A Systematic Review and Meta-Analysis of the Efficacy of Evening Primrose Oil for Mastalgia Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Trial Selection
2.4. Data Extraction
2.5. Assessment of Risk of Bias
2.6. Statistical Analysis
2.7. Grading Quality of Evidence
2.8. Patient and Public Involvement
3. Results
3.1. Trial Selection
3.2. Characteristics of the Trials
3.3. Participants
3.4. Interventions
3.5. Outcomes
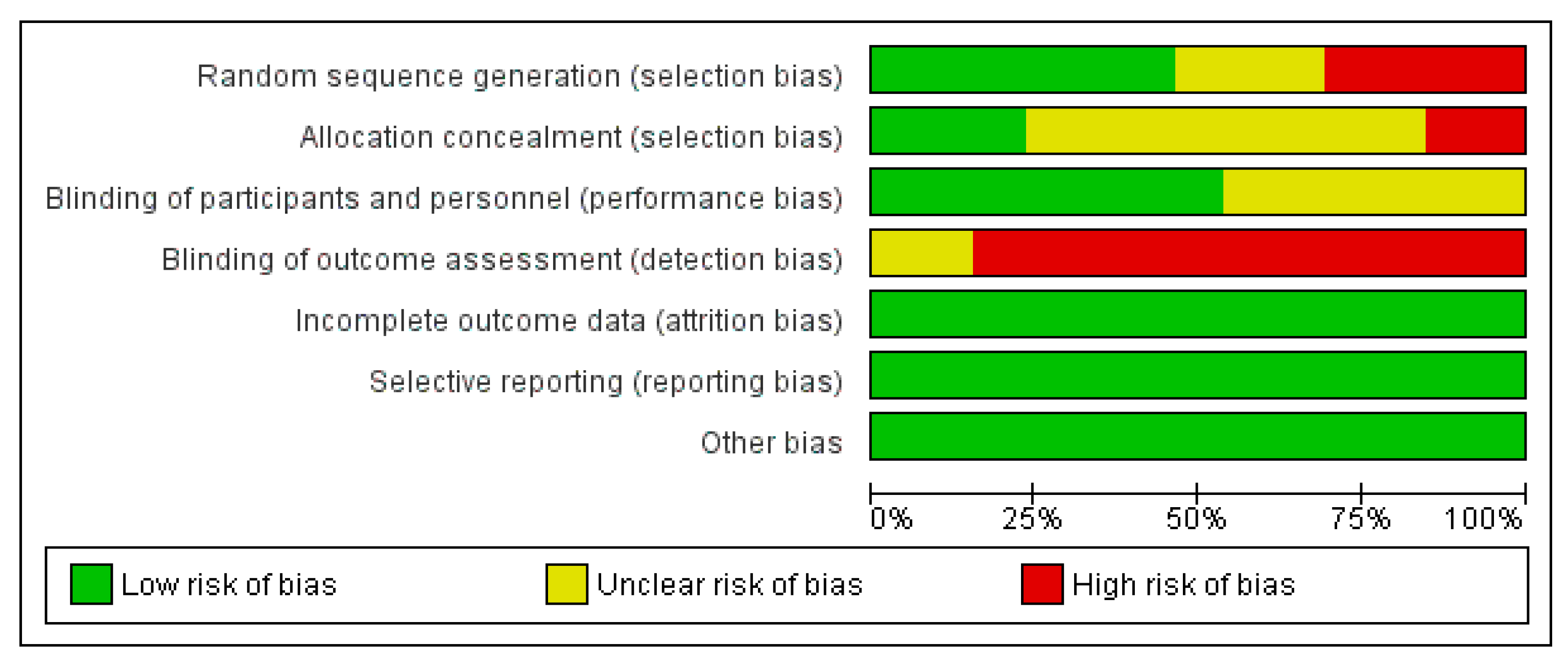
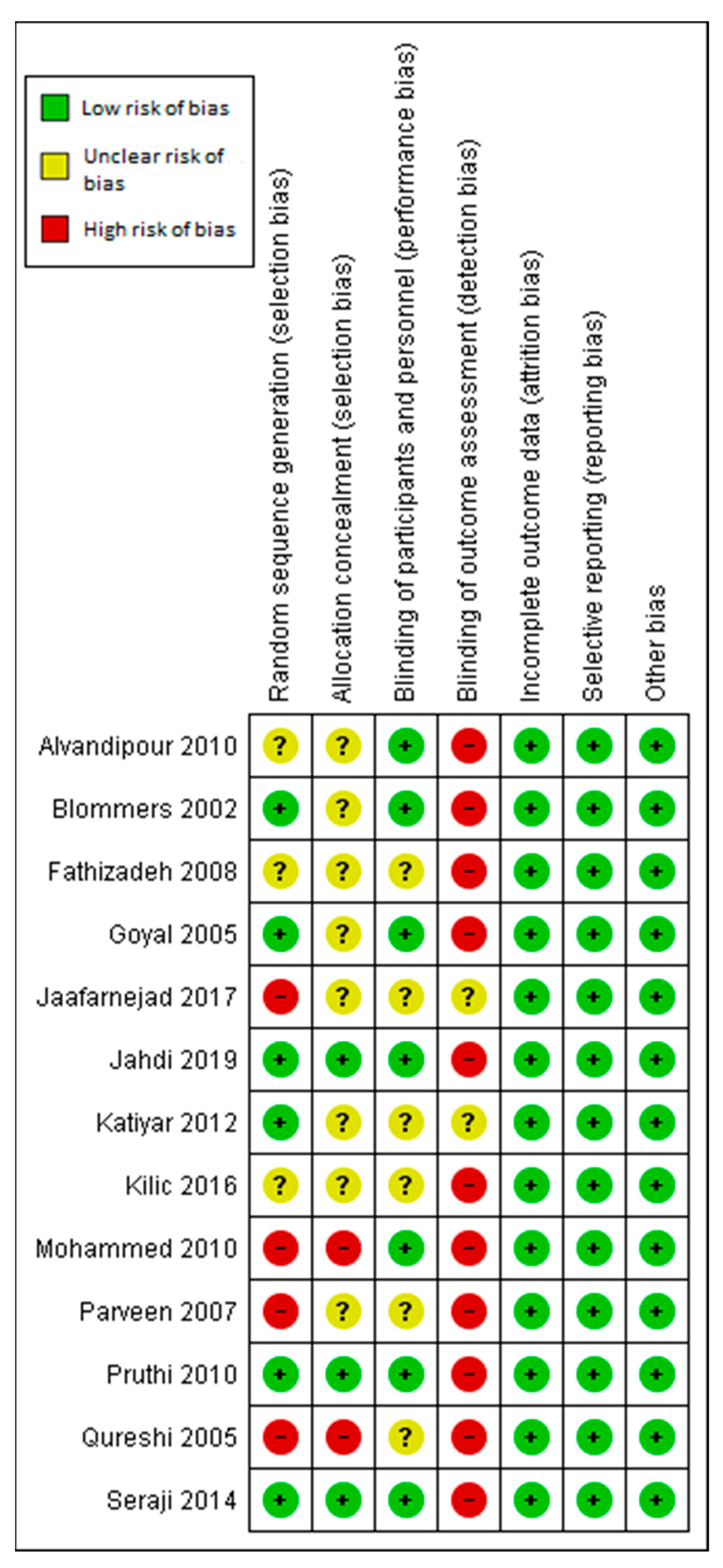
3.6. Assessment of the Risk of Bias
3.6.1. Allocation
3.6.2. Blinding
3.6.3. Incomplete Outcome Data
3.6.4. Selective Reporting
3.6.5. Other Potential Sources of Bias
3.7. Clinical Outcomes
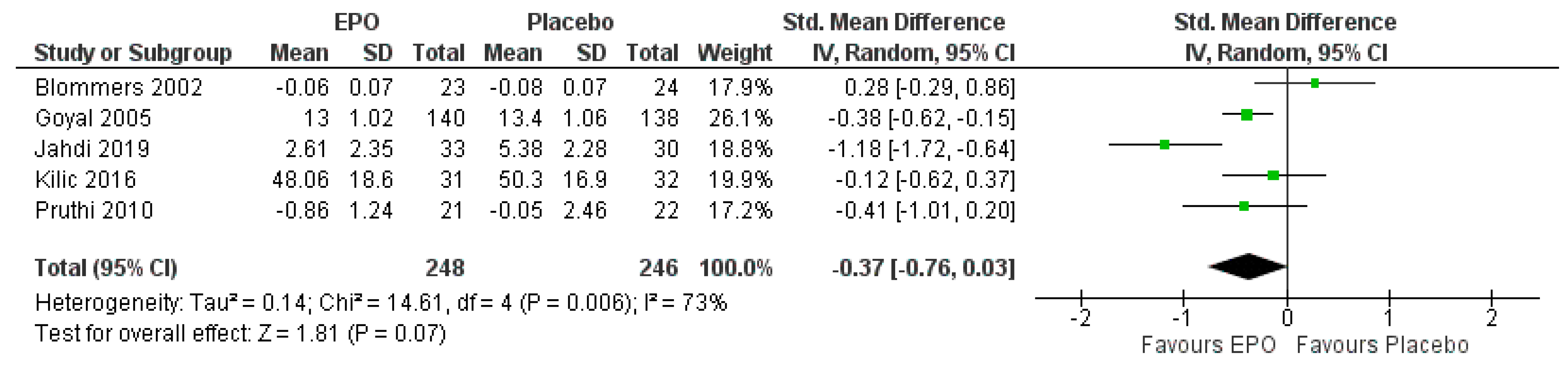
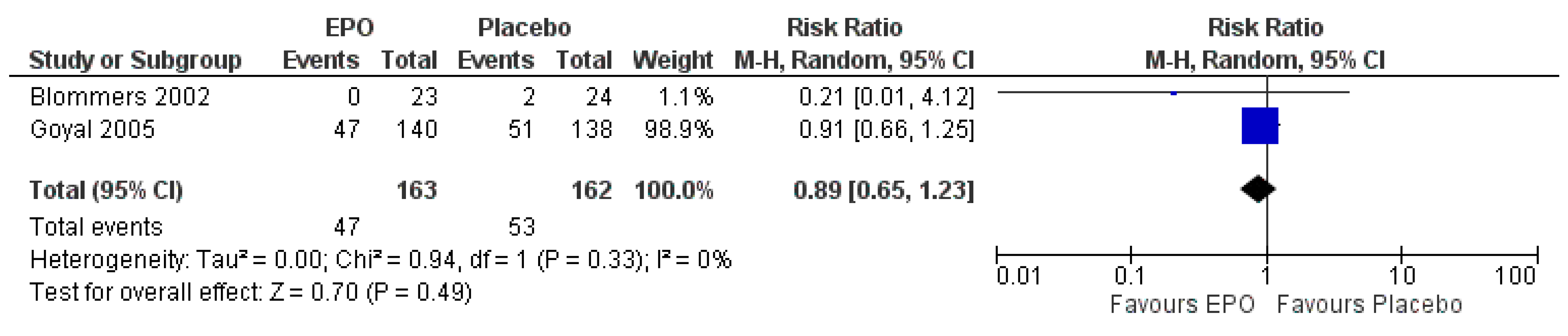
3.7.1. Comparison between EPO and the Placebo
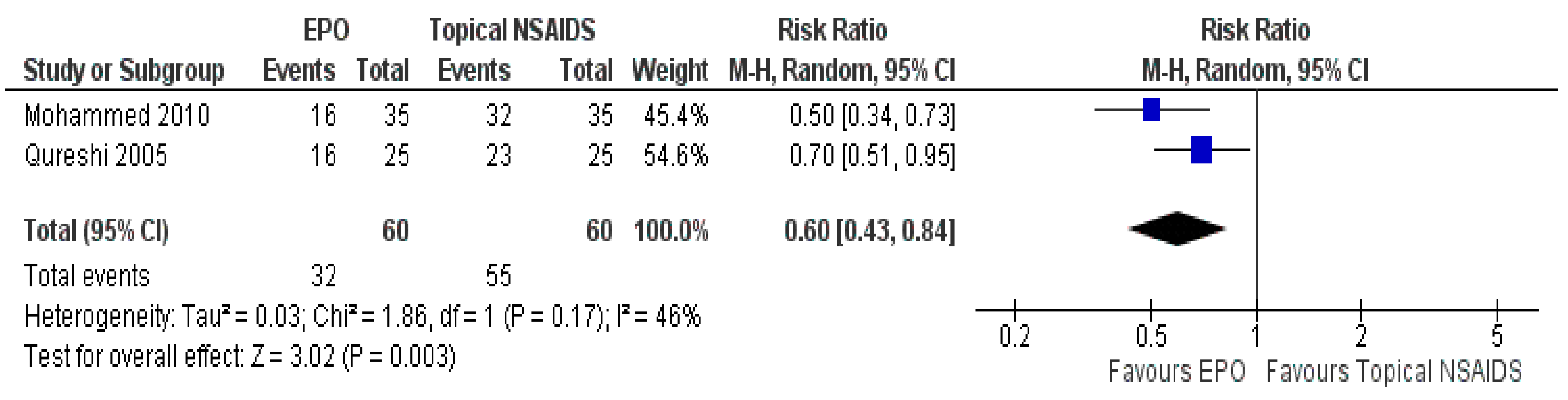
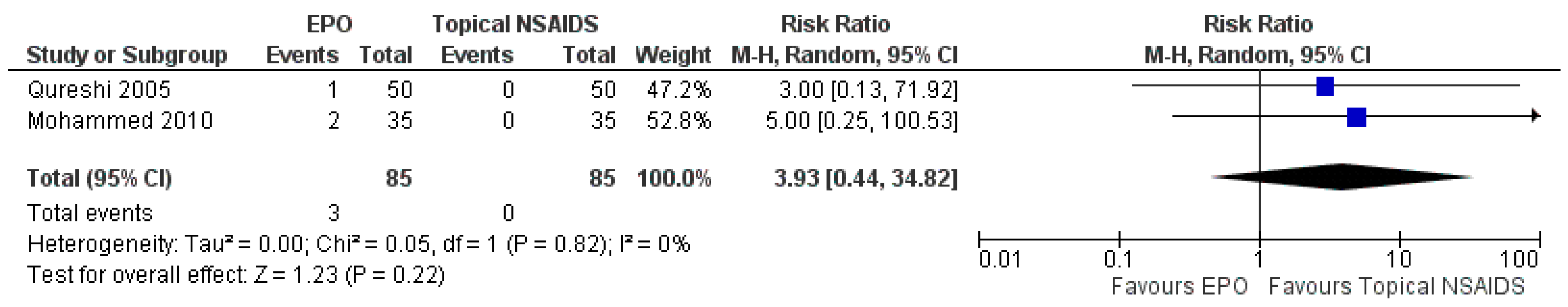
3.7.2. Comparison between EPO and Topical NSAIDs
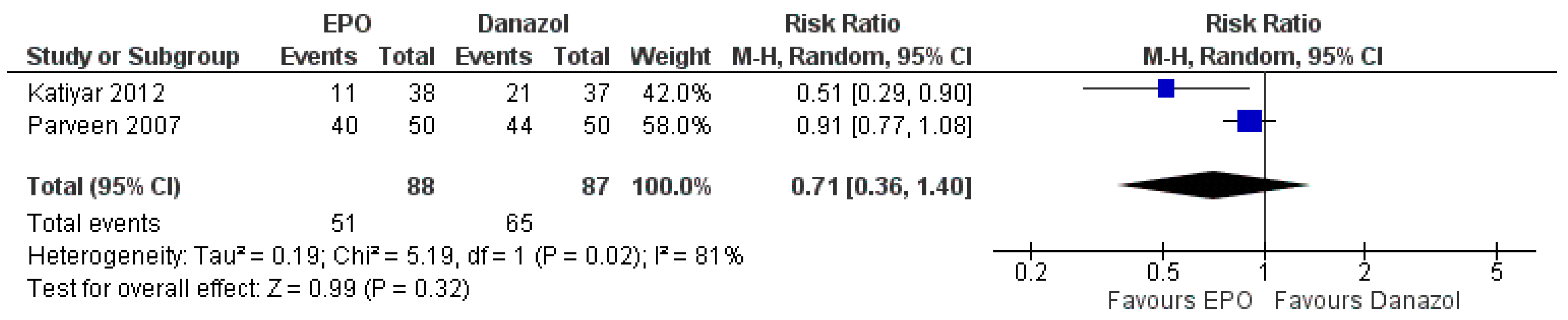
3.7.3. Comparison between EPO and Danazol
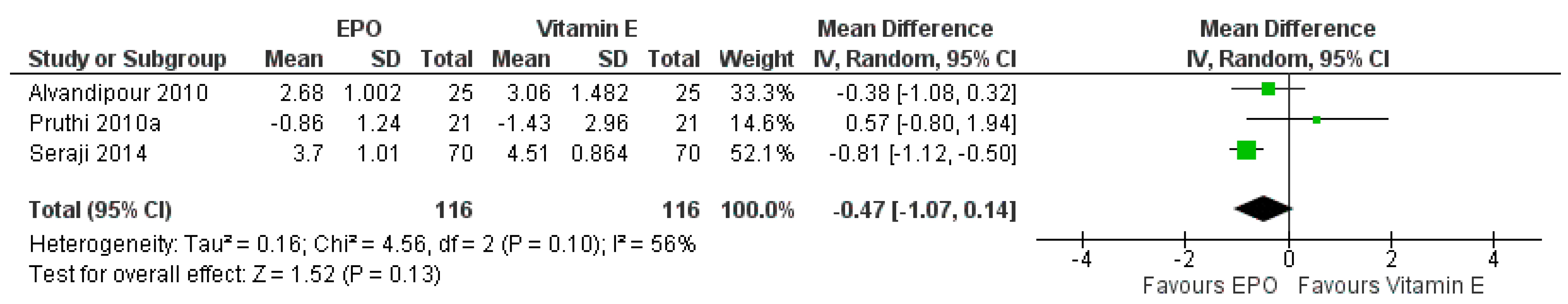
3.7.4. Comparison between EPO and Vitamin E
4. Discussion
4.1. Summary of the Main Result
4.2. Overall Completeness and Applicability of the Evidence
4.3. Quality of the Evidence
4.4. Potential Biases in the Review Process
4.5. Agreements and Disagreements with Other Studies or Reviews
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Millet, A.V.; Dirbas, F.M. Clinical management of breast pain: A review. Obstet. Gynecol. Surv. 2002, 57, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Mansel, R.E.; Goyal, A.; Preece, P.; Leinster, S.; Maddox, P.R.; Gateley, C.; Kubista, E.; von Fournier, D. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am. J. Obstet. Gynecol. 2004, 191, 1942–1949. [Google Scholar] [CrossRef]
- Santen, R.J.; Mansel, R. Benign breast disorders. N. Engl. J. Med. 2005, 353, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ader, D.N.; Browne, M.W. Prevalence and impact of cyclic mastalgia in a United States clinic-based sample. Am. J. Obstet. Gynecol. 1997, 177, 126–132. [Google Scholar] [CrossRef]
- Rosolowich, V.; Saettler, E.; Szuck, B.; Breast Disease, C. Mastalgia. J. Obstet. Gynaecol. Can. 2006, 28, 49–57. [Google Scholar] [CrossRef]
- Pruthi, S.; Wahner-Roedler, D.L.; Torkelson, C.J.; Cha, S.S.; Thicke, L.S.; Hazelton, J.H.; Bauer, B.A. Vitamin E and Evening Primrose Oil for management of cyclical mastalgia: A randomized Pilot Study. Altern. Med. Rev. 2010, 15, 59–67. [Google Scholar] [PubMed]
- Gautam, S.; Srivastava, A.; Kataria, K.; Dhar, A.; Ranjan, P.; Kumar, J. New Breast Pain Chart for Objective Record of Mastalgia. Indian J. Surg. 2016, 78, 245–248. [Google Scholar] [CrossRef]
- Eren, T.; Aslan, A.; Ozemir, I.A.; Baysal, H.; Sagiroglu, J.; Ekinci, O.; Alimoglu, O. Factors Effecting Mastalgia. Breast Care (Basel) 2016, 11, 188–193. [Google Scholar] [CrossRef]
- Wisbey, J.R.; Kumar, S.; Mansel, R.E.; Peece, P.E.; Pye, J.K.; Hughes, L.E. Natural history of breast pain. Lancet 1983, 2, 672–674. [Google Scholar] [CrossRef]
- Mansel, R.E.; Webster, D.J.T.; Sweetland, H.M. Breast Pain and Nodularity; Elsevier: Amsterdam, The Netherlands, 2009; p. 365. [Google Scholar]
- Gregory, P.L.; Biswas, A.C.; Batt, M.E. Musculoskeletal problems of the chest wall in athletes. Sports Med. 2002, 32, 235–250. [Google Scholar] [CrossRef]
- Kumar, S.; Mansel, R.E.; Scanlon, M.F.; Hughes, L.E.; Edwards, C.A.; Woodhead, J.S.; Newcombe, R.G. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br. J. Surg. 1984, 71, 870–873. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Chapkin, R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F.; Manku, M.S. Premenstrual syndrome and premenstrual breast pain (cyclical mastalgia): Disorders of essential fatty acid (EFA) metabolism. Prostaglandins Leukot. Essent. Fat. Acids 1989, 37, 255–261. [Google Scholar] [CrossRef]
- Bamford, J.T.; Gibson, R.W.; Renier, C.M. Atopic eczema unresponsive to evening primrose oil (linoleic and gamma-linolenic acids). J. Am. Acad. Dermatol. 1985, 13, 959–965. [Google Scholar] [CrossRef]
- Barber, A.J. Evening primrose oil: A panacea. Pharm. J. 1988, 240, 723–725. [Google Scholar]
- Higgins, J.P.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Updated July 2019; The Cochrane Collaboration: London, UK, 2019. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Br. Med. J. 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Stewart, J.C.M.; Royle, G.T.; Taylor, I. A randomized controlled trial of evening primrose oil for mastalgia. In Recent Advances in the Study of Benign Breast Disease: The Proceeding of the 4rd International Breast Syymposium; Mansel, R.E., Ed.; The Parthenon Publishing Group: London, UK, 1992; pp. 89–96. [Google Scholar]
- Pye, J.K.; Mansel, R.E.; Hughes, L.E. Clinical experience of drug treatment of mastalgia. Lancet 1985, 326, 373–377. [Google Scholar] [CrossRef]
- Preece, P.E.; Hanslip, J.I.; Gilbert, L.; Walker, D.; Pashby, N.L. Evening Primrose Oil (Efamol) for Mastalgia; Eden Press: Montreal, QC, Canada, 1982; pp. 147–154. [Google Scholar]
- Sharma, N.; Gupta, A.; Jha, P.K.; Rajput, P. Mastalgia cured. Randomized trial comparing centchroman to evening primrose oil. Breast J. 2012, 18, 509–510. [Google Scholar] [CrossRef]
- Qureshi, S.; Sultan, N. Topical nonsteroidal anti-inflammatory drugs versus oil of evening primrose in the treatment of mastalgia. R. Coll. Surg. Edinb. Irel. 2005, 3, 7–10. [Google Scholar] [CrossRef]
- Jafarnezhad, F.; Moghaddam, E.A.; Emami, S.A. Compare the effect of flaxseed, evening primrose oil and vitamin E on duration of periodic breast pain. J. Educ. Health Promot. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Blommers, J.; de Lange-De Klerk, E.S.; Kuik, D.J.; Bezemer, P.D.; Meijer, S. Evening primrose oil and fish oil for severe chronic mastalgia: A randomized, double-blind, controlled trial. Am. J. Obstet. Gynecol. 2002, 187, 1389–1394. [Google Scholar] [CrossRef]
- Kılıç, M.Ö.; Sen, M.; Içen, D. The comparison of evening primrose oil, fructus agni casti, and reassurance in the treatment of mastalgia. Int. J. Surg. Med. 2016, 2, 83–88. [Google Scholar] [CrossRef]
- Parveen, S.; Sarwar, G.; Ali, M.; Channa, G.A. Danazol versus oil of evening primrose in the treatment of mastalgia. Pak. J. Surg. 2007, 23, 10–13. [Google Scholar]
- Goyal, A.; Mansel, R.E. A Randomized Multicentre Study of Gamolenic Acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005, 11, 41–47. [Google Scholar] [CrossRef]
- Mohammed, M.K. Non-hormonal treatment for mastalgia- a topical non-steroidal anti-inflammatory agent versus Evening Primrose Oil: Prospective comparative analysis. Iraqi J. Community Med. 2010, 4, 282–286. [Google Scholar]
- Fathizadeh, N.; Takfallah, L.; Ehsanpour, S.; Namnabati, M.; Askari, S. Effects of evening primrose oil and vitamin E on the severity of periodical breast pain. Iran. J. Nurs. Midwifery Res. 2009, 13, 90–93. [Google Scholar]
- Alvandipour, M.; Tayebi, P.; Alizadeh Navaie, R.; Khodabakhshi, H. Comparison between effect of evening primrose oil and vitamin E in treatment of cyclic mastalgia. J. Babol Univ. Med Sci. 2011, 13, 7–11. [Google Scholar]
- Jahdi, F.; Tolouei, R.; Neisani Samani, L.; Hashemian, M.; Haghani, H.; Mojab, F.; Memarzadeh, M. Effect of Evening Primrose Oil and Vitamin B6 on Pain Control of Cyclic Mastalgia Associated with Fibrocystic Breast Changes: A Triple-Blind Randomized Controlled Trial. Shiraz E-Med. J. 2018, 20, e81243. [Google Scholar] [CrossRef]
- Seraji, A.; Salehi, A.; Momeni, H.; Kerami, A.; Naeimi, N. The effects of evening primrose and vitex agnus on pain scale of the women with cyclic mastalgia a clinical trial. Complementary Med. J. 2014, 3, 639–653. [Google Scholar]
- Katiyar, S.; Nigam, S.K.; Omar, P. Clinocopathological profile of Mastalgia in an around Kanpur. J. Evol. Med. Dent. Sci. 2012, 1, 565–569. [Google Scholar]
- Sarayloo, K.; Najmabadi, K.M.; Ghazanfarpour, M. Effects of the evening primrose oil on women’s mastalgia: A systemic review of randomized controlled trials. Malays. J. Nurs. 2017, 9, 28–35. [Google Scholar]
- Kataria, K.; Dhar, A.; Srivastava, A.; Kumar, S.; Goyal, A. A systematic review of current understanding and management of mastalgia. Indian J. Surg. 2014, 76, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Mansel, R.E.; Arvind, N.; Prasad, K.; Dhar, A.; Chabra, A. Evidence-based management of Mastalgia: A meta-analysis of randomised trials. Breast 2007, 16, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Mehra Golshan, A.B.C.; Chen, W. Breast Pain. 2020. Available online: https://www.uptodate.com/contents/breast-pain?search=breast%20pain&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H11 (accessed on 8 February 2021).
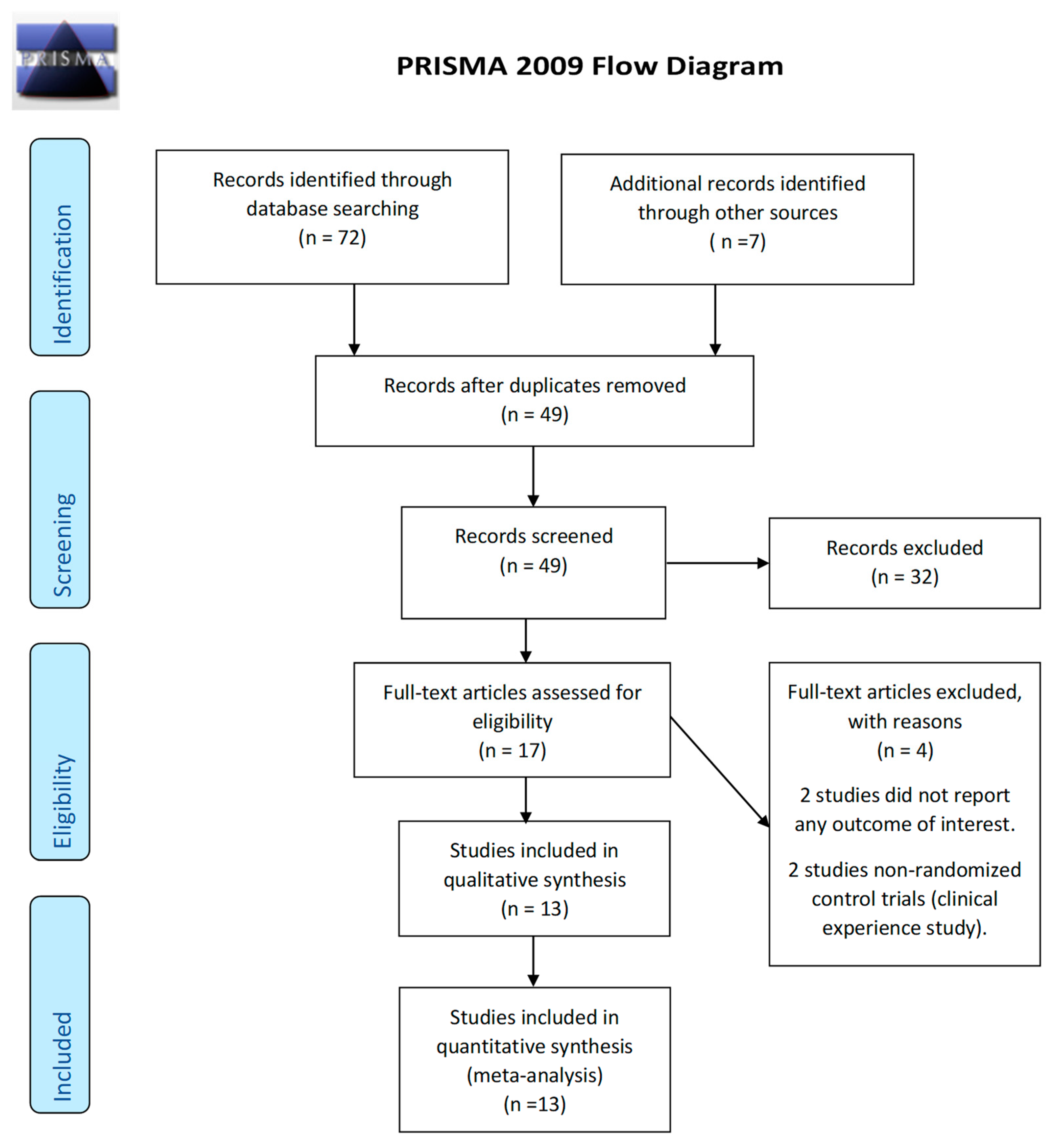
| Studies | Setting | Size, n | Age | Evening Primrose Oil Dose (g)/Day | Comparison Dose/Day | Time of Treatment (Months) | Outcome |
|---|---|---|---|---|---|---|---|
| Karachi, Pakistan | 50 | 15–50 | 1 | Topical NSAIDs (Piroxicam gel 0.5%) | 3 |
|
| Karachi, Pakistan | 100 | 15–50 | 1 | Danazol; 200 mg | 3 |
|
| Mashad, Iran | 90 | 18–45 | 1 | Vitamin E; 400 IU | 2 |
|
| Amsterdam, The Netherlands | 120 | 18–45 | 3 | Corn oil plus wheat-germ oil; 3 g | 6 |
|
| Ankara, Turkey | 128 | 18–60 | 1 | Starch tablet; 20 mg | 3 |
|
| Minnesota, USA | 85 | 19–56 | 3 | Corn oil; 6 g | 6 |
|
| Ghaemshar, Iran | 100 | 18–50 | 2 | Vitamin E; 400 IU | 6 |
|
| Kanpur, India | 80 | 15–55 | 3 | Danazol; 200 mg | 2 |
|
| Isfahan, Iran | 66 | 18–40 | 3 | Vitamin E; 1800 mg | 2 |
|
| Cardiff, UK | 555 | 18–55 | 4 | Coconut oil; 500 mg | 12 |
|
| Baghdad, Iraq | 70 | 17–48 | 3 | Topical NSAIDs | 3 |
|
| Tehran, Iran | 94 | 18–50 | 2 | Placebo capsule | 3 |
|
| Arak, Iran | 214 | 18–50 | 2 | Vitamin E; 400 IU | 2 |
|
| EPO Compared to Placebo for Mastalgia Treatment | ||||||
|---|---|---|---|---|---|---|
| Patient or Population: Women with Mastalgia Setting: Outpatient Clinic Intervention: Evening Primrose Oil Comparison: Placebo | ||||||
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | No of Participants (Studies) | Certainty of the Evidence (Grade) | Comments | |
| Risk with Placebo | Risk with EPO | |||||
| The severity of pain | The mean severity of pain was 0 | SMD 0.37 lower (0.76 lower to 0.03 higher) | - | 525 (5 RCTs) | ⊕⊕⊕⊕ HIGH | Risk of bias: not serious |
| Inconsistency: not serious | ||||||
| Indirectness: not serious | ||||||
| Imprecision: not serious | ||||||
| The number of patients responding to treatment | Study population | RR 1.26 (0.86 to 1.84) | 63 (1 RCT) | ⊕⊕⊝⊝ LOW | Risk of bias: not serious | |
| 563 per 1000 | 709 per 1000 (484 to 1000) | Inconsistency: not serious | ||||
| Indirectness: not serious | ||||||
| Imprecision: not serious | ||||||
| The occurrence of adverse events | Study population | RR 0.89 (0.65 to 1.23) | 325 (2 RCTs) | ⊕⊕⊕⊝ MODERATE | Risk of bias: not serious | |
| 327 per 1000 | 291 per 1000 (213 to 402) | Inconsistency: not serious | ||||
| Indirectness: serious | ||||||
| Imprecision: | ||||||
| not serious | ||||||
| Quality of life | The mean quality of life was 0 | MD 4 higher (6.52 lower to 14.52 higher) | - | 63 (1 RCT) | ⊕⊕⊝⊝ LOW | Risk of bias: not serious |
| Inconsistency: serious | ||||||
| Indirectness: not serious | ||||||
| Imprecision: | ||||||
| serious | ||||||
| EPO Compared to Topical NSAIDs for Mastalgia Treatment | ||||||
|---|---|---|---|---|---|---|
| Patient or Population: Women with Mastalgia Setting: Outpatient Clinic Intervention: Evening Primrose Oil Comparison: Topical NSAIDs | ||||||
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | No of Participants (Studies) | Certainty of the Evidence (Grade) | Comments | |
| Risk with Topical NSAIDs | Risk with EPO | |||||
| The number of patients responding to treatment | Study population | RR 0.60 (0.43 to 0.84) | 120 (2 RCTs) | ⊕⊕⊕⊕ HIGH | Risk of bias: not serious | |
| 917 per 1000 | 550 per 1000 (394 to 770) | Inconsistency: not serious | ||||
| Indirectness: not serious | ||||||
| Imprecision: not serious | ||||||
| The occurrence of adverse events | Study population | RR 3.93 (0.44 to 34.82) | 170 (2 RCTs) | ⊕⊕⊕⊕ HIGH | Risk of bias: not serious | |
| 0 per 1000 | 0 per 1000 (0 to 0) | Inconsistency: not serious | ||||
| Indirectness: not serious | ||||||
| Imprecision: not serious | ||||||
| EPO Compared to Danazol for Mastalgia Treatment | ||||||
|---|---|---|---|---|---|---|
| Patient or Population: Women with Mastalgia Setting: Outpatient Clinic Intervention: Evening Primrose Oil Comparison: Danazol | ||||||
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | No of Participants (Studies) | Certainty of the Evidence (Grade) | Comments | |
| Risk with Danazol | Risk with Comparison EPO | |||||
| The number of patients responded to treatment | Study population | RR 0.71 (0.36 to 1.40) | 175 (2 RCTs) | ⊕⊕⊕⊝ MODERATE | Risk of bias: not serious | |
| 747 per 1000 | 530 per 1000 (269 to 1000) | Inconsistency: serious | ||||
| Indirectness: not serious | ||||||
| Imprecision: not serious | ||||||
| The occurrence of adverse events | Study population | RR 0.38 (0.16 to 0.88) | 100 (1 RCT) | ⊕⊕⊝⊝ LOW | Risk of bias: not serious | |
| 320 per 1000 | 122 per 1000 (51 to 282) | Inconsistency: not serious | ||||
| Indirectness: not serious | ||||||
| Imprecision: serious | ||||||
| EPO Compared to Vitamin E for Mastalgia Treatment | ||||||
|---|---|---|---|---|---|---|
| Patient or Population: Women with Mastalgia Setting: Outpatient Clinic Intervention: Evening Primrose Oil Comparison: Vitamin E | ||||||
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | No Of Participants (Studies) | Certainty of the Evidence (Grade) | Comments | |
| Risk with Vitamin E | Risk with EPO | |||||
| The severity of pain | The mean severity of pain was 0 | MD 0.47 lower (1.07 lower to 0.14 higher) | - | 305 (3 RCTs) | ⊕⊕⊕⊝ MODERATE | Risk of bias: not serious |
| Inconsistency: not serious | ||||||
| Indirectness: not serious | ||||||
| Imprecision: serious | ||||||
| The number of patients responded to treatment | Study population | RR 2.30 (1.19 to 4.43) | 61 (1 RCT) | ⊕⊕⊝⊝ LOW | Risk of bias: not serious | |
| 267 per 1000 | 613 per 1000 (317 to 1000) | Inconsistency: not serious | ||||
| Indirectness: not serious | ||||||
| Imprecision: not serious | ||||||
| The occurrence of adverse events | Study population | RR 3.21 (0.14 to 75.61) | 58 (1 RCT) | ⊕⊕⊝⊝ LOW | Risk of bias: not serious | |
| 0 per 1000 | 0 per 1000 (0 to 0) | Inconsistency: not serious | ||||
| Indirectness: not serious | ||||||
| Imprecision: not serious | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Adni, L.L.; Norhayati, M.N.; Mohd Rosli, R.R.; Muhammad, J. A Systematic Review and Meta-Analysis of the Efficacy of Evening Primrose Oil for Mastalgia Treatment. Int. J. Environ. Res. Public Health 2021, 18, 6295. https://doi.org/10.3390/ijerph18126295
Ahmad Adni LL, Norhayati MN, Mohd Rosli RR, Muhammad J. A Systematic Review and Meta-Analysis of the Efficacy of Evening Primrose Oil for Mastalgia Treatment. International Journal of Environmental Research and Public Health. 2021; 18(12):6295. https://doi.org/10.3390/ijerph18126295
Chicago/Turabian StyleAhmad Adni, Lina Liana, Mohd Noor Norhayati, Ritzzaleena Rosli Mohd Rosli, and Juliawati Muhammad. 2021. "A Systematic Review and Meta-Analysis of the Efficacy of Evening Primrose Oil for Mastalgia Treatment" International Journal of Environmental Research and Public Health 18, no. 12: 6295. https://doi.org/10.3390/ijerph18126295
APA StyleAhmad Adni, L. L., Norhayati, M. N., Mohd Rosli, R. R., & Muhammad, J. (2021). A Systematic Review and Meta-Analysis of the Efficacy of Evening Primrose Oil for Mastalgia Treatment. International Journal of Environmental Research and Public Health, 18(12), 6295. https://doi.org/10.3390/ijerph18126295






