Effects of Functional Training on Sarcopenia in Elderly Women in the Presence or Absence of ACE Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Functional Training Program
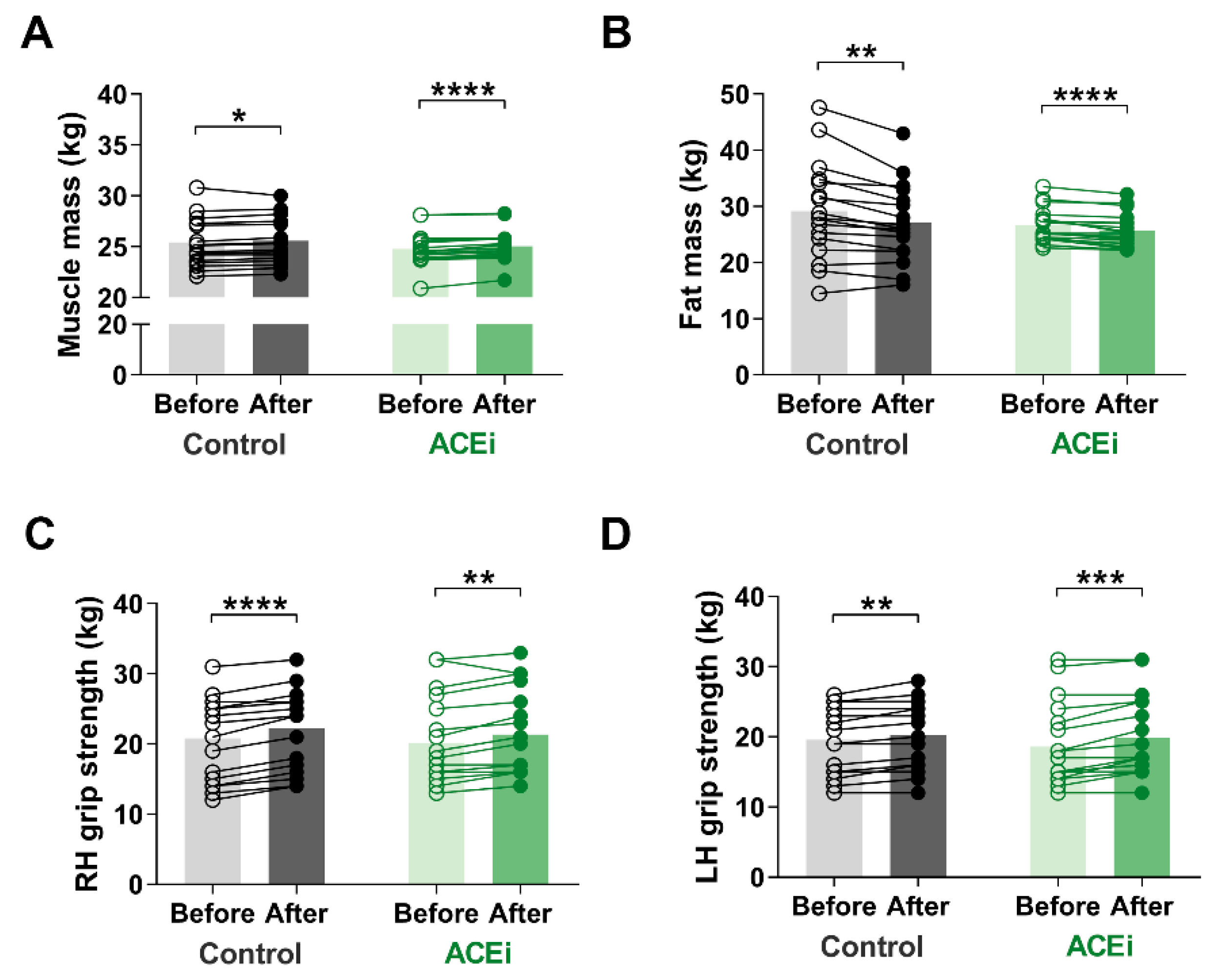
2.3. Body Composition Assessment
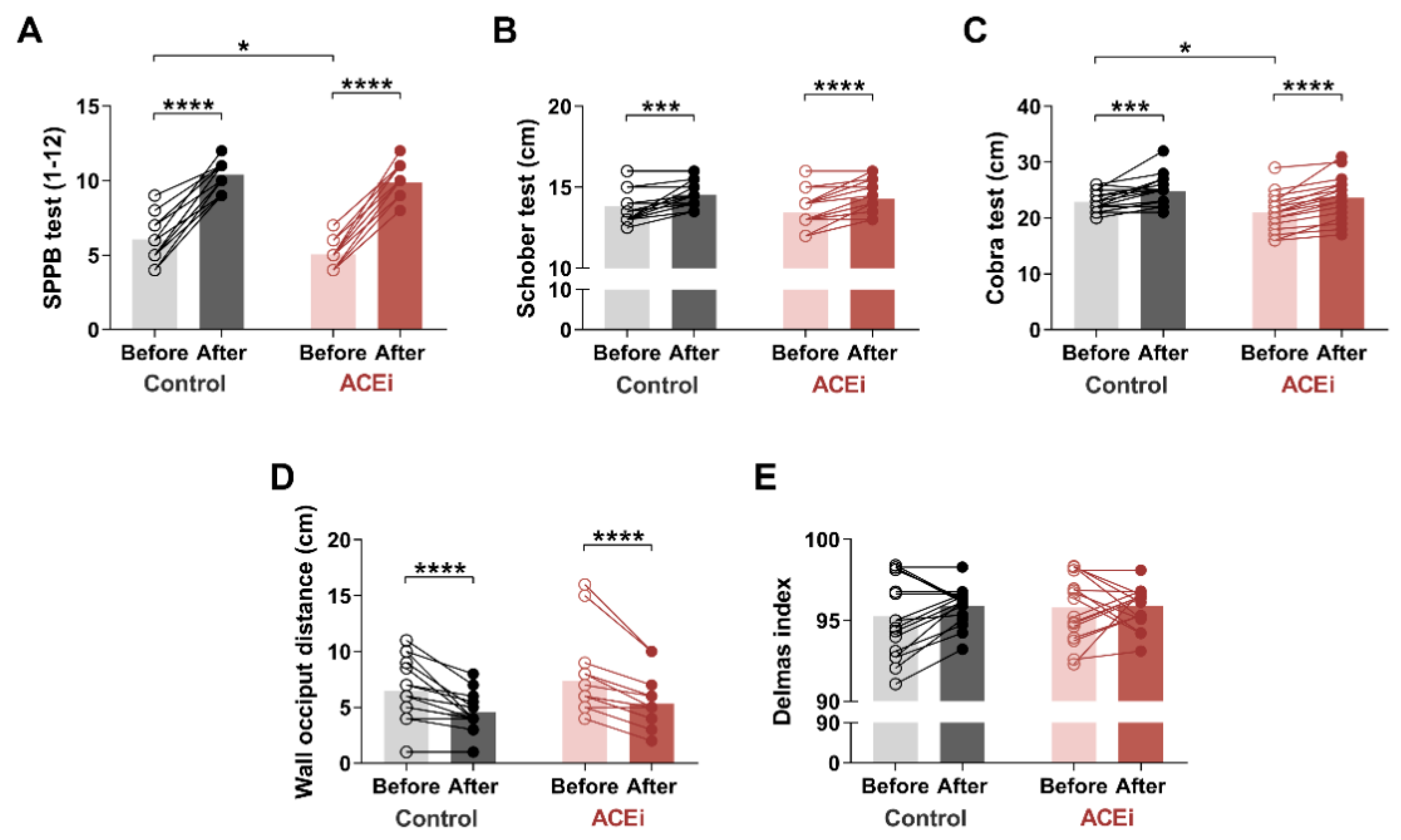
2.4. Short Physical Performance Battery Test
2.5. Handgrip Strength Measurement
2.6. Posture Characterization Delmas Index
2.7. Cobra Test
2.8. Occiput Wall Distance Test
2.9. Schober Test
2.10. Statistical Analyses
3. Results
3.1. The Effects of Six-Month Training Program on Body Weight and Body Mass Index (BMI) in Elderly Females with Sarcopenia
3.2. Six-Month Training Program Improves Muscle Mobility and Posture in Elderly Females with Sarcopenia
3.3. Six-Month Training Program Enhances Muscle Mass and Function in Elderly Females with Sarcopenia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Band, M.M.; Sumukadas, D.; Struthers, A.D.; Avenell, A.; Donnan, P.T.; Kemp, P.R.; Smith, K.T.; Hume, C.L.; Hapca, A.; Witham, M.D. Leucine and ACE inhibitors as therapies for sarcopenia (LACE trial): Study protocol for a randomised controlled trial. Trials 2018, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Spira, D.; Walston, J.; Buchmann, N.; Nikolov, J.; Demuth, I.; Steinhagen-Thiessen, E.; Eckardt, R.; Norman, K. Angiotensin-converting enzyme inhibitors and parameters of sarcopenia: Relation to muscle mass, strength and function: Data from the Berlin Aging Study-II (BASE-II). Drugs Aging 2016, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Sumukadas, D.; Witham, M.D.; Struthers, A.D.; McMurdo, M.E. Effect of perindopril on physical function in elderly people with functional impairment: A randomized controlled trial. CMAJ 2007, 177, 867–874. [Google Scholar] [CrossRef]
- Di Bari, M.; van de Poll-Franse, L.V.; Onder, G.; Kritchevsky, S.B.; Newman, A.; Harris, T.B.; Williamson, J.D.; Marchionni, N.; Pahor, M.; Health, A.; et al. Antihypertensive medications and differences in muscle mass in older persons: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004, 52, 961–966. [Google Scholar]
- De Spiegeleer, A.; Beckwee, D.; Bautmans, I.; Petrovic, M.; The Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Drugs Aging 2018, 35, 719–734. [Google Scholar] [CrossRef]
- Gaedtke, A.; Morat, T. TRX Suspension Training: A New Functional Training Approach for Older Adults—Development, Training Control and Feasibility. Int. J. Exerc. Sci. 2015, 8, 224–233. [Google Scholar]
- Young, K.J.; Je, C.W.; Hwa, S.T. Effect of proprioceptive neuromuscular facilitation integration pattern and swiss ball training on pain and balance in elderly patients with chronic back pain. J. Phys. Ther. Sci. 2015, 27, 3237–3240. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Seeman, T.E.; Tinetti, M.E.; Nevitt, M.C.; Berkman, L.F. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 1994, 6, 410–419. [Google Scholar] [CrossRef]
- Lee, S.H.; Gong, H.S. Measurement and Interpretation of Handgrip Strength for Research on Sarcopenia and Osteoporosis. J. Bone Metab. 2020, 27, 85–96. [Google Scholar] [CrossRef]
- Clarkson, H.M. Musculoskeletal Assessment: Joint Range of Motion and Manual Muscle Strength; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Antonelli-Incalzi, R.; Pedone, C.; Cesari, M.; Di Iorio, A.; Bandinelli, S.; Ferrucci, L. Relationship between the occiput-wall distance and physical performance in the elderly: A cross sectional study. Aging Clin. Exp. Res. 2007, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Chale, A.; Cloutier, G.J.; Hau, C.; Phillips, E.M.; Dallal, G.E.; Fielding, R.A. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.C.; van Loon, L.J. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef]
- Yoon, D.H.; Lee, J.Y.; Song, W. Effects of resistance exercise training on cognitive function and physical performance in cognitive frailty: A randomized controlled trial. J. Nutr. Health Aging 2018, 22, 944–951. [Google Scholar] [CrossRef]
- Stoever, K.; Heber, A.; Eichberg, S.; Brixius, K. Influences of resistance training on physical function in older, obese men and women with sarcopenia. J. Geriatr. Phys. Ther. 2018, 41, 20–27. [Google Scholar] [CrossRef]
- Moriyama, Y.; Hara, M.; Aratani, S.; Ishikawa, H.; Kono, K.; Tamaki, M. The association between six month intra-dialytic resistance training and muscle strength or physical performance in patients with maintenance hemodialysis: A multicenter retrospective observational study. BMC Nephrol. 2019, 20, 172. [Google Scholar] [CrossRef]
- Dhillon, R.J.; Hasni, S. Pathogenesis and management of sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Balogun, S.; Winzenberg, T.; Wills, K.; Scott, D.; Jones, G.; Aitken, D.; Callisaya, M.L. Prospective associations of low muscle mass and function with 10-year falls risk, incident fracture and mortality in community-dwelling older adults. J. Nutr. Health Aging 2017, 21, 843–848. [Google Scholar] [CrossRef]
- Visser, M.; Simonsick, E.M.; Colbert, L.H.; Brach, J.; Rubin, S.M.; Kritchevsky, S.B.; Newman, A.B.; Harris, T.B.; Health ABC Study. Type and intensity of activity and risk of mobility limitation: The mediating role of muscle parameters. J. Am. Geriatr. Soc. 2005, 53, 762–770. [Google Scholar] [CrossRef]
- Sumukadas, D.; Band, M.; Miller, S.; Cvoro, V.; Witham, M.; Struthers, A.; McConnachie, A.; Lloyd, S.M.; McMurdo, M. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.R.; Humphries, S.E.; Montgomery, H.E. The ACE I/D polymorphism and human physical performance. Trends Endocrinol. Metab. 2000, 11, 416–420. [Google Scholar] [CrossRef]
- Coelho, A.V.; Probst, S.V.; Nogari, M.V.; Teixeira, C.D.; Felcar, M.J.; Santos, C.D.; Gomes, M.V.M.; Andraus, C.A.R.; Fernandes, P.B.K. Angiotensin-II blockage, muscle strength, and exercise capacity in physically independent older adults. J. Phys. Ther. Sci. 2016, 28, 547–552. [Google Scholar] [CrossRef]
- Springer, J.; von Haehling, S. ACE inhibitors and sarcopenia: Covering all the BASEs? Drugs Aging 2016, 33, 839–840. [Google Scholar] [CrossRef]
- Onder, G.; Vedova, D.C.; Pahor, M. Effects of ACE inhibitors on skeletal muscle. Curr. Pharm. Des. 2006, 12, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Papp, G.; Szabó, K.; Jámbor, I.; Mile, M.; Berki, R.A.; Arany, A.C.; Makra, G.; Szodoray, P.; Csiki, Z.; Balogh, L. Regular exercise may restore certain age-related alterations of adaptive immunity and rebalance immune regulation. Front. Immunol. 2021, 16, 12. [Google Scholar]
- Buford, T.W.; Manini, T.M.; Hsu, F.C.; Cesari, M.; Anton, S.D.; Nayfield, S.; Stafford, R.S.; Church, T.S.; Pahor, M.; Carter, C.S. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J. Am. Geriatr. Soc. 2012, 60, 1244–1252. [Google Scholar] [CrossRef]
| Schedule | Exercises | Intensity Level 1 (Month 1–3) | Intensity Level 2 (Month 4–6) |
|---|---|---|---|
| 15 min | warm-up with treadmill/elliptic trainer/bicycle | 50% HRmax | 55% HRmax |
| 20 min | TRX squat | Three sets of 12–15 reps | - |
| TRX single leg squat | - | Three sets of 10 reps per foot | |
| TRX low rows | Three sets of 12–15 reps (range 10–45°) | - | |
| TRX single arm low rows | - | Three sets of 10 reps per arm (range 10–45°) | |
| TRX push up | Three sets of 12–15 reps (range 10–45°) | - | |
| TRX push up on one leg | - | Three sets of 10 reps per leg (range 10–45°) | |
| TRX standing hip drop | Three sets of 10 reps | Three sets of 15 reps | |
| 10 min | Fitball exercises | in pairs with professional aid | Individually |
| 10 min | Stretching | within the normal range of motion | within the maximum range of motion |
| Control Group | Before Training | After Training | p-Values |
| Body weight (kg) | 72.09 ± 9.14 | 71.81 ± 8.76 | n.s. |
| Height (cm) | 163.35 ± 5.27 | 163.35 ± 5.27 | n.s. |
| Body Mass Index (BMI) | 26.96 ± 2.63 | 26.85 ± 2.45 | n.s. |
| ACEi-Treated Group | Before Training | After Training | p-Values |
| Body weight [kg] | 71.08 ± 7.51 | 70.69 ± 7.27 | 0.014 |
| Height [cm] | 164.17 ± 6.34 | 164.17 ± 6.34 | n.s. |
| BMI | 26.33 ± 2.63 | 26.20 ± 1.70 | 0.029 |
| Control Group | Before Training | After Training | p-Values |
| Standing balance (score) | 2 (1.5–2.0) | 4 (3.0–4.0) | <0.0001 |
| Walk (score) | 1 (1.0–2.0) | 3 (3.0–3.5) | <0.0001 |
| Chair stands (score) | 3 (2.0–3.0) | 4 (3.5–4.0) | 0.0002 |
| SPPB (score) | 6 (5.0–7.0) | 11 (9.5–11.0) | <0.0001 |
| ACEi-Treated Group | Before Training | After Training | p-Values |
| Standing balance [score] | 1 (1.0–2.0) | 3.5 (3.0–4.0) | <0.0001 |
| Walk [score] | 1 (1.0–2.0) | 3 (2.0–4.0) | <0.0001 |
| Chair stands [score] | 2 (2.0–3.0) | 3 (3.0–4.0) | <0.0001 |
| SPPB [score] | 5 (4.0–5.25) | 10 (9.0–11.0) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mile, M.; Balogh, L.; Papp, G.; Pucsok, J.M.; Szabó, K.; Barna, L.; Csiki, Z.; Lekli, I. Effects of Functional Training on Sarcopenia in Elderly Women in the Presence or Absence of ACE Inhibitors. Int. J. Environ. Res. Public Health 2021, 18, 6594. https://doi.org/10.3390/ijerph18126594
Mile M, Balogh L, Papp G, Pucsok JM, Szabó K, Barna L, Csiki Z, Lekli I. Effects of Functional Training on Sarcopenia in Elderly Women in the Presence or Absence of ACE Inhibitors. International Journal of Environmental Research and Public Health. 2021; 18(12):6594. https://doi.org/10.3390/ijerph18126594
Chicago/Turabian StyleMile, Marianna, László Balogh, Gábor Papp, József Márton Pucsok, Krisztina Szabó, Lilla Barna, Zoltán Csiki, and István Lekli. 2021. "Effects of Functional Training on Sarcopenia in Elderly Women in the Presence or Absence of ACE Inhibitors" International Journal of Environmental Research and Public Health 18, no. 12: 6594. https://doi.org/10.3390/ijerph18126594
APA StyleMile, M., Balogh, L., Papp, G., Pucsok, J. M., Szabó, K., Barna, L., Csiki, Z., & Lekli, I. (2021). Effects of Functional Training on Sarcopenia in Elderly Women in the Presence or Absence of ACE Inhibitors. International Journal of Environmental Research and Public Health, 18(12), 6594. https://doi.org/10.3390/ijerph18126594






