Decrease in All-Cause 30-Day Mortality after Bacteraemia over a 15-Year Period: A Population-Based Cohort Study in Denmark in 2000–2014
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Bacteraemia Study Cohort
2.3. Identification and Susceptibility Testing
2.4. Empiric Treatment
2.5. Background Populations and the Statistical Analysis
3. Results
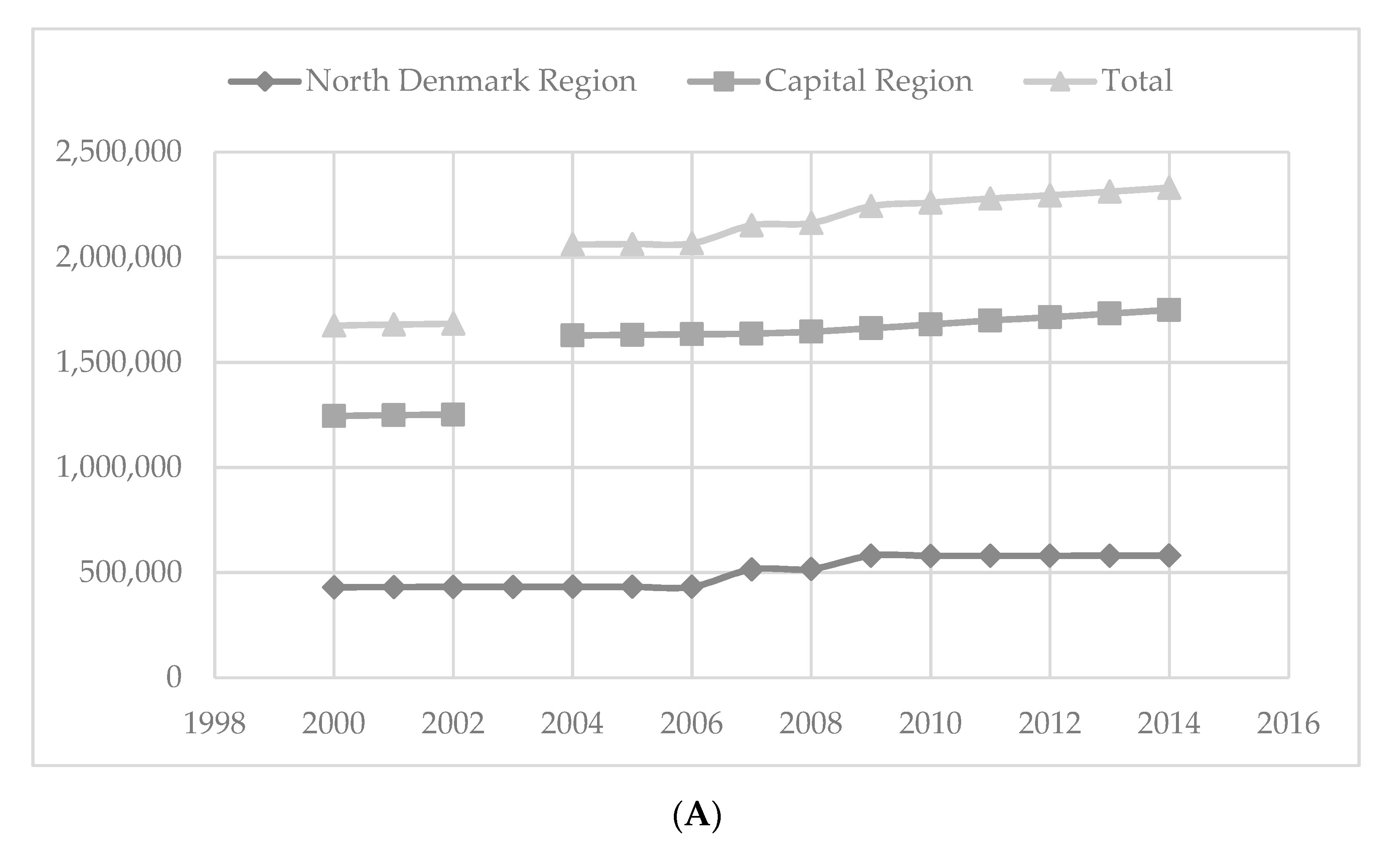
3.1. Study Population
3.2. Calculations in Relation to Background Population
3.3. Total Number of Patients and Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sogaard, M.; Norgaard, M.; Dethlefsen, C.; Schonheyder, H.C. Temporal Changes in the Incidence and 30-Day Mortality associated with Bacteremia in Hospitalized Patients from 1992 through 2006: A Population-based Cohort Study. Clin. Infect. Dis. 2011, 52, 61–69. [Google Scholar] [CrossRef]
- Wilson, J.; Elgohari, S.; Livermore, D.M.; Cookson, B.; Johnson, A.; Lamagni, T.; Chronias, A.; Sheridan, E. Trends among pathogens reported as causing bacteraemia in England, 2004–2008. Clin. Microbiol. Infect. 2011, 17, 451–458. [Google Scholar] [CrossRef]
- Skogberg, K.; Lyytikäinen, O.; Ollgren, J.; Nuorti, J.P.; Ruutu, P. Population-based burden of bloodstream infections in Finland. Clin. Microbiol. Infect. 2012, 18, E170–E176. [Google Scholar] [CrossRef]
- Madsen, K.M.; Schønheyder, H.C.; Kristensen, B.; Sørensen, H.T. Secular trends in incidence and mortality of bacteraemia in a Danish county 1981–1994. APMIS 1999, 107, 346–352. [Google Scholar] [CrossRef]
- Lam, J.C.; Gregson, D.B.; Robinson, S.; Somayaji, R.; Conly, J.M.; Parkins, M.D. Epidemiology and Outcome Determinants of Staphylococcus aureus Bacteremia Revisited: A Population-Based Study. Infection 2019, 47, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Holmbom, M.; Giske, C.G.; Fredrikson, M.; Östholm Balkhed, Å.; Claesson, C.; Nilsson, L.E.; Hoffmann, M.; Hanberger, H. 14-Year Survey in a Swedish County Reveals a Pronounced Increase in Bloodstream Infections (BSI). Comorbidity-An Independent Risk Factor for Both BSI and Mortality. PLoS ONE 2016, 11, e0166527. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Al-Hasan, M.N. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Gradel, K.O.; Nielsen, S.L.; Pedersen, C.; Knudsen, J.D.; Østergaard, C.; Arpi, M.; Jensen, T.G.; Kolmos, H.J.; Søgaard, M.; Lassen, A.T.; et al. Low Completeness of Bacteraemia Registration in the Danish National Patient Registry. PLoS ONE 2015, 10, e0131682. [Google Scholar] [CrossRef]
- Laupland, K.B. Incidence of bloodstream infection: A review of population-based studies. Clin. Microbiol. Infect. 2013, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Frank, L. Epidemiology. When an entire country is a cohort. Science 2000, 287, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Gradel, K.; Arpi, M.; Knudsen, J.; Schonheyder, H.; Ostergaard, C.; Sogaard, M. The Danish Collaborative Bacteraemia Network (DACOBAN) database. Clin. Epidemiol. 2014, 6, 301. [Google Scholar] [CrossRef]
- Trick, W.E.; Zagorski, B.M.; Tokars, J.I.; Vernon, M.O.; Welbel, S.F.; Wisniewski, M.F.; Richards, C.; Weinstein, R.A. Computer Algorithms To Detect Bloodstream Infections. Emerg. Infect. Dis. 2004, 10, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Menchinelli, G.; Liotti, F.M.; Fiori, B.; De Angelis, G.; D’Inzeo, T.; Giordano, L.; Posteraro, B.; Sabbatucci, M.; Sanguinetti, M.; Spanu, T. In vitro Evaluation of BACT/ALERT® VIRTUO®, BACT/ALERT 3D®, and BACTECTM FX Automated Blood Culture Systems for Detection of Microbial Pathogens Using Simulated Human Blood Samples. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Kahlmeter, G.; Brown, D.F.J.; Goldstein, F.W.; MacGowan, A.P.; Mouton, J.W.; Odenholt, I.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; Soriano, F.; et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) Technical Notes on antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2006, 12, 501–503. [Google Scholar] [CrossRef]
- Knudsen, J.D.; Boel, J.; Olsen, B.R.; Benfield, T.; Arpi, M.; Frimodt-Møller, N.; Jarløv, J.O.; Østergaard, C. Antibiotics-Dosage, Precausions and Treatment Recommendations-Handbook; Sundhedsfagligt Råd for Klinisk Mikrobiologi, Region Hovedstaden: Copenhagen, Denmark, 2018. [Google Scholar]
- Statistik, D. Population Denmark. Available online: https://www.dst.dk/da/Statistik/emner/befolkning-og-valg/befolkning-og-befolkningsfremskrivning/folketal (accessed on 20 October 2020).
- Wikipedia Formation of Regions. Available online: https://en.wikipedia.org/wiki/Counties_of_Denmark (accessed on 20 October 2020).
- Andrews, D.W.K. Chi-square diagnostic tests for econometric models. J. Econom. 1988, 37, 135–156. [Google Scholar] [CrossRef]
- Cuzick, J. A wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.C.; Drefahl, S.; Ahlbom, A.; Lambe, M.; Modig, K. Trends in life expectancy: Did the gap between the healthy and the ill widen or close? BMC Med. 2020, 18, 41. [Google Scholar] [CrossRef]
- Nielsen, S.L.; Pedersen, C.; Jensen, T.G.; Gradel, K.O.; Kolmos, H.J.; Lassen, A.T. Decreasing incidence rates of bacteremia: A 9-year population-based study. J. Infect. 2014, 69, 51–59. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Søgaard, M.; Engebjerg, M.C.; Lundbye-Christensen S, S.H. Changes in blood culture methodology have an impact on time trends of bacteraemia: A 26-year regional study. Epidemiol. Infect. 2011, 139, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Kern, W.V.; Rieg, S. Burden of bacterial bloodstream infection—A brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef]
- Thønnings, S.; Jansåker, F.; Gradel, K.O.; Styrishave, B.; Knudsen, J.D. Cefuroxime compared to piperacillin/tazobactam as empirical treatment of Escherichia coli bacteremia in a low Extended-spectrum beta-lactamase (ESBL) prevalence cohort. Infect. Drug Resist. 2019, 12, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.; Melzer, M. Strategy to reduce E. coli bacteraemia based on cohort data from a London teaching hospital. Postgrad. Med. J. 2018, 94, 212–215. [Google Scholar] [CrossRef]
- Freund, Y.; Khoury, A.; Möckel, M.; Karamercan, M.; Dodt, C.; Leach, R.; Bloom, B.; Garcia-Castrillo, L. European Society of Emergency Medicine position paper on the 1-hour sepsis bundle of the Surviving Sepsis Campaign: Expression of concern. Eur. J. Emerg. Med. 2019, 26, 232–233. [Google Scholar] [CrossRef]
- Bruce, M.G.; Singleton, R.; Bulkow, L.; Rudolph, K.; Zulz, T.; Gounder, P.; Hurlburt, D.; Bruden, D.; Hennessy, T. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine 2015, 33, 4813–4819. [Google Scholar] [CrossRef]
- Martinez-Roig, A. Invasive Pneumococcal disease in children 5 years after conjugate vaccine introduction-Eight States, 1998–2005. Pediatr. Catalana 2008, 68, 175–176. [Google Scholar]
- Kwong, J.C.; Maaten, S.; Upshur, R.E.G.; Patrick, D.M.; Marra, F. The Effect of Universal Influenza Immunization on Antibiotic Prescriptions: An Ecological Study. Clin. Infect. Dis. 2009, 49, 750–756. [Google Scholar] [CrossRef]
- Uslan, D.Z. Age- and Sex-Associated Trends in Bloodstream Infection. Arch. Intern. Med. 2007, 167, 834. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Special Eurobarometer 445: Antimicrobial Resistance; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Di Gennaro, F.; Marotta, C.; Amicone, M.; Bavaro, D.F.; Bernaudo, F.; Frisicale, E.M.; Kurotschka, P.K.; Mazzari, A.; Veronese, N.; Murri, R.; et al. Italian young doctors’ knowledge, attitudes and practices on antibiotic use and resistance: A national cross-sectional survey. J. Glob. Antimicrob. Resist. 2020, 23, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Moore, L.S.P.; Castro-Sánchez, E.; Spanoudaki, E.; Grady, C.; Holmes, A.H.; Drumright, L.N. A needs assessment study for optimising prescribing practice in secondary care junior doctors: The Antibiotic Prescribing Education among Doctors (APED). BMC Infect. Dis. 2016, 16, 456. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holm, M.K.A.; Jansåker, F.; Gradel, K.O.; Nielsen, R.T.; Østergaard Andersen, C.; Jarløv, J.O.; Schønheyder, H.C.; Dahl Knudsen, J. Decrease in All-Cause 30-Day Mortality after Bacteraemia over a 15-Year Period: A Population-Based Cohort Study in Denmark in 2000–2014. Int. J. Environ. Res. Public Health 2021, 18, 5982. https://doi.org/10.3390/ijerph18115982
Holm MKA, Jansåker F, Gradel KO, Nielsen RT, Østergaard Andersen C, Jarløv JO, Schønheyder HC, Dahl Knudsen J. Decrease in All-Cause 30-Day Mortality after Bacteraemia over a 15-Year Period: A Population-Based Cohort Study in Denmark in 2000–2014. International Journal of Environmental Research and Public Health. 2021; 18(11):5982. https://doi.org/10.3390/ijerph18115982
Chicago/Turabian StyleHolm, Mona Katrine Alberthe, Filip Jansåker, Kim Oren Gradel, Rikke Thoft Nielsen, Christian Østergaard Andersen, Jens Otto Jarløv, Henrik Carl Schønheyder, and Jenny Dahl Knudsen. 2021. "Decrease in All-Cause 30-Day Mortality after Bacteraemia over a 15-Year Period: A Population-Based Cohort Study in Denmark in 2000–2014" International Journal of Environmental Research and Public Health 18, no. 11: 5982. https://doi.org/10.3390/ijerph18115982
APA StyleHolm, M. K. A., Jansåker, F., Gradel, K. O., Nielsen, R. T., Østergaard Andersen, C., Jarløv, J. O., Schønheyder, H. C., & Dahl Knudsen, J. (2021). Decrease in All-Cause 30-Day Mortality after Bacteraemia over a 15-Year Period: A Population-Based Cohort Study in Denmark in 2000–2014. International Journal of Environmental Research and Public Health, 18(11), 5982. https://doi.org/10.3390/ijerph18115982





