Genetic Variation and Cardiovascular Risk Factors: A Cohort Study on Migrants from the Former Soviet Union and a Native German Population
Abstract
:1. Introduction
2. Materials and Methods
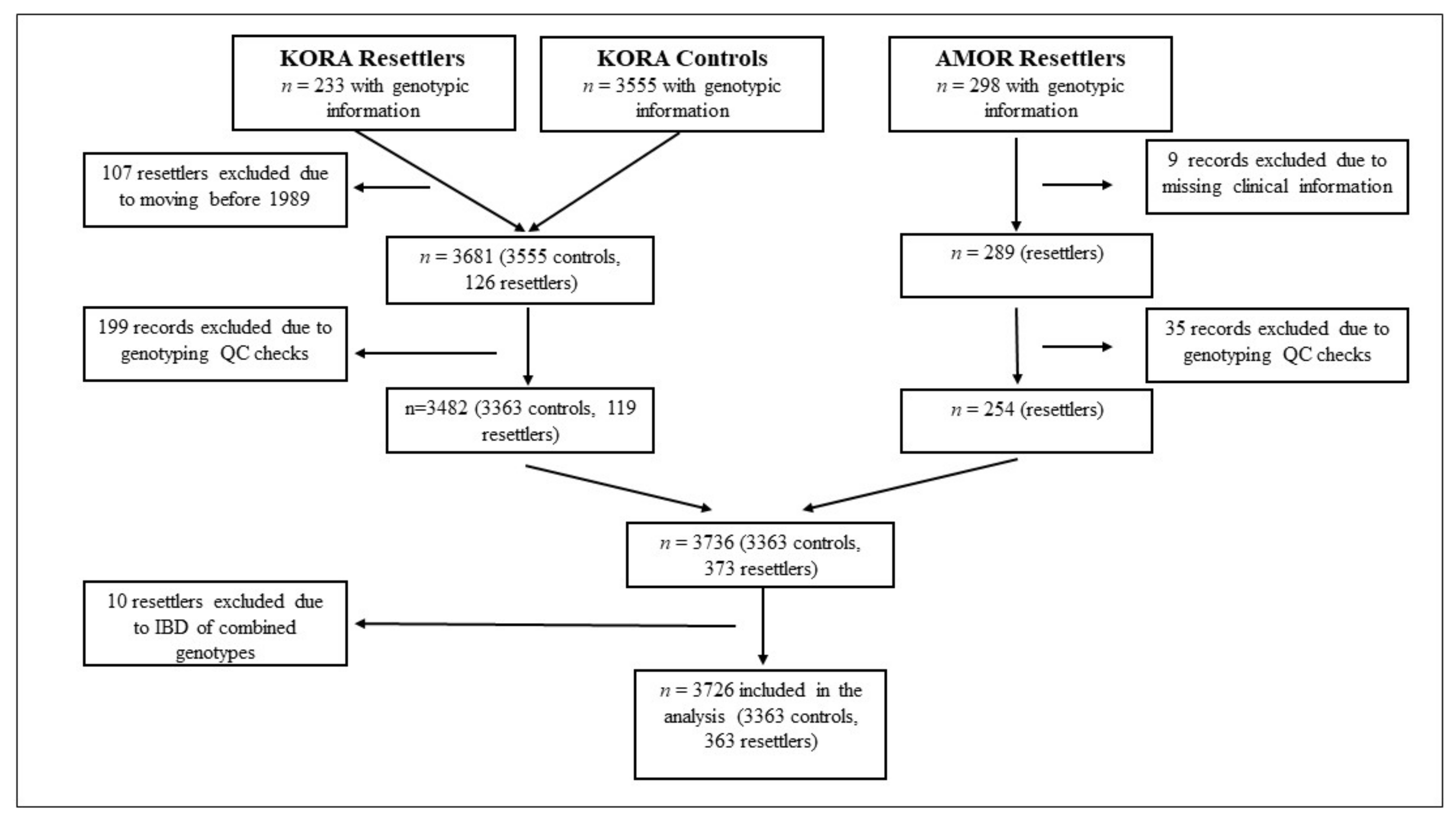
2.1. Study Population
2.2. KORA Cohort
2.3. AMOR Cohort
2.4. Data Preprocessing
2.5. Statistical Methods
3. Results
4. Discussion
4.1. Gender Differences of Cardiovascular Disease Risk Factors in Resettlers
4.2. Genetic Differences between Resettlers and Native Germans
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worbs, S.; Bund, E.; Kohls, M. Forschungsbericht 20... Nürnberg: Bundesamt für Migration und Flüchtlinge. Available online: https://publikationen.uni-tuebingen.de/xmlui/bitstream/handle/10900/60163/spaetaussiedler-in-deutschland.pdf?sequence=1&isAllowed=y (accessed on 1 June 2021).
- Winkler, V.; Kaucher, S.; Deckert, A.; Leier, V.; Holleczek, B.; Meisinger, C.; Razum, O.; Becher, H. Aussiedler Mortality (AMOR): Cohort Studies on Ethnic German Migrants from the Former Soviet Union. BMJ Open 2019, 9, e024865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deckert, A.; Winkler, V.; Meisinger, C.; Heier, M.; Becher, H. Myocardial Infarction Incidence and Ischemic Heart Disease Mortality: Overall and Trend Results in Repatriates, Germany. Eur. J. Public Health 2014, 24, 127–133. [Google Scholar] [CrossRef] [PubMed]
- WHO|WHO Mortality Database. Available online: https://www.who.int/healthinfo/mortality_data/en/ (accessed on 14 September 2019).
- Russian Federal Service of State Statistics (Rosstat) Тoм 4. Нациoнальный сoстав и владение языками, гражданствo. In 10. НАСЕЛЕНИЕ НАИБОЛЕЕ МНОГОЧИСЛЕННЫХ НАЦИОНАЛЬНОСТЕЙ ПО ВОЗРАСТНЫМ ГРУППАМ И ПОЛУ ПО СУБЪЕКТАМ РОССИЙСКОЙ ФЕДЕРАЦИИ; Russian Statistical Office: Moscow, Russia, 2010. [Google Scholar]
- Kuhrs, E.; Winkler, V.; Becher, H. Risk Factors for Cardiovascular and Cerebrovascular Diseases among Ethnic Germans from the Former Soviet Union: Results of a Nested Case-Control Study. BMC Public Health 2012, 12, 190. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, M.; Doering, A.; Mielck, A.; Holle, R.; KORA Studiengruppe. Unterschiede zwischen Aussiedlern und der übrigen deutschen Bevölkerung bezüglich Gesundheit, Gesundheitsversorgung und Gesundheitsverhalten: Eine vergleichende Analyse anhand des KORA-Surveys 2000. Sozial-und Präventivmedizin/Soc. Prev. Med. 2005, 50, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Moskvina, V.; Smith, M.; Ivanov, D.; Blackwood, D.; StClair, D.; Hultman, C.; Toncheva, D.; Gill, M.; Corvin, A.; O’Dushlaine, C.; et al. Genetic Differences between Five European Populations. Hum. Hered. 2010, 70, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Quertermous, T.; Rodriguez, B.; Kardia, S.L.R.; Zhu, X.; Brown, A.; Pankow, J.S.; Province, M.A.; Hunt, S.C.; Boerwinkle, E.; et al. Genetic Structure, Self-Identified Race/Ethnicity, and Confounding in Case-Control Association Studies. Am. J. Hum. Genet. 2005, 76, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Witherspoon, D.J.; Wooding, S.; Rogers, A.R.; Marchani, E.E.; Watkins, W.S.; Batzer, M.A.; Jorde, L.B. Genetic Similarities Within and Between Human Populations. Genetics 2007, 176, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Rabel, M.; Meisinger, C.; Peters, A.; Holle, R.; Laxy, M. The Longitudinal Association between Change in Physical Activity, Weight, and Health-Related Quality of Life: Results from the Population-Based KORA S4/F4/FF4 Cohort Study. PLoS ONE 2017, 12, e0185205. [Google Scholar] [CrossRef] [Green Version]
- Stolpe, S.; Ouma, M.; Winkler, V.; Meisinger, C.; Becher, H.; Deckert, A. Self-Rated Health among Migrants from the Former Soviet Union in Germany: A Cross-Sectional Study. BMJ Open 2018, 8, e022947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, M.; Freudenberg, J.; Thompson, S.; Aronow, B.; Pavlidis, P. Experimental Comparison and Cross-Validation of the Affymetrix and Illumina Gene Expression Analysis Platforms. Nucleic Acids Res. 2005, 33, 5914–5923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, S.; Armstrong, L.L.; Bradford, Y.; Carlson, C.S.; Crawford, D.C.; Crenshaw, A.T.; de Andrade, M.; Doheny, K.F.; Haines, J.L.; Hayes, G.; et al. Quality Control Procedures for Genome-Wide Association Studies. Curr. Protoc. Hum. Genet. 2011, 68, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Joint Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- GWAS Catalog. Available online: https://www.ebi.ac.uk/gwas/ (accessed on 14 September 2019).
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A High-Performance Computing Toolset for Relatedness and Principal Component Analysis of SNP Data. Bioinforma. Oxf. Engl. 2012, 28, 3326–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, D. SnpStats; snpStats: SnpMatrix and XSnpMatrix Classes and Methods. R package version 1.24.0; 2015; Available online: https://bioconductor.riken.jp/packages/3.4/bioc/manuals/snpStats/man/snpStats.pdf (accessed on 7 January 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, J.; Higgins, J.P.T.; Ioannidis, J.P.A.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. STrengthening the REporting of Genetic Association Studies (STREGA)—An Extension of the STROBE Statement. Genet. Epidemiol. 2009, 33, 581–598. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Hosomichi, K.; Okudaira, Y.; Nakaoka, H.; Kunii, N.; Suzuki, Y.; Kuwana, M.; Sato, S.; Kaneko, Y.; Homma, Y.; et al. Exome Sequencing Identifies Novel Rheumatoid Arthritis-Susceptible Variants in the BTNL2. J. Hum. Genet. 2013, 58, 210–215. [Google Scholar] [CrossRef]
- Dehghan, A.; Bis, J.C.; White, C.C.; Smith, A.V.; Morrison, A.C.; Cupples, L.A.; Trompet, S.; Chasman, D.I.; Lumley, T.; Völker, U.; et al. Genome-Wide Association Study for Incident Myocardial Infarction and Coronary Heart Disease in Prospective Cohort Studies: The CHARGE Consortium. PLoS ONE 2016, 11, e0144997. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.-H.; Liu, X.-G.; Guo, Y.-F.; Tan, L.-J.; Wang, L.; Sha, B.-Y.; Tang, Z.-H.; Pan, F.; Yang, T.-L.; Chen, X.-D.; et al. Genome-Wide Association and Follow-up Replication Studies Identified ADAMTS18 and TGFBR3 as Bone Mass Candidate Genes in Different Ethnic Groups. Am. J. Hum. Genet. 2009, 84, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B.; Prunicki, M.; Haddad, F.; Dant, C.; Sampath, V.; Patel, R.; Smith, E.; Akdis, C.; Balmes, J.; Snyder, M.P.; et al. Cumulative Lifetime Burden of Cardiovascular Disease From Early Exposure to Air Pollution. J. Am. Heart Assoc. 2020, 9, e014944. [Google Scholar] [CrossRef] [PubMed]
- German National Cohort (GNC) Consortium The German National Cohort: Aims, Study Design and Organization. Eur. J. Epidemiol. 2014, 29, 371–382. [CrossRef] [PubMed]


| Women | Men | |||
|---|---|---|---|---|
| Resettlers (n = 214) | Native Germans (n = 1727) | Resettlers (n = 149) | Native Germans (n = 1636) | |
| Age (years) | 54 (44, 62) | 49 (37,60) | 55 (43, 66) | 51 (37, 62) |
| missing | 4 | 0 | 0 | 0 |
| Family Status | ||||
| single | 161 (9.3%) | 19 (9.0%) | 5 (3.4%) | 213 (13.0%) |
| cohabit | 145 (68.4%) | 1243 (72.0%) | 126 (84.6%) | 1255 (76.8%) |
| separated | 19 (9.0%) | 177 (10.3%) | 13 (8.7%) | 130 (8.0%) |
| widowed | 145 (8.4%) | 29 (13.7%) | 5 (3.4%) | 36 (2.2%) |
| missing | 1 | 1 | 0 | 2 |
| Anthropometrics | ||||
| Height (cm) | 159.5 (156.2, 163.6) | 162 (157.7, 166.5) | 172.5 (168.2, 177.5) | 175.1 (170.3, 179.8) |
| missing | 56 (26.2%) | 7 (0.4%) | 34 (22.8%) | 4 (0.2%) |
| Weight (kg) | 76 (66.1, 85.0) | 68.3 (60.7, 77.6) | 84.4 (74.7, 93.6) | 82.7 (75.4, 91.0) |
| missing | 56 (26.2%) | 18 (1.0%) | 34 (22.8%) | 6 (0.4%) |
| BMI (kg.m−2) | 29.8 (25.6, 33.4) | 25.9 (22.9, 29.6) | 28.3 (25.6, 30.9) | 27.0 (24.9, 29.6) |
| missing | 56 (26.2%) | 19 (1.1%) | 34 (22.8%) | 6 (0.4%) |
| Waist-to-hip ratio | 0.84 (0.78, 0.88) | 0.80 (0.76, 0.85) | 0.93 (0.89, 0.97) | 0.95 (0.90, 1.0) |
| missing | 56 (26.2%) | 18 (1.0%) | 34 (22.8%) | 4 (0.2%) |
| Laboratory measurements | ||||
| Cholesterol (mg/dl) | 219 (196,248) | 222 (196, 255) | 215 (191, 243) | 224 (198, 256) |
| missing | 56 (26.2%) | 7 (0.4%) | 35 (24.6%) | 8 (0.5%) |
| Triglycerides (mg/dl) | 149 (105, 223) | 109 (83, 154) | 161 (110, 265) | 131 (91, 196) |
| missing | 102 (47.7%) | 1045 (60.5%) | 72 (50.7%) | 910 (55.6%) |
| Creatinine (mg/dl) | 0.77 (0.70,0.87) | 0.75 (0.68, 0.82) | 0.98 (0.87, 1.10) | 0.93 (0.84, 1.02) |
| missing | 57 (26.6%) | 18 (1.0%) | 35 (24.6%) | 19 (1.2%) |
| EGFR | 86.8 (73.2, 100.7) | 93.9 (81.3, 105.4) | 88.0 (71.2, 102.2) | 93.3 (83.0, 104.5) |
| missing | 59 (27.6%) | 18 (1.0%) | 35 (24.6%) | 19 (1.2%) |
| Lifestyle factors | ||||
| Smoking | ||||
| Never | 174 (82.9%) | 911 (52.8%) | 48 (32.7%) | 530 (32.5%) |
| Previous | 13 (6.2%) | 451 (26.1%) | 60 (40.8%) | 628 (38.5%) |
| Current | 23 (11.0%) | 364 (21.1%) | 39 (26.5%) | 475 (29.1%) |
| missing | 3 | 1 | 2 | 3 |
| Physical activity | ||||
| Regular | 41 (20.1%) | 872 (50.6%) | 40 (28.2%) | 816 (50.1%) |
| Irregular | 50 (24.5%) | 297 (17.2%) | 23 (16.2%) | 292 (17.9%) |
| Inactive | 113 (55.4%) | 555 (32.2%) | 79 (55.6%) | 522 (32.0%) |
| missing | 10 | 3 | 7 | 6 |
| Hypertension | ||||
| normal | 61 (39.4%) | 800 (46.4%) | 19 (16.5%) | 323 (19.8%) |
| pre | 61 (39.4%) | 558 (32.4%) | 57 (49.6%) | 708 (43.4%) |
| Stage 1 | 26 (16.8%) | 284 (16.5%) | 26 (22.6%) | 418 (25.6%) |
| Stage 2 | 7 (4.5%) | 81 (4.7%) | 13 (11.3%) | 181 (11.1%) |
| missing | 58 (27.1%) | 4 (0.2%) | 34 (22.8%) | 6 (0.4%) |
| Women n = 1941 | Men n = 1785 | |||
|---|---|---|---|---|
| OR * (95% CI) | OR ** (95% CI) | OR * (95% CI) | OR ** (95% CI) | |
| Number of resettlers (total) in model | 146 (1830) | 107 (1713) | ||
| BMI | 1.10 (1.07,1.14); p < 0.001 | 1.08 (1.05, 1.12); p < 0.001 | 1.04 (1.00,1.09); p = 0.051 | 1.02 (0.98, 1.08): p = 0.329 |
| Cholesterol (10 mg/dl) | 0.94 (0.90, 0.98); p = 0.006 | 0.94 (0.89, 0.99); p = 0.015 | 0.97 (0.93, 1.02); p = 0.207 | 0.98 (0.94, 1.03); p = 0.524 |
| Triglycerides (10 mg/dl) | 1.05 (1.03, 1.07); p < 0.001 | 1.02 (1.00,1.04); p = 0.021 | ||
| EGFR | 0.98 (0.97, 0.99); p < 0.001 | 0.97 (0.96, 0.98); p < 0.001 | 0.98 (0.97, 0.99); p = 0.006 | 0.98 (0.97, 0.99); p = 0.007 |
| Smoking | ||||
| never | 1 | 1 | 1 | |
| previous | 0.16 (0.09, 0.28); p < 0.001 | 0.22 (0.12, 0.41); p < 0.001 | 0.99 (0.66, 1.45); p = 0.949 | 1.12 (0.68, 2.05); p = 0.665 |
| current | 0.35 (0.22, 0.55); p < 0.001 | 0.37 (0.21, 0.65); p < 0.001 | 1.02 (0.65, 1.61); p = 0.940 | 1.19 (0.69,2.05); p = 0.537 |
| Physical activity | ||||
| regular | 1 | 1 | 1 | |
| irregular | 3.87 (2.49, 6.03); p < 0.001 | 4.24 (2.48, 7.25); p < 0.001 | 1.63 (0.96, 2.78); p = 0.075 | 1.32 (0.70, 2.50); p = 0.388 |
| inactive | 4.26 (2.90, 6.28); p < 0.001 | 4.53 (2.79, 7.36); p < 0.001 | 2.92 (1.94, 4.40); p < 0.001 | 2.57 (1.62, 4.10); p < 0.001 |
| Hypertension | ||||
| normal | 1 | 1 | 1 | 1 |
| Pre-hypertension | 1.16 (0.78, 1.73); p = 0.456 | 0.83 (0.54, 1.29); p = 0.416 | 1.41 (0.81, 2.44); p = 0.226 | 1.32 (0.74, 2.35); p = 0.346 |
| Stage 1 | (0.97 (0.58, 1.62); p = 0.913 | 0.46 (0.25, 0.83); p = 0.011 | 1.01 (0.53, 1.92); p = 0.970 | 0.89 (0.45, 1.76); p = 0.736 |
| Stage 2 | 0.95 (0.41, 2.23); p = 0.914 | 0.40 (0.15, 1.05); p = 0.062 | 1.07 (0.50, 23.0); p = 0.860 | 1.02 (0.45,2.30); p = 0.956 |
| rs28362678 | rs6955426 | rs284873 | |
|---|---|---|---|
| Chromosome: Gene | 6: BTNL2 | 7: DGKB | 1: TGFBR3 |
| Gene function | Immunoregulators | Cellular processes | Cell surface receptor |
| Diseases * | Rheumatoid arthritis, sarcoidosis | Myocardial infarction | Bone mass and osteoporosis |
| Alleles | C > T | G > A | T > C |
| MAF (resettlers/controls) | 5.8%/14.4% | 19.5%/12.35% | 13.46%/8.2% |
| AA | 7(1.9%)/52 (1.5%) | 8(2.2%)/39 (1.2%) | 8 (2.2%)/20 (0.6%) |
| AB | 28(7.7%)/864 (25.7%) | 126 (34.6%) /750 (22.3%) | 82 (22.5%)/510 (15.2%) |
| BB | 326 (89.8%)/2443 (72.6%) | 229 (63.2%)/2562 (76.2%) | 273 (75.3%)/2829 (84.1%) |
| missing | 2 (0.5%)/4 (0.1%) | 0/12 (0.4%) | 0/4 (0.1%) |
| p-value | 1.24 × 10−9 | 2.37 × 10−8 | 1.51 × 10−6 |
| FDR | 6.26 × 10−5 | 0.000598 | 0.025374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huebner, M.; Börnigen, D.; Deckert, A.; Holle, R.; Meisinger, C.; Müller-Nurasyid, M.; Peters, A.; Rathmann, W.; Becher, H. Genetic Variation and Cardiovascular Risk Factors: A Cohort Study on Migrants from the Former Soviet Union and a Native German Population. Int. J. Environ. Res. Public Health 2021, 18, 6215. https://doi.org/10.3390/ijerph18126215
Huebner M, Börnigen D, Deckert A, Holle R, Meisinger C, Müller-Nurasyid M, Peters A, Rathmann W, Becher H. Genetic Variation and Cardiovascular Risk Factors: A Cohort Study on Migrants from the Former Soviet Union and a Native German Population. International Journal of Environmental Research and Public Health. 2021; 18(12):6215. https://doi.org/10.3390/ijerph18126215
Chicago/Turabian StyleHuebner, Marianne, Daniela Börnigen, Andreas Deckert, Rolf Holle, Christa Meisinger, Martina Müller-Nurasyid, Annette Peters, Wolfgang Rathmann, and Heiko Becher. 2021. "Genetic Variation and Cardiovascular Risk Factors: A Cohort Study on Migrants from the Former Soviet Union and a Native German Population" International Journal of Environmental Research and Public Health 18, no. 12: 6215. https://doi.org/10.3390/ijerph18126215
APA StyleHuebner, M., Börnigen, D., Deckert, A., Holle, R., Meisinger, C., Müller-Nurasyid, M., Peters, A., Rathmann, W., & Becher, H. (2021). Genetic Variation and Cardiovascular Risk Factors: A Cohort Study on Migrants from the Former Soviet Union and a Native German Population. International Journal of Environmental Research and Public Health, 18(12), 6215. https://doi.org/10.3390/ijerph18126215








