frame
2.2. Study Design
2.3. Environmental Sampling
2.4. Swab Processing
2.5. Statistical Analysis
3. Results
3.1. Recovery of Bacteria from the High-Touch Surfaces
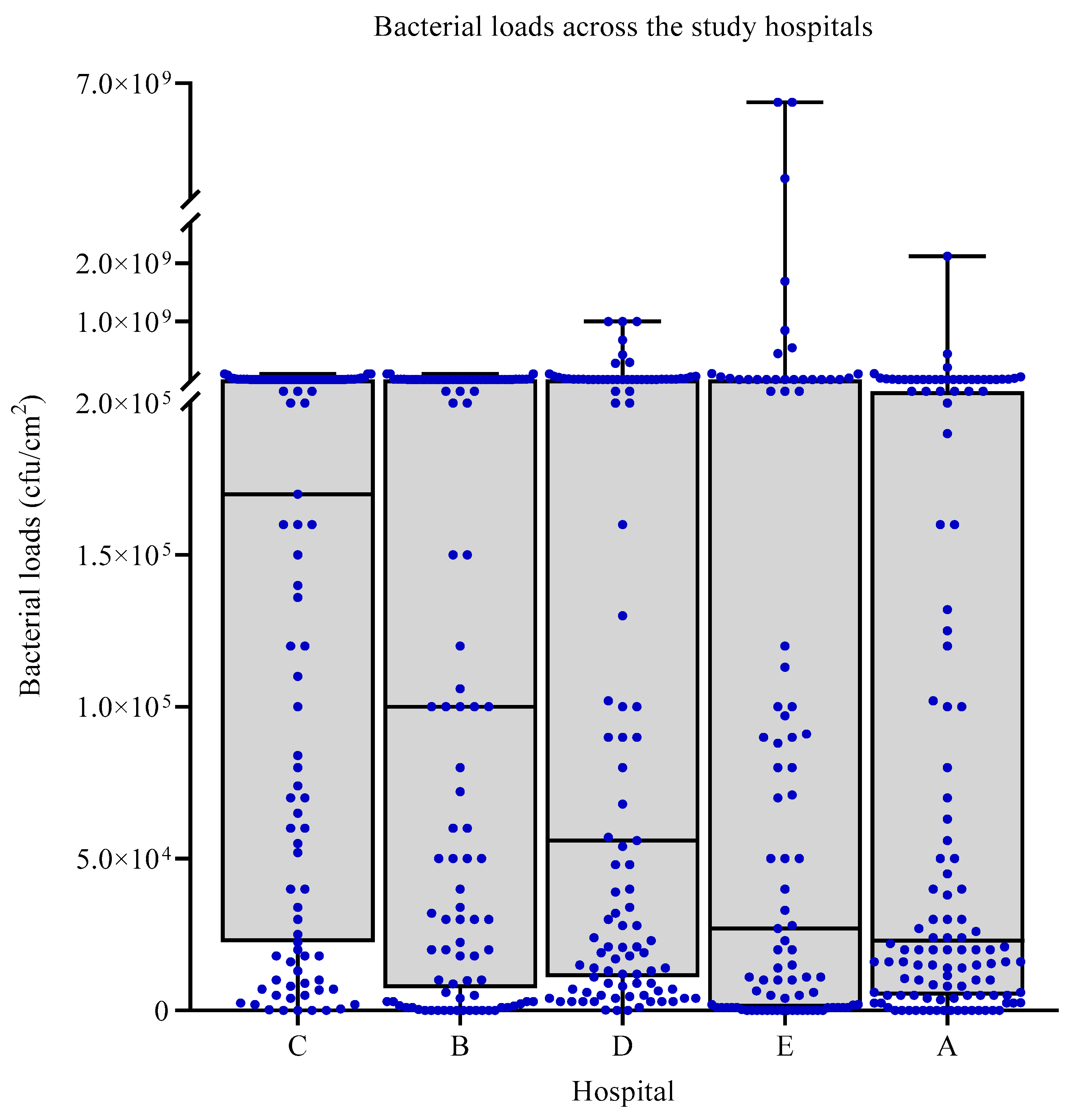
3.2. Bacterial Loads across the Study Hospitals
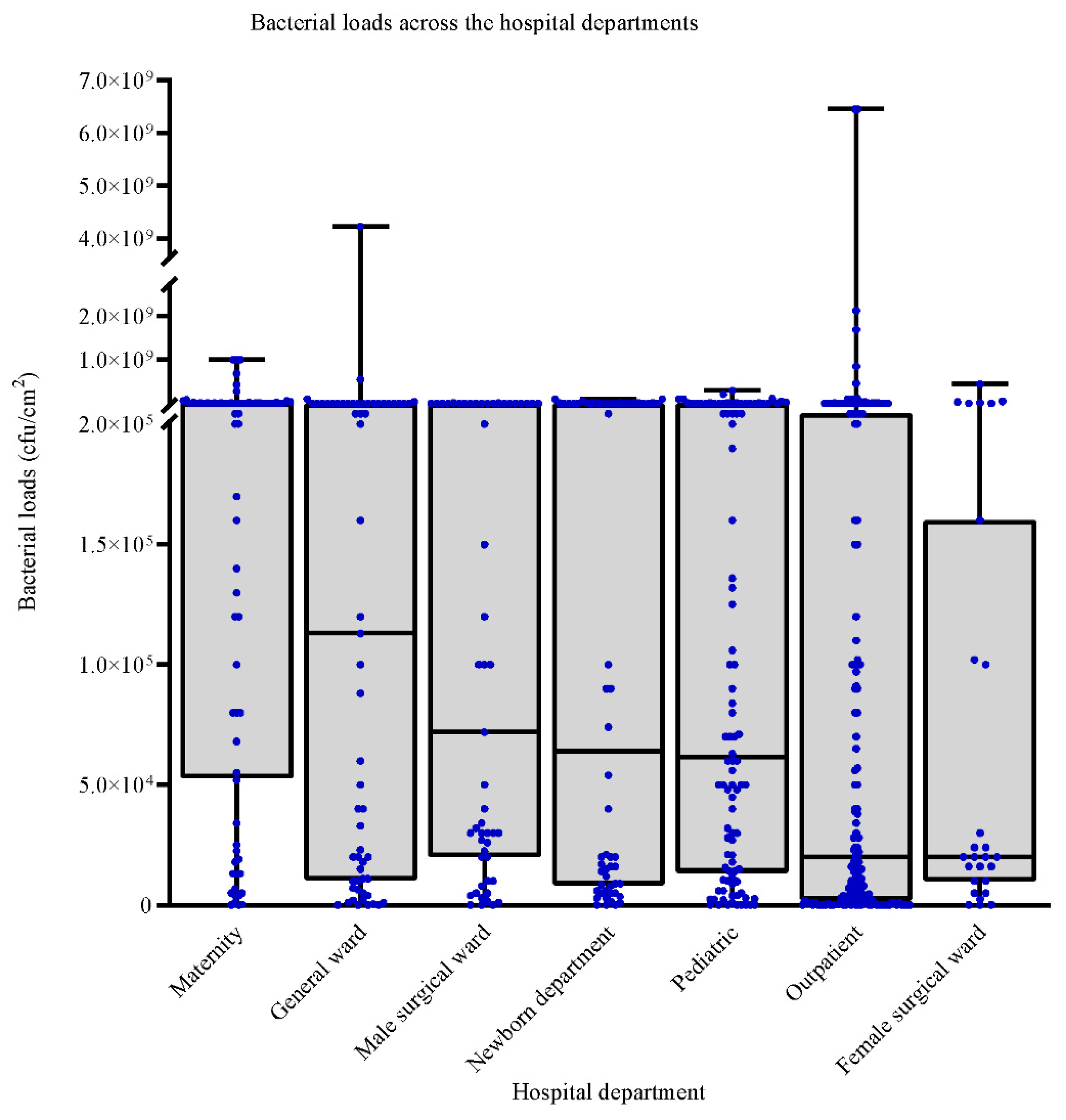
3.3. Bacterial Loads across the Sampled Hospitals Departments
3.4. IPC Practices in the Hospitals
3.5. Impact of Biosafety Practices on Bacterial Loads in the Study Hospitals
3.6. Influence of Cleaning Practices on Bacterial Loads in the Study Hospitals
3.7. Influence of Storage, Disposal, and Biosafety Practices on Bacterial Loads in the Study Hospitals
3.8. Multivariate Analysis of the Influence of Hospital IPC Practices and Environmental Conditions on Bacterial Loads in the Study Hospitals
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, A.M.; Nilsen, R.M.; Eriksen, H.M.; Cox, R.J.; Harthug, S. Mortality related to hospital-associated infections in a tertiary hospital; repeated cross-sectional studies between 2004–2011. Antimicrob. Resist. Infect. Control 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Vrijens, F.; Hulstaert, F.; Devriese, S.; Van De Sande, S. Hospital-acquired infections in Belgian acute-care hospitals: An estimation of their global impact on mortality, length of stay and healthcare costs. Epidemiol. Infect. 2011, 140, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Benenson, S.; Cohen, M.J.; Schwartz, C.; Revva, M.; Moses, A.E.; Levin, P.D. Is it financially beneficial for hospitals to prevent nosocomial infections? BMC Health Serv. Res. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Nejad, S.B.; Allegranzi, B.; Syed, S.B.; Ellis, B.; Pittet, D. Health-care-associated infection in Africa: A systematic review. Bull. World Health Organ. 2011, 89, 757–765. [Google Scholar] [CrossRef]
- Ndegwa, M.L. Hospital-Acquired Infections Surveillance in Three Kenyan Hospitals, 2010–2012. Open Forum Infect. Dis. 2015, 2, 2014–2015. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J. Hosp. Infect. 2004, 56, 10–15. [Google Scholar] [CrossRef]
- Jung, J.; Choi, H.-S.; Lee, J.-Y.; Ryu, S.; Kim, S.-K.; Hong, M.; Kwak, S.; Kim, H.; Lee, M.-S.; Sung, H.; et al. Outbreak of carbapenemase-producing Enterobacteriaceae associated with a contaminated water dispenser and sink drains in the cardiology units of a Korean hospital. J. Hosp. Infect. 2020, 104, 476–483. [Google Scholar] [CrossRef]
- Leitner, E.; Zarfel, G.; Luxner, J.; Herzog, K.; Pekard-Amenitsch, S.; Hoenigl, M.; Valentin, T.; Feierl, G.; Grisold, A.J.; Högenauer, C.; et al. Contaminated Handwashing Sinks as the Source of a Clonal Outbreak of KPC-2-Producing Klebsiella oxytoca on a Hematology Ward. Antimicrob. Agents Chemother. 2014, 59, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Creamer, E.; Humphreys, H. The contribution of beds to healthcare-associated infection: The importance of adequate decontamination. J. Hosp. Infect. 2008, 69, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Donskey, C.J. Does improving surface cleaning and disinfection reduce health care-associated infections? Am. J. Infect. Control 2013, 41, S12–S19. [Google Scholar] [CrossRef]
- Dancer, S.J.; White, L.F.; Lamb, J.; Girvan, E.K.; Robertson, C. Measuring the effect of enhanced cleaning in a UK hospital: A prospective cross-over study. BMC Med. 2009, 7, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.E. Use of audit tools to evaluate the efficacy of cleaning systems in hospitals. Am. J. Infect. Control 2003, 31, 181–187. [Google Scholar] [CrossRef]
- Eckstein, B.C.; Adams, D.A.; Eckstein, E.C.; Rao, A.; Sethi, A.K.; Yadavalli, G.K.; Donskey, C.J. Reduction of Clostridium Difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect. Dis. 2007, 7, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L.; Dumigan, D.G.; Golebiewski, M.; Balogun, O.; Rizvani, R. Monitoring the Effectiveness of Hospital Cleaning Practices by Use of an Adenosine Triphosphate Bioluminescence Assay. Infect. Control Hosp. Epidemiol. 2009, 30, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Galvin, S.; Dolan, A.; Cahill, O.; Daniels, S.; Humphreys, H. Microbial monitoring of the hospital environment: Why and how? J. Hosp. Infect. 2012, 82, 143–151. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Storr, J.; Donaldson, L. ‘Clean Care is Safer Care’: The Global Patient Safety Challenge 2005–2006. Int. J. Infect. Dis. 2006, 10, 419–424. [Google Scholar] [CrossRef]
- National Infection Prevention and Control Guidelines for Health Care Services in Kenya. 2010. Available online: http://guidelines.health.go.ke:8000/media/infection_control_policy.pdf (accessed on 5 May 2021).
- Gichuhi, A.W.; Kamau, S.M.; Nyangena, E.; Otieno-Ayayo, Z.N. Health Care Workers Adherence to Infection Prevention Practices and Control Measures: A Case of a Level Four District Hospital in Kenya. Am. J. Nurs. Sci. 2015, 4, 39. [Google Scholar] [CrossRef][Green Version]
- Collins, B. The hospital environment: How clean should a hospital be? J. Hosp. Infect. 1988, 11, 53–56. [Google Scholar] [CrossRef]
- Dharan, S.; Mourouga, P.; Copin, P.; Bessmer, G.; Tschanz, B.; Pittet, D. Routine disinfection of patients’ environmental surfaces. Myth or reality? J. Hosp. Infect. 1999, 42, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, G.; Dolinger, A.; Rogo, K.; Mwaura, N.; Wafula, F.; Coarasa, J.; Goicoechea, A.; Das, J. Observations of infection prevention and control practices in primary health care, Kenya. Bull. World Health Organ. 2017, 95, 503–516. [Google Scholar] [CrossRef]
- Vermeil, T.; Peters, A.; Kilpatrick, C.; Pires, D.; Allegranzi, B.; Pittet, D. Hand hygiene in hospitals: Anatomy of a revolution. J. Hosp. Infect. 2019, 101, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Widmer, A.E. No time for handwashing!? Handwashing versus alcoholic rub: Can we afford 100% compliance? Infect. Control Hosp. Epidemiol. 2010, 18, 205–208. [Google Scholar] [CrossRef]
- Davies, S. Fragmented management, hospital contract cleaning and infection control. Policy Polit. 2010, 38, 445–463. [Google Scholar] [CrossRef]
- Deshpande, A.; Cadnum, J.L.; Fertelli, D.; Sitzlar, B.; Thota, P.; Mana, T.S.; Jencson, A.; Alhmidi, H.; Koganti, S.; Donskey, C.J. Are hospital floors an underappreciated reservoir for transmission of health care-associated pathogens? Am. J. Infect. Control 2017, 45, 336–338. [Google Scholar] [CrossRef]
- Scott, E.; Bloomfield, S.F. Investigations of the effectiveness of detergent washing, drying and chemical disinfection on contamination of cleaning cloths. J. Appl. Bacteriol. 1990, 68, 279–283. [Google Scholar] [CrossRef]
- Ayliffe, G.A.J.; Collins, B.J.; Lowbury, E.J.L.; Babb, J.R.; Lilly, H.A. Ward floors and other surfaces as reservoirs of hospital infection. Epidemiol. Infect. 1967, 65, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Pittet, D. Healthcare-Associated Infection in Developing Countries: Simple Solutions to Meet Complex Challenges. Infect. Control Hosp. Epidemiol. 2007, 28, 1323–1327. [Google Scholar] [CrossRef]
- Wesangula, E.; Kamau, R.; Kamau, V.; Kiberenge, F.; Gituku, J. Infection prevention and control—Bridging the knowledge gap among Kenyan health care workers. Int. J. Infect. Dis. 2016, 45, 289. [Google Scholar] [CrossRef][Green Version]
- Guidelines for Environmental Infection Control in Health-Care Facilities. Available online: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf (accessed on 4 April 2021).
| Hospital Infection Control Practice | Total No. of Items or Equipment Sampled (%) (n = 559) | Median Bacterial Loads (IQR) | p-Value |
|---|---|---|---|
| Detergents and/or disinfectants used to clean and/or decontaminate the hospital surfaces | |||
| Bleach only | 248 (44.4) | 9.0 × 104 (1.2 × 104–1.0 × 106) | 0.002 |
| Bleach and Soap | 311 (55.6) | 5.0 × 104 (5.0 × 103–5.0 × 105) | |
| Frequency of cleaning floors daily | |||
| Once | 134 (24.0) | 5.65 × 104 (1.15 × 104–1.3 × 106) | 0.004 |
| Twice | 341 (61.0) | 5.0 × 104 (5.0 × 103–5.0 × 105) | |
| Thrice | 68 (12.2) | 1.0 × 105 (2.0 × 104–1.0 × 106) | |
| Four times | 16 (2.8) | 2.0 × 106 (5.0 × 105–1.0 × 107) | |
| Frequency of cleaning high-touch surfaces daily | |||
| Once | 149 (26.7) | 5.3 × 104 (7.0 × 103–1.0 × 106) | 0.004 |
| Twice | 270 (48.3) | 7.0 × 104 (9.8 × 103–1.0 × 106) | |
| Thrice | 23 (4.1) | 7.1 × 104 (1.4 × 104–8.0 × 105) | |
| Four times | 55 (9.8) | 1.0 × 104 (0–2.4 × 105) | |
| Five times | 42 (7.5) | 1.5 × 105 (3.0 × 104–1.0 × 106) | |
| Rarely | 20 (3.6) | 1.7 × 104 (6.0 × 103–1.0 × 105) | |
| Mops and cleaning cloths stored wet | |||
| Yes | 84 (15.0) | 1.8 × 105 (2.1 × 104–2.0 × 106) | 0.02 |
| No | 475 (85.0) | 5.0 × 104 (6.6 × 103–8.0 × 105) | |
| Availability of running water | |||
| Yes | 462 (82.6) | 6.4 × 104 (9.0 × 103–1.0 × 106) | 0.13 |
| No | 97 (17.4) | 3.7 × 104 (1.7 × 103–7.3 × 105) | |
| Availability of hand wash station in the department | |||
| Yes | 435 (77.8) | 5.0 × 104 (8.8 × 103–1.0 × 106) | 0.94 |
| No | 124 (22.2) | 9.0 × 104 (5.0 × 103–1.0 × 106) | |
| Availability of soap at the handwash stations | |||
| Yes | 377 (67.4) | 8.2 × 104 (1.2 × 104–1.0 × 106) | 0.01 |
| No | 182 (32.6) | 2.8 × 104 (3.0 × 103–6.1 × 105) | |
| Availability of gloves for clinician use | |||
| Yes | 504 (90.2) | 7.0 × 104 (1.0 × 104–1.0 × 106) | <0.001 |
| No | 55 (9.8) | 1.0 × 104 (0–2.4 × 105) | |
| Availability of laboratory coats or gowns clinicians and support staff | |||
| Yes | 404 (72.3) | 6.7 × 104 (1.0 × 104–1.0 × 106) | 0.02 |
| No | 155 (27.7) | 5.0 × 104 (1.6 × 103–5.0 × 105) | |
| Personal protective garments were retained in the facility | |||
| Yes | 94 (16.8) | 5.0 × 104 (1.0 × 103–1.0 × 106) | 0.13 |
| No | 465 (83.2) | 6.3 × 104 (1.0 × 104–1.0 × 106) | |
| Clinician laboratory coats or gowns were washed within the facility | |||
| Yes | 169 (30.2) | 2.1 × 104 (3.5 × 103–5,0 × 105) | 0.01 |
| No | 390 (69.8) | 8.8 × 104 (1.1 × 104–1.0 × 106) | |
| Patient sheets and gowns were washed daily or when soiled | |||
| Yes | 504 (90.2) | 7.0 × 104 (1.0 × 104–1.0 × 106) | <0.001 |
| No | 55 (9.8) | 1.0 × 104 (0–2.4 × 105) | |
| Hospital Storage, Disposal, and Biosafety Practice | Total No. of Items or Equipment Sampled (%) (n = 559) | Median Bacterial Loads (IQR) | p Value |
|---|---|---|---|
| Dirty linen or gowns transported in a designated container | |||
| Yes | 524 (93.7) | 6.3 × 104 (9.0 × 103–1.0 × 106) | 0.03 |
| No | 35 (6.3) | 2.0 × 104 (3.0 × 102–2.2 × 105) | |
| Availability of containers for needle disposal | |||
| Yes | 524 (93.7) | 6.3 × 104 (9.0 × 103–1.0 × 106) | 0.03 |
| No | 35 (6.3) | 2.0 × 104 (3.0 × 102–2.2 × 105) | |
| Biosafety waste collected in designated bins | |||
| Yes | 523 (93.6) | 6.15 × 104 (9.0 × 103–1.0 × 106) | 0.03 |
| No | 36 (6.4) | 3.5 × 104 (3.0 × 102–2.2 × 105) | |
| Biosafety waste removed at least daily from the area | |||
| Yes | 496 (88.7) | 7.0 × 104 (9.0 × 103–1.0 × 106) | 0.03 |
| No | 63 (11.2) | 2.0 × 104 (3.5 × 103–2.8 × 105) | |
| Availability of quarantine or isolation rooms in the department | |||
| Yes | 302 (54.0) | 8.0 × 105 (6.75 × 103–1.0 × 106) | 0.16 |
| No | 257 (46.0) | 4.5 × 104 (9.0 × 103–4.8 × 105) | |
| Beds greater than 1 m apart | |||
| Yes | 92 (16.5) | 1.13 × 105 (2.0 × 104–1.18 × 106) | 0.04 |
| No | 467 (83.5) | 5.0 × 104 (6.0 × 103–8.0 × 105) | |
| The department was well ventilated | |||
| Yes | 337 (60.3) | 7.0 × 104 (9.0 × 103–8.0 × 105) | 0.74 |
| No | 222 (39.7) | 5.0 × 104 (7.0 × 103–1.2 × 106) | |
| Movement of people limited or restricted into the departments | |||
| Yes | 366 (65.5) | 8.0 × 104 (1.0 × 104–1.0 × 106) | 0.01 |
| No | 193 (34.5) | 3.7 × 104 (3.0 × 103–5.0 × 105) | |
| Hospital Infection Control Practices and Environmental Conditions | IRR (95% C.I.) | Standard Error | p-Value |
|---|---|---|---|
| Detergents and/or disinfectants used to clean and/or decontaminate the hospital surfaces | |||
| Bleach | 0.67 (0.19–2.29) | 0.42 | 0.53 |
| Bleach/Soap | Reference | ||
| Frequency of cleaning floors daily | |||
| Once | Reference | ||
| Twice | 0.73 (0.21–2.50) | 0.46 | 0.65 |
| Thrice | 0.04 (0.01–0.10) | 0.02 | <0.001 * |
| Four times | 0.21 (0.07–0.59) | 0.11 | 0.004 * |
| Frequency of cleaning high-touch surfaces daily | |||
| Once | Reference | ||
| Twice | 0.21 (0.06–0.76) | 0.14 | 0.02 * |
| Thrice | 0.11 (0.04–0.32) | 0.06 | <0.001 * |
| Four times | 4.18 (1.55–11.23) | 2.1 | 0.005 # |
| Five times | 0.02 (0.01–0.04) | 0.01 | <0.001 * |
| Rarely | 0.06 (0.02–0.16) | 0.03 | <0.001 * |
| Mops and cleaning cloths stored wet (Yes) | 0.08 (0.02–0.30) | 0.05 | <0.001 * |
| Availability of running water (Yes) | 0.19 (0.08–0.47) | 0.08 | <0.001 * |
| Availability of hand wash station in the department (Yes) | 0.25 (0.07–0.94) | 0.17 | 0.04 * |
| Availability of soap at the hand wash stations (Yes) | 0.21 (0.12–0.39) | 0.06 | <0.001 * |
| Availability of gloves for clinician use (Yes) | 0.10 (0.04–0.24) | 0.04 | 0.001 * |
| Availability of laboratory coats or gowns clinicians and support staff (Yes) | 0.33 (0.10–1.07) | 0.19 | 0.06 |
| Personal protective garments were retained in the facility (Yes) | 5.84 (3.09–11.02) | 1.89 | <0.001 # |
| Clinician laboratory coats or gowns were washed within the facility (Yes) | 2.97 (7.60–11.64) | 2.07 | 0.12 |
| Patient sheets and gowns were washed daily or when soiled (Yes) | 0.10 (0.04–0.24) | 0.04 | 0.001 * |
| Dirty linen or gowns transported in a designated container (Yes) | 72.11 (20.22–257.14) | 46.11 | <0.001 # |
| Availability of containers for needle disposal (Yes) | 74.11 (19.46–282.27) | 50.6 | <0.001 # |
| Biosafety waste collected in designated bins (Yes) | 74.1 (23.70–231.70) | 43.1 | <0.001 # |
| Biosafety waste removed at least daily from the area (Yes) | 71.80 (20.75–248.45) | 45.5 | <0.001 # |
| Availability of quarantine or isolation rooms in the department (Yes) | 6.37 (1.61–25.16) | 4.46 | 0.008 # |
| Beds greater than 1 m apart (Yes) | 0.99 (0.35–2.79) | 0.52 | 1 |
| The department was well ventilated (Yes) | 2.70 (0.50–14.33) | 2.3 | 0.24 |
| Movement of people limited or restricted into the department (Yes) | 0.23 (0.04–1.20) | 0.19 | 0.08 |
| Hospital Infection Control Practices and Environmental Conditions | IRR (95% C.I.) | Robust Standard Error | p-Value |
|---|---|---|---|
| Mops and cleaning cloths stored wet (Yes) | 4.48 (1.84–10.88) | 2.03 | 0.001 # |
| Frequency of cleaning and/or decontaminating the floor per day | |||
| Twice | 1.30 (0.95–1.80) | 0.21 | 0.10 |
| Thrice | 0.40 (0.17–0.90) | 0.17 | 0.02 * |
| Frequency of cleaning and/or decontaminating high-touch surfaces per day | |||
| Thrice | 1.53 (0.97–2.42) | 0.36 | 0.06 |
| Five times | 0.64 (0.46–0.89) | 0.11 | 0.008 * |
| Availability of hand wash station in the department (Yes) | 0.88 (0.64–1.22) | 0.15 | 0.47 |
| Availability of soap at the hand wash station (Yes) | 0.66 (0.50–0.86) | 0.09 | 0.003 * |
| Are clinician laboratory coats or gowns washed within the facility? (Yes) | 1.08 (0.69–1.69) | 0.24 | 0.72 |
| Personal protective garments were retained in the facility (Yes) | 0.63 (0.23–1.72) | 0.32 | 0.37 |
| Biosafety waste collected in designated bins (Yes) | 0.87 (0.46–1.67) | 0.29 | 0.69 |
| Biosafety waste removed at least daily from the department (Yes) | 1.04 (0.61–1.76) | 0.27 | 0.87 |
| Movement of people is limited/restricted into the department (Yes) | 0.98 (0.69–1.37) | 0.16 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odoyo, E.; Matano, D.; Georges, M.; Tiria, F.; Wahome, S.; Kyany’a, C.; Musila, L. Ten Thousand-Fold Higher than Acceptable Bacterial Loads Detected in Kenyan Hospital Environments: Targeted Approaches to Reduce Contamination Levels. Int. J. Environ. Res. Public Health 2021, 18, 6810. https://doi.org/10.3390/ijerph18136810
Odoyo E, Matano D, Georges M, Tiria F, Wahome S, Kyany’a C, Musila L. Ten Thousand-Fold Higher than Acceptable Bacterial Loads Detected in Kenyan Hospital Environments: Targeted Approaches to Reduce Contamination Levels. International Journal of Environmental Research and Public Health. 2021; 18(13):6810. https://doi.org/10.3390/ijerph18136810
Chicago/Turabian StyleOdoyo, Erick, Daniel Matano, Martin Georges, Fredrick Tiria, Samuel Wahome, Cecilia Kyany’a, and Lillian Musila. 2021. "Ten Thousand-Fold Higher than Acceptable Bacterial Loads Detected in Kenyan Hospital Environments: Targeted Approaches to Reduce Contamination Levels" International Journal of Environmental Research and Public Health 18, no. 13: 6810. https://doi.org/10.3390/ijerph18136810
APA StyleOdoyo, E., Matano, D., Georges, M., Tiria, F., Wahome, S., Kyany’a, C., & Musila, L. (2021). Ten Thousand-Fold Higher than Acceptable Bacterial Loads Detected in Kenyan Hospital Environments: Targeted Approaches to Reduce Contamination Levels. International Journal of Environmental Research and Public Health, 18(13), 6810. https://doi.org/10.3390/ijerph18136810






