The Gender Gap in the Diagnostic-Therapeutic Journey of the Infertile Couple
Abstract
1. Introduction
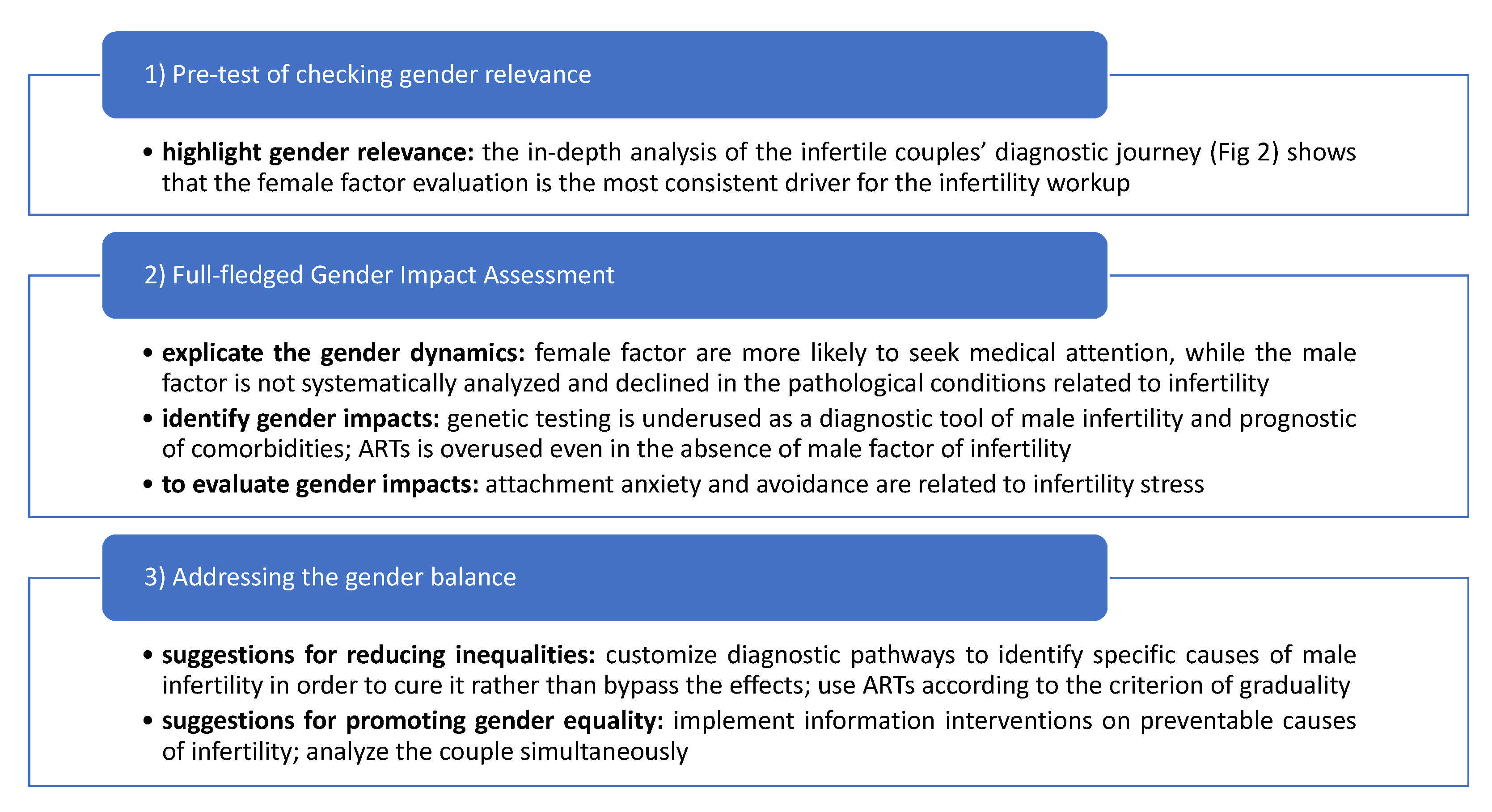
2. Materials and Methods
- Pretest of checking gender relevance.
- Full-fledged gender impact assessment (GIA), identifying and evaluating gender impacts.
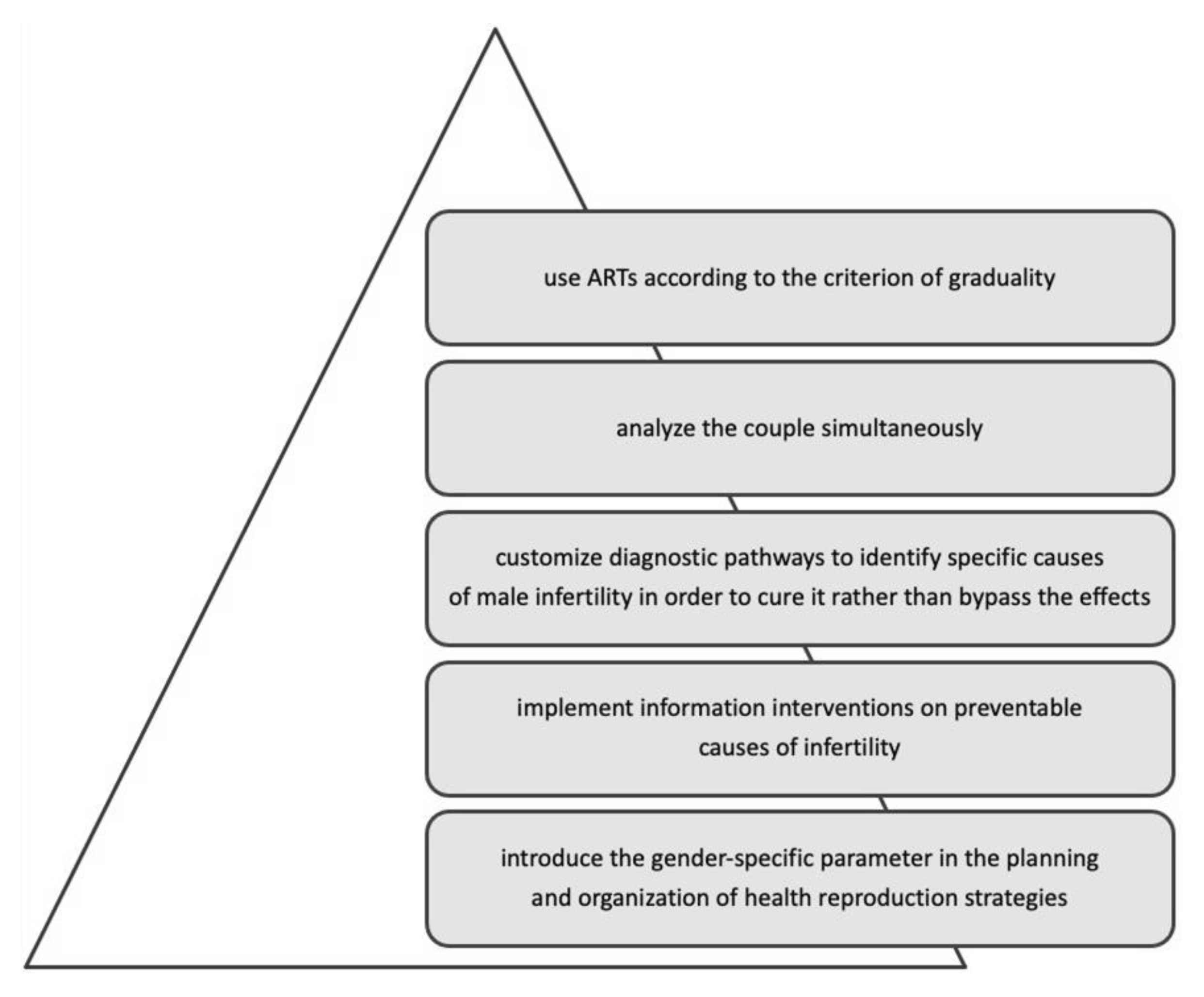
- Addressing the gender balance, giving suggestions for reducing gender inequalities and improving gender equality.
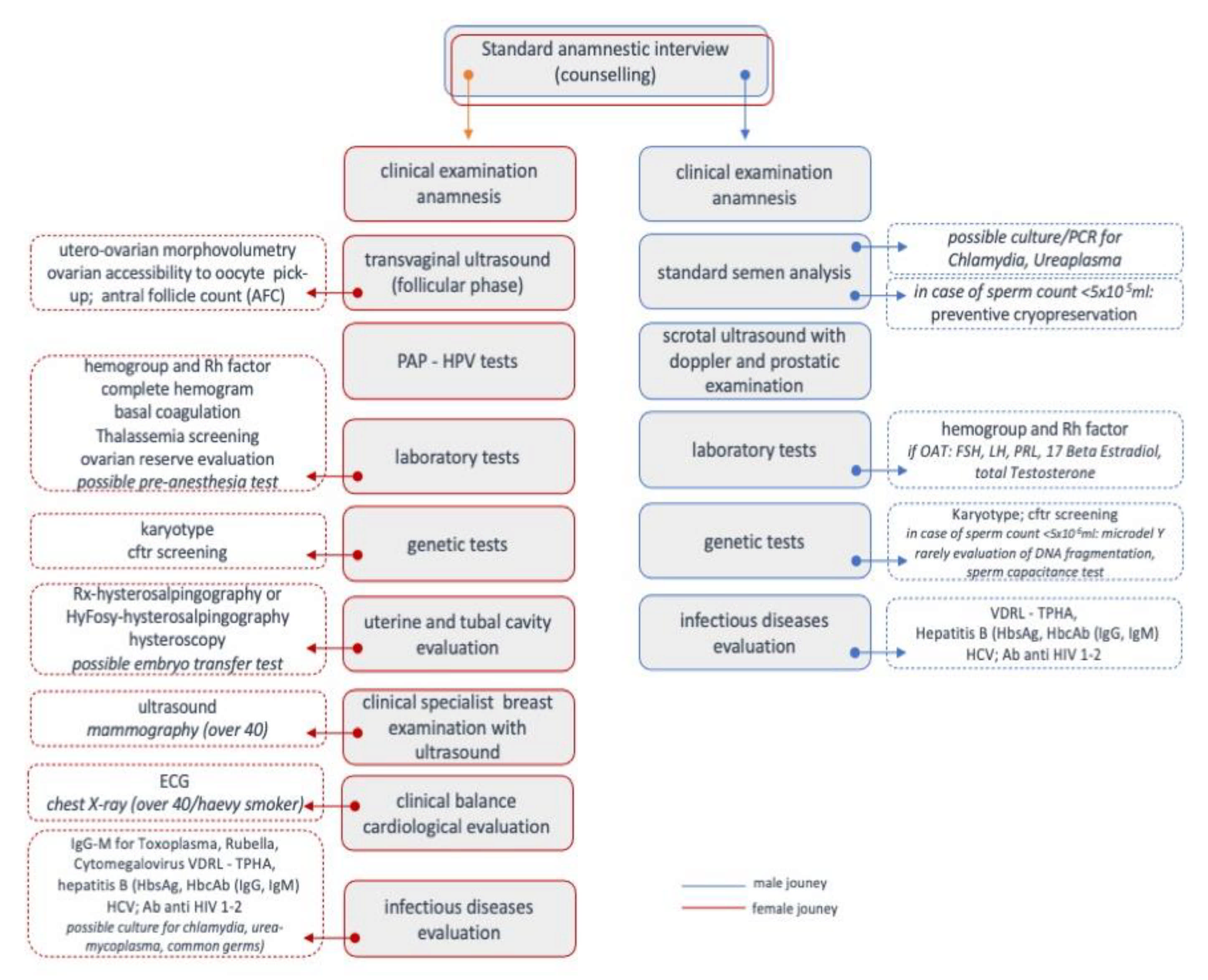
- Characteristics of the subjects: age/date of birth, nationality, educational qualification, profession, religion, and relationship with ART.
- Characteristics of the families of origin: age of parents; profession; living distances; welfare needs; years of marriage and procreative research; sequential reconstruction of the family story accompanied by age, marital status, and presence of children; the possible presence of cases of abortion or sterility in the family; and any genetically transmitted diseases or infections.
- Story of the couple: years of engagement, marriage/cohabitation, and coital frequency; any previous relationships; significant experiences faced together; the story of the personal process of procreative waiting and health; causes of infertility and any surgical intervention (e.g., varicocele, endometriosis, etc.); and previous ART.
- Psychological interview: biopsychosocial data collection, its usefulness, and other contacts with psychologists in the past.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, U.I.; Gabrielsen, J.S.; Lipshultz, L.I. Cutting-Edge Evaluation of Male Infertility. Urol. Clin. N. Am. 2020, 47, 129–138. [Google Scholar] [CrossRef]
- Choy, J.T.; Eisenberg, M.L. Male infertility as a window to health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Sylvest, R.; Fürbringer, J.K.; Schmidt, L.; Pinborg, A. Infertile men’s needs and assessment of fertility care. Upsala J. Med. Sci. 2016, 121, 276–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peterson, B.D.; Newton, C.R.; Rosen, K.H.; Skaggs, G.E. Gender differences in how men and women who are referred for IVF cope with infertility stress. Hum. Reprod. 2006, 21, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.Y.; Wu, L.H.; Loke, A.Y. Gender differences in experiences with and adjustments to infertility: A literature review. Int. J. Nurs. Stud. 2015, 52, 1640–1652. [Google Scholar] [CrossRef] [PubMed]
- Greil, A.L.; Shreffler, K.M.; Schmidt, L.; McQuillan, J. Variation in distress among women with infertility: Evidence from a population-based sample. Hum. Reprod. 2011, 26, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Mason, R.; Lagosky, S.; Rochon, P.A. Integrating and evaluating sex and gender in health research. Health Res. Policy Syst. 2016, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Baggio, G.; Corsini, A.; Floreani, A.; Giannini, S.; Zagonel, V. Gender medicine: A task for the third millennium. Clin. Chem. Lab. Med. 2013, 51, 713–727. [Google Scholar] [CrossRef]
- Pinnelli, A.; Di Cesare, M. Human fertility: Sociodemographic aspects. Contraception 2005, 72, 303–307. [Google Scholar] [CrossRef]
- Popay, J. Whose theory is it anyway? J. Epidemiol. Community Health 2006, 60, 571–572. [Google Scholar] [CrossRef][Green Version]
- Nachtigall, R.D.; Becker, G.; Wozny, M. The effects of gender-specific diagnosis on men’s and women’s response to infertility. Fertil. Steril. 1992, 57, 113–121. [Google Scholar] [CrossRef]
- Casu, G.; Zaia, V.; Fernandes Martins, M.; Parente Barbosa, C.; Gremigni, P. A dyadic mediation study on social support, coping, and stress among couples starting fertility treatment. J. Fam. Psychol. 2019, 33, 315–326. [Google Scholar] [CrossRef]
- Halcomb, L. Men and infertility: Insights from the sociology of gender. Sociol. Compass 2018, 12, e12624. [Google Scholar] [CrossRef]
- Dudgeon, M.R.; Inhorn, M.C. Men’s influences on women’s reproductive health: Medical anthropological perspectives. Soc. Sci. Med. 2004, 59, 1379–1395. [Google Scholar] [CrossRef]
- Fledderjohann, J.; Barnes, L.W. Reimagining infertility: A critical examination of fertility norms, geopolitics and survey bias. Health Policy Plan. 2018, 33, 34–40. [Google Scholar] [CrossRef]
- Bíziková, L.; Sedová, T.; Szapuová, M. Why Gendered Science Matters. How to Include Gender Dimension into Research Projects. 2007. Available online: http://www.cec-wys.org/prilohy/aedc08b1/manual%20main%20body%20final.pdf (accessed on 2 April 2021).
- Ovseiko, P.V.; Greenhalgh, T.; Adam, P.; Grant, J.; Hinrichs-Krapels, S.; Graham, K.E.; Valentine, P.A.; Sued, O.; Boukhris, O.F.; Al Olaqi, N.M.; et al. A global call for action to include gender in research impact assessment. Health Res. Policy Syst. 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Klinge, I. Bringing gender expertise to biomedical and health-related research. Gend. Med. 2007, 4, S59–S63. [Google Scholar] [CrossRef]
- European Commission. Toolkit for Gender in Research: Checklist for Gender in Research. European Commission. 2011. Available online: http://bookshop.europa.eu/en/toolkit-gender-in-eufunded-research-pbKINA24840/ (accessed on 2 April 2021).
- Di Resta, C.; Ferrari, D.; Viganò, M.; Moro, M.; Sabetta, E.; Minerva, M.; Ambrosio, A.; Locatelli, M.; Tomaiuolo, R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination—An Analysis of Serological Response and Side Effects. Vaccines 2021, 9, 522. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: A committee opinion. Fertil. Steril. 2015, 103, e18–e25. [CrossRef]
- Cariati, F.; D’Argenio, V.; Tomaiuolo, R. The evolving role of genetic tests in reproductive medicine. J. Transl. Med. 2019, 17, 267. [Google Scholar] [CrossRef]
- Fainberg, J.; Kashanian, J.A. Recent advances in understanding and managing male infertility. F1000Research 2019, 8, 670. [Google Scholar] [CrossRef]
- O’Neill, C.L.; Chow, S.; Rosenwaks, Z.; Palermo, G.D. Development of ICSI. Reproduction 2018, 156, F51–F58. [Google Scholar] [CrossRef]
- Geng, T.; Cheng, L.; Ge, C.; Zhang, Y. The effect of ICSI in infertility couples with non-male factor: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2020, 37, 2929–2945. [Google Scholar] [CrossRef]
- Korkmaz, C.; Tekin, Y.B.; Sakinci, M.; Ercan, C.M. Effects of maternal ageing on ICSI outcomes and embryo development in relation to oocytes morphological characteristics of birefringent structures. Zygote 2015, 23, 550–555. [Google Scholar] [CrossRef]
- Gennarelli, G.; Carosso, A.; Canosa, S.; Filippini, C.; Cesarano, S.; Scarafia, C.; Brunod, N.; Revelli, A.; Benedetto, C. ICSI Versus Conventional IVF in Women Aged 40 Years or More and Unexplained Infertility: A Retrospective Evaluation of 685 Cycles with Propensity Score Model. J. Clin. Med. 2019, 8, 1694. [Google Scholar] [CrossRef]
- Tannus, S.; Son, W.Y.; Gilman, A.; Younes, G.; Shavit, T.; Dahan, M.H. The role of intracytoplasmic sperm injection in non-male factor infertility in advanced maternal age. Hum. Reprod. 2017, 32, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Annual Capri Workshop Group; Albertini, D.F.; Crosignani, P.; Dumoulin, J.; Evers, J.L.H.; Leridon, H.; Mastenbroek, S.; Painter, R.; Pinborg, A.; Somigliana, E.; et al. IVF, from the past to the future: The inheritance of the Capri Workshop Group. Hum. Reprod. Open 2020, 3, hoaa040. [Google Scholar] [CrossRef]
- Ferlin, A.; Foresta, C. Infertility: Practical Clinical Issues for Routine Investigation of the Male Partner. J. Clin. Med. 2020, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.; Boivin, J.; Dancet, E.; De Klerk, C.; Emery, M.; Lewis-Jones, C.; Thorn, P.; Broeck, U.V.D.; Venetis, C.; Verhaak, C.; et al. ESHRE guideline: Routine psychosocial care in infertility and medically assisted reproduction—A guide for fertility staff. Hum. Reprod. 2015, 30, 2476–2485. [Google Scholar] [CrossRef]
- Donarelli, Z.; Lo Coco, G.; Gullo, S.; Salerno, L.; Marino, A.; Sammartano, F.; Allegra, A. The Fertility Quality of Life Questionnaire (FertiQoL) Relational subscale: Psychometric properties and discriminant validity across gender. Hum. Reprod. 2016, 31, 2061–2071. [Google Scholar] [CrossRef]
- Donarelli, Z.; Salerno, L.; Lo Coco, G.; Allegra, A.; Marino, A.; Kivlighan, D.M. From telescope to binoculars. Dyadic outcome resulting from psychological counselling for infertile couples undergoing ART. J. Reprod. Infant Psychol. 2019, 37, 13–25. [Google Scholar] [CrossRef]
- Matthiesen, S.M.; Frederiksen, Y.; Ingerslev, H.J.; Zachariae, R. Stress, distress and outcome of assisted reproductive technology (ART): A meta-analysis. Hum. Reprod. 2011, 26, 2763–2776. [Google Scholar] [CrossRef]
- Hegyi, B.E.; Kozinszky, Z.; Badó, A.; Dombi, E.; Németh, G.; Pásztor, N. Anxiety and depression symptoms in infertile men during their first infertility evaluation visit. J. Psychosom. Obstet. Gynecol. 2019, 40, 311–317. [Google Scholar] [CrossRef]
- Hvidt, J.; Knudsen, U.B.; Zachariae, R.; Ingerslev, H.J.; Philipsen, M.T.; Frederiksen, Y. Associations of bedtime, sleep duration, and sleep quality with semen quality in males seeking fertility treatment: A preliminary study. Basic Clin. Androl. 2020, 30, 5. [Google Scholar] [CrossRef]
- Viganò, P.; Chiaffarino, F.; Bonzi, V.; Salonia, A.; Ricci, E.; Papaleo, E.; Mauri, P.A.; Parazzini, F. Sleep disturbances and semen quality in an Italian cross-sectional study. Basic Clin. Androl. 2017, 27, 16. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Nordkap, L.; Priskorn, L.; Hansen, Å.M.; Bang, A.K.; Holmboe, S.A.; Schmidt, L.; Jensen, T.K.; Jørgensen, N. Psychological stress, stressful life events, male factor infertility, and testicular function: A cross-sectional study. Fertil. Steril. 2020, 113, 865–875. [Google Scholar] [CrossRef]
- Newton, C.R.; Sherrard, W.; Glavac, I. The Fertility Problem Inventory: Measuring perceived infertility-related stress. Fertil. Steril. 1999, 72, 54–62. [Google Scholar] [CrossRef]
- Slade, P.; Emery, J.; Lieberman, B.A. A prospective, longitudinal study of emotions and relationships in in-vitro fertilization treatment. Hum. Reprod. 1997, 12, 183–190. [Google Scholar] [CrossRef]
- Verhaak, C.M.; Smeenk, J.M.; Eugster, A.; van Minnen, A.; Kremer, J.A.; Kraaimaat, F.W. Stress and marital satisfaction among women before and after their first cycle of in vitro fertilization and intracytoplasmic sperm injection. Fertil. Steril. 2001, 76, 525–531. [Google Scholar] [CrossRef]
- Lund, R.; Sejbaek, C.S.; Christensen, U.; Schmidt, L. The impact of social relations on the incidence of severe depressive symptoms among infertile women and men. Hum. Reprod. 2009, 24, 2810–2820. [Google Scholar] [CrossRef]
- Jordan, C.; Revenson, T.A. Gender differences in coping with infertility: A meta-analysis. J. Behav. Med. 1999, 22, 341–358. [Google Scholar] [CrossRef]
- Glover, L.; Abel, P.D.; Gannon, K. Male subfertility: Is pregnancy the only issue? Psychological responses matter too-and are different in men. BMJ 1998, 316, 1405–1406. [Google Scholar] [CrossRef]
- Peterson, B.D.; Pirritano, M.; Christensen, U.; Schmidt, L. The impact of partner coping in couples experiencing infertility. Hum. Reprod. 2008, 23, 1128–1137. [Google Scholar] [CrossRef]
- Van den Broeck, U.; D’Hooghe, T.; Enzlin, P.; Demyttenaere, K. Predictors of psychological distress in patients starting IVF treatment: Infertility-specific versus general psychological characteristics. Hum. Reprod. 2010, 25, 1471–1480. [Google Scholar] [CrossRef]
- Donarelli, Z.; Lo Coco, G.; Gullo, S.; Marino, A.; Volpes, A.; Allegra, A. Are attachment dimensions associated with infertility-related stress in couples undergoing their first IVF treatment? A study on the individual and cross-partner effect. Hum. Reprod. 2012, 27, 3215–3225. [Google Scholar] [CrossRef]
- Schmidt, L.; Holstein, B.E.; Boivin, J.; Sångren, H.; Tjørnhøj-Thomsen, T.; Blaabjerg, J.; Hald, F.; Andersen, A.N.; Rasmussen, P.E. Patients’ attitudes to medical and psychosocial aspects of care in fertility clinics: Findings from the Copenhagen Multi-centre Psychosocial Infertility (COMPI) Research Programme. Hum. Reprod. 2003, 18, 628–637. [Google Scholar] [CrossRef]
- Knoll, N.; Schwarzer, R.; Pfuller, B.; Kienle, R. Transmission of depressive symptoms: A study with couples undergoing assisted-reproduction treatment. Eur. Psychol. 2009, 14, 7–17. [Google Scholar] [CrossRef]
- Moura-Ramos, M.; Santos, T.A.; Canavarro, M.C. The Role of Attachment Anxiety and Attachment Avoidance on the Psychosocial Well-being of Infertile Couples. J. Clin. Psychol. Med. Settings 2017, 24, 132–143. [Google Scholar] [CrossRef]
- Peterson, B.D.; Sejbaek, C.S.; Pirritano, M.; Schmidt, L. Are severe depressive symptoms associated with infertility-related distress in individuals and their partners? Hum. Reprod. 2014, 29, 76–82. [Google Scholar] [CrossRef]
- D’Argenio, V.; Cariati, F.; Tomaiuolo, R. One4Two®: An Integrated Molecular Approach to Optimize Infertile Couples’ Journey. Genes 2021, 12, 60. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, K. Demarginalizing the Intersection of Race and Sex: A Black Feminist Critique of Antidiscrimination Doctrine. Feminist Theory and Antiracist Politics. Univ. Chic. Leg. Forum. 1989, 140, 139–167. [Google Scholar]
- Damaskos, P.; Amaya, B.; Gordon, R.; Walters, C.B. Intersectionality and the LGBT Cancer Patient. Semin. Oncol. Nurs. 2018, 34, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Bolte, G. Advance gender Study Group. Intersectionality-based quantitative health research and sex/gender sensitivity: A scoping review. Int. J. Equity Health 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Heard, E.; Fitzgerald, L.; Wigginton, B.; Mutch, A. Applying intersectionality theory in health promotion research and practice. Health Promot. Int. 2020, 35, 866–876. [Google Scholar] [CrossRef]
- Wilson, Y.; White, A.; Jefferson, A.; Danis, M. Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. Am. J. Bioeth. 2019, 19, 8–19. [Google Scholar] [CrossRef]
- Cho, H.L. Can Intersectionality Help Lead to More Accurate Diagnosis? Am. J. Bioeth. 2019, 19, 37–39. [Google Scholar] [CrossRef]
- Hentemann, M.A.; Briskemyr, S.; Bertheussen, K. Blastocyst transfer and gender: IVF versus ICSI. J. Assist. Reprod. Genet. 2009, 26, 433–436. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
| AS IS (Problems) | TO BE (Solutions) |
|---|---|
| The erroneous concept that full investigation for infertile men is not needed | Male fertility experts should always be involved in the diagnostic process of the infertile couple |
| Male infertility is usually defined only based on semen analysis | Assessment should embrace:
|
| Semen reporting is still performed in many laboratories that do not have adequate preparation | Semen should be evaluated according to the World Health Organization (WHO) manual and preferably performed in laboratories that have expertise in reproductive medicine |
A common malpractice is:
| Solutions are:
|
| Multiple cycles of IVF/ICSI can last for years and the male figure must not be neglected during the months of treatment, limiting itself to the sole observation of the seminal fluid values | In addition, given the strong association between infertility, cryptorchidism, testicular hypotrophy, and microlithiasis with testicular cancer, recurring scrotal ultrasonography is a great opportunity to identify suspected testis masses and nodules |
Genetic variables are studied:
| Solutions are:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gullo, G.; Cucinella, G.; Perino, A.; Gullo, D.; Segreto, D.; Laganà, A.S.; Buzzaccarini, G.; Donarelli, Z.; Marino, A.; Allegra, A.; et al. The Gender Gap in the Diagnostic-Therapeutic Journey of the Infertile Couple. Int. J. Environ. Res. Public Health 2021, 18, 6184. https://doi.org/10.3390/ijerph18126184
Gullo G, Cucinella G, Perino A, Gullo D, Segreto D, Laganà AS, Buzzaccarini G, Donarelli Z, Marino A, Allegra A, et al. The Gender Gap in the Diagnostic-Therapeutic Journey of the Infertile Couple. International Journal of Environmental Research and Public Health. 2021; 18(12):6184. https://doi.org/10.3390/ijerph18126184
Chicago/Turabian StyleGullo, Giuseppe, Gaspare Cucinella, Antonio Perino, Domenico Gullo, Daniela Segreto, Antonio Simone Laganà, Giovanni Buzzaccarini, Zaira Donarelli, Angelo Marino, Adolfo Allegra, and et al. 2021. "The Gender Gap in the Diagnostic-Therapeutic Journey of the Infertile Couple" International Journal of Environmental Research and Public Health 18, no. 12: 6184. https://doi.org/10.3390/ijerph18126184
APA StyleGullo, G., Cucinella, G., Perino, A., Gullo, D., Segreto, D., Laganà, A. S., Buzzaccarini, G., Donarelli, Z., Marino, A., Allegra, A., Maranto, M., Carosso, A. R., Garofalo, P., & Tomaiuolo, R. (2021). The Gender Gap in the Diagnostic-Therapeutic Journey of the Infertile Couple. International Journal of Environmental Research and Public Health, 18(12), 6184. https://doi.org/10.3390/ijerph18126184












