The Negative Impact of Varicocele on Basic Semen Parameters, Sperm Nuclear DNA Dispersion and Oxidation-Reduction Potential in Semen
Abstract
1. Introduction
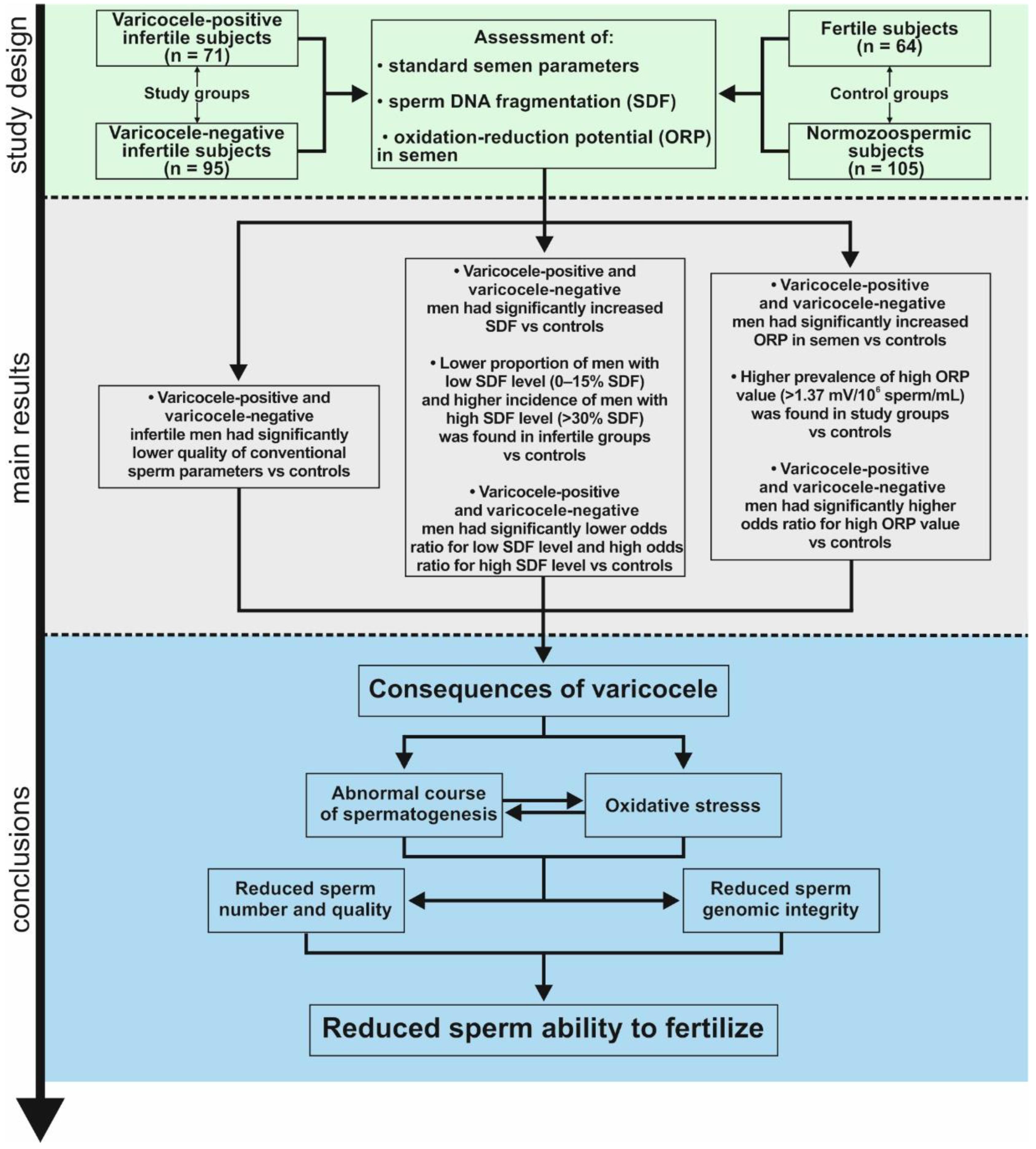
2. Materials and Methods
2.1. Study Groups
2.2. Manual Semen Analysis
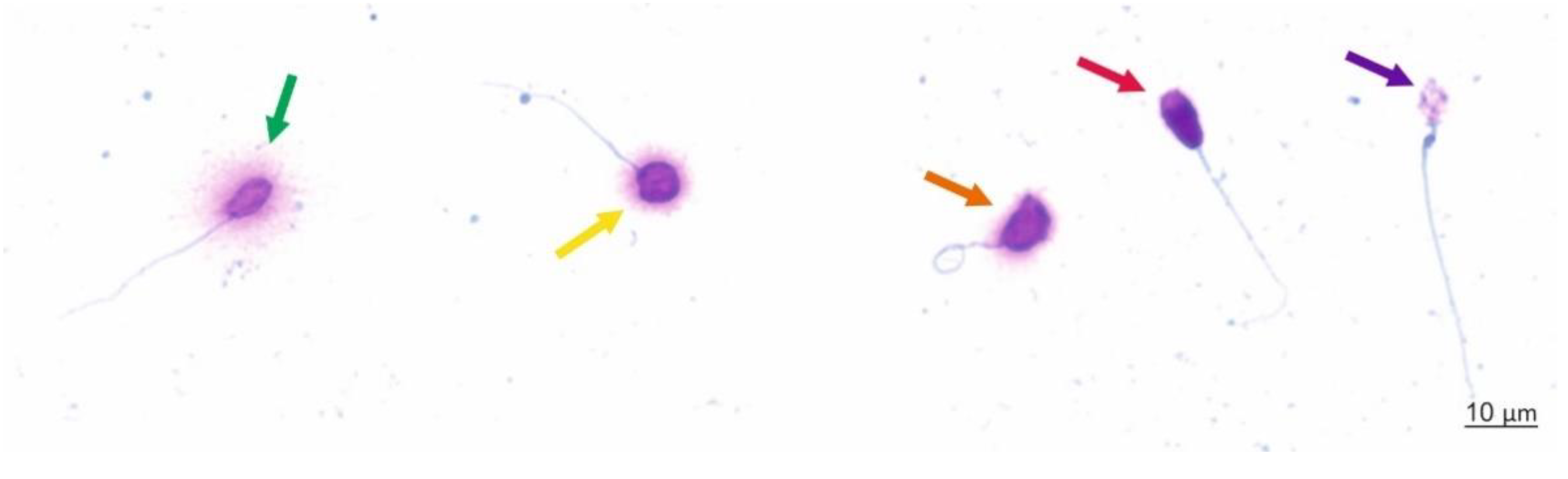
2.3. Sperm Chromatin Dispersion (SCD) Test (Halosperm Test)
Scoring Criteria
2.4. Measurement of Oxidation-Reduction Potential in Semen
2.5. Data Analysis
3. Results
3.1. Age and Standard Semen Parameters
3.2. Sperm DNA Dispersion
3.3. Oxidation-Reduction Potential in Semen
3.4. Correlations between Study Parameters
4. Discussion
4.1. The Detrimental Effect of Varicoceles on Conventional Sperm Characteristics
4.2. The Detrimental Effect of Varicoceles on Nuclear Sperm DNA
4.3. The Detrimental Effect of Varicoceles on the Oxidation-Reduction Potential in Semen
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Parekh, N.; Selvam, M.K.P.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male oxidative stress infertility (MOSI): Proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J. Men’s Heal. 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Agarwal, A.; Barbăroșie, C.; Ambar, R.; Finelli, R. The impact of single-and double-strand dna breaks in human spermatozoa on assisted reproduction. Int. J. Mol. Sci. 2020, 21, 3882. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Freeman, S.; Bertolotto, M.; Richenberg, J.; Belfield, J.; Dogra, V.; Huang, D.Y.; Lotti, F.; Markiet, K.; Nikolic, O.; Ramanathan, S.; et al. Ultrasound evaluation of varicoceles: Guidelines and recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for detection, classification, and grading. Eur. Radiol. 2020, 30, 11–25. [Google Scholar] [CrossRef]
- Lewis, S.E.M.; Esteves, S.C. What does a varicocele do to a man’s fertility? There is much more than meets the eye. Int. Braz. J. Urol. 2021, 47, 284–286. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Carvalho, J.; Corona, G.; Jones, T.H.; Kadioglu, A.; Martinez-Salamanca, J.I.; Minhas, S.; Serefoglu, E.C.; Verze, P.; et al. Sexual and Reproductive Health EAU Guidelines; European Association of Urology: Arnhem, The Netherlands, 2021. [Google Scholar]
- Panner Selvam, M.K.; Agarwal, A. Sperm and seminal plasma proteomics: Molecular changes associated with varicocele-mediated male infertility. World J. Mens Health 2020, 37, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Su, J.S.; Farber, N.J.; Vij, S.C. Pathophysiology and treatment options of varicocele: An overview. Andrologia 2021, 53. [Google Scholar] [CrossRef]
- Ren, W.; Qu, J.; Xue, B.; Hu, J.; Zu, X. Infertility duration and pre-operative sperm progressive motility are significant factors of spontaneous pregnancy after varicocele repair. Am. J. Reprod. Immunol. 2020, 84, 1–7. [Google Scholar] [CrossRef]
- Qiu, D.; Shi, Q.; Pan, L. Efficacy of varicocelectomy for sperm DNA integrity improvement: A meta-analysis. Andrologia 2021, 53. [Google Scholar] [CrossRef]
- Asafu-Adjei, D.; Judge, C.; Deibert, C.M.; Li, G.; Stember, D.; Stahl, P.J. Systematic Review of the Impact of Varicocele Grade on Response to Surgical Management. J. Urol. 2020, 203, 48–56. [Google Scholar] [CrossRef]
- Birowo, P.; Wijaya, J.R.; Atmoko, W.; Rasyid, N. The effects of varicocelectomy on the DNA fragmentation index and other sperm parameters: A meta-analysis. Basic Clin. Androl. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.; Ibrahim, E.; Intasqui, P.; Belardin, L.B.; Antoniassi, M.P.; Lynne, C.M.; Brackett, N.L.; Bertolla, R.P. Seminal inflammasome activity in the adult varicocele. Hum. Fertil. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.; Farber, N.; Vij, S. Conventional semen analysis and advanced sperm function tests in diagnosis and management of varicocele. Andrologia 2021, 53. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Mohamed, O.; Mahmoud, O.; Alsagheer, G.A.; Reyad, A.M.; Abolyosr, A.; Abdel-Kader, M.S.; Saber-Khalaf, M. The impact of varicocelectomy on sperm DNA fragmentation and pregnancy rate in subfertile men with normal semen parameters: A pilot study. Arab J. Urol. 2021, 19, 186–190. [Google Scholar] [CrossRef]
- Lara-Cerrillo, S.; Gual-Frau, J.; Benet, J.; Abad, C.; Prats, J.; Amengual, M.J.; García-Peiró, A. Microsurgical varicocelectomy effect on sperm telomere length, DNA fragmentation and seminal parameters. Hum. Fertil. 2020, 1–7. [Google Scholar] [CrossRef]
- Maheshwari, A.; Muneer, A.; Lucky, M.; Mathur, R.; McEleny, K. A review of varicocele treatment and fertility outcomes. Hum. Fertil. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Afshar, K.; Domes, T. Varicocele. Can. Urol. Assoc. J. 2018, 12, 34–36. [Google Scholar] [CrossRef]
- Moazzam, A.; Sharma, R.; Agarwal, A. Relationship of spermatozoal DNA fragmentation with semen quality in varicocele-positive men. Andrologia 2015, 47, 935–944. [Google Scholar] [CrossRef]
- Ata-abadi, N.S.; Mowla, S.J.; Aboutalebi, F.; Dormiani, K.; Kiani-Esfahani, A.; Tavalaee, M.; Nasr-Esfahani, M.H. Hypoxia-related long noncoding RNAs are associated with varicocele-related male infertility. PLoS ONE 2020, 15, 1–12. [Google Scholar] [CrossRef]
- Esteves, S.C.; Santi, D.; Simoni, M. An update on clinical and surgical interventions to reduce sperm DNA fragmentation in infertile men. Andrology 2020, 8, 53–81. [Google Scholar] [CrossRef]
- Esteves, S.C.; Zini, A.; Coward, R.M.; Evenson, D.P.; Gosálvez, J.; Lewis, S.E.M.; Sharma, R.; Humaidan, P. Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia 2021, 53, 1–41. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia 2021, 53, 1–15. [Google Scholar] [CrossRef]
- Ammar, O.; Tekeya, O.; Hannachi, I.; Sallem, A.; Haouas, Z.; Mehdi, M. Increased Sperm DNA Fragmentation in Infertile Men with Varicocele: Relationship with Apoptosis, Seminal Oxidative Stress, and Spermatic Parameters. Reprod. Sci. 2021, 28, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2021, 53. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, J.T.; Belardin, L.B.; Okada, F.K.; Antoniassi, M.P.; Fraietta, R.; Bertolla, R.P.; Intasqui, P. Oxidative origin of sperm DNA fragmentation in the adult varicocele. Int. Braz. J. Urol. 2021, 47, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Yeste, M.; Becerra-Tomás, N.; Aston, K.I.; James, E.R.; Salas-Huetos, A. Clinical implications of sperm DNA damage in IVF and ICSI: Updated systematic review and meta-analysis. Biol. Rev. 2021. [Google Scholar] [CrossRef]
- Tanaka, T.; Kobori, Y.; Terai, K.; Inoue, Y.; Osaka, A.; Yoshikawa, N.; Shimomura, Y.; Suzuki, K.; Minami, T.; Iwahata, T.; et al. Seminal oxidation–reduction potential and sperm DNA fragmentation index increase among infertile men with varicocele. Hum. Fertil. 2020, 1–5. [Google Scholar] [CrossRef]
- Abdelbaki, S.A.; Sabry, J.H.; Al-Adl, A.M.; Sabry, H.H. The impact of coexisting sperm DNA fragmentation and seminal oxidative stress on the outcome of varicocelectomy in infertile patients: A prospective controlled study. Arab J. Urol. 2017, 15, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Al Omrani, B.; Al Eisa, N.; Javed, M.; Al Ghedan, M.; Al Matrafi, H.; Al Sufyan, H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod. Biol. Endocrinol. 2018, 16, 1–6. [Google Scholar] [CrossRef]
- Evenson, D.P. Evaluation of sperm chromatin structure and DNA strand breaks is an important part of clinical male fertility assessment. Transl. Androl. Urol. 2017, 6, S495–S500. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Han, M.; Ding, J.; Wang, F.; Wang, G.; Shen, L.; Wang, J.; Zheng, B.; Meng, Q.; Wang, W.; et al. Importance of a semen analysis report for determining the relationship between SCSA sperm DNA fragmentation index and assisted reproductive technology pregnancy rate. Reprod. Biol. 2020, 20, 460–464. [Google Scholar] [CrossRef]
- Leach, M.; Aitken, R.J.; Sacks, G. Sperm DNA fragmentation abnormalities in men from couples with a history of recurrent miscarriage. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 379–383. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Trieu, T.S.; Tran, T.O.; Luong, T.L.A. Evaluation of sperm DNA fragmentation index, Zinc concentration and seminal parameters from infertile men with varicocele. Andrologia 2019, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Sabanegh, E. Symptomatic male with subclinical varicocele found on ultrasound evaluation. Asian J. Androl. 2016, 18, 313–314. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization Press: Geneva, Switzerland, 2010. [Google Scholar]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory assessment of oxidative stress in semen. Arab J. Urol. 2018, 16, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Alkan, İ.; Yüksel, M.; Canat, H.L.; Atalay, H.A.; Can, O.; Özveri, H.; Başar, M.M. Superoxide Anion Production by the Spermatozoa of Men with Varicocele: Relationship with Varicocele Grade and Semen Parameters. World J. Mens Health 2018, 36, 255. [Google Scholar] [CrossRef] [PubMed]
- Finelli, R.; Pallotti, F.; Cargnelutti, F.; Faja, F.; Carlini, T.; Rizzo, F.; Lenzi, A.; Paoli, D.; Lombardo, F. Sperm DNA damage and cytokines in varicocele: A case-control study. Andrologia 2021, 1–6. [Google Scholar] [CrossRef]
- Alargkof, V.; Kersten, L.; Stanislavov, R.; Kamenov, Z.; Nikolinakos, P. Relationships between sperm DNA integrity and bulk semen parameters in Bulgarian patients with varicocele. Arch. Ital. Urol. Androl. 2019, 91. [Google Scholar] [CrossRef]
- Pallotti, F.; Paoli, D.; Carlini, T.; Vestri, A.R.; Martino, G.; Lenzi, A.; Lombardo, F. Varicocele and semen quality: A retrospective case–control study of 4230 patients from a single centre. J. Endocrinol. Invest. 2018, 41, 185–192. [Google Scholar] [CrossRef]
- Redmon, J.B.; Drobnis, E.Z.; Sparks, A.; Wang, C.; Swan, S.H. Semen and reproductive hormone parameters in fertile men with and without varicocele. Andrologia 2019, 51, 1–7. [Google Scholar] [CrossRef]
- Gill, K.; Rosiak-Gill, A.; Jakubik, J.; Patorski, L.; Lukaszuk, M.; Piasecka, M. The higher risk for sperm DNA damage in infertile men. Ginekol. Pol. 2019, 90. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Jakubik, J.; Rosiak-Gill, A.; Kups, M.; Lukaszuk, M.; Kurpisz, M.; Fraczek, M.; Piasecka, M. Utility and Predictive Value of Human Standard Semen Parameters and Sperm DNA Dispersion for Fertility Potential. Int. J. Environ. Res. Public Health 2019, 16, 2004. [Google Scholar] [CrossRef] [PubMed]
- Jakubik-Uljasz, J.; Gill, K.; Rosiak-Gill, A.; Piasecka, M. Relationship between sperm morphology and sperm DNA dispersion. Transl. Androl. Urol. 2020, 9, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Arafa, M.; Mahdi, M.; Agarwal, A.; Al Said, S.; Al-Emadi, I.; El Ansari, W.; Alattar, A.; Al Rumaihi, K.; Elbardisi, H. Oxidation–reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab J. Urol. 2018, 16, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and male fertility: From molecular studies to clinical evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef]
| Parameters | Total (n = 335) | Varicocele Positive-Infertile Men (1) (n = 71) | Varicocele Negative-Infertile Men (2) (n = 95) | Healthy Volunteers with Normozoospermia (3) (n = 105) | Men with Proven Fertility (4) (n = 64) | p 1 vs. 2 | p 1 vs. 3 | p 1 vs. 4 | p 2 vs. 3 | p 2 vs. 4 | p 3 vs. 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 32.00 (20.00–49.00) 31.84 ± 5.66 | 32.00 (22.00–48.00) 32.61 ± 5.02 | 34.00 (24.00–49.00) 34.37 ± 5.37 | 28.00 (20.00–44.00) 28.80 ± 5.23 | 32.00 (22.00–47.00) 32.39 ± 5.13 | NS | p = 0.000038 | NS | p < 0.000001 | NS | p = 0.000462 |
| Semen volume (mL) | 3.00 (0.50–10.00) 3.47 ± 1.53 | 3.00 (1.00–9.00) 3.43 ± 1.68 | 3.00 (0.50–8.00) 3.19 ± 1.43 | 3.50 (0.75–10.00) 3.89 ± 1.63 | 3.00 (0.50–8.00) 3.25 ± 1.74 | NS | NS | NS | p = 0.011929 | NS | p = 0.012485 |
| Sperm concentration (×106/mL) | 25.38 (0.05–146.50) 29.16 ± 25.59 | 8.90 (0.25–146.50) 17.85 ± 23.90 | 10.50 (0.05–104.50) 17.39 ± 19.84 | 32.00 (8.25–119.50) 38.36 ± 21.61 | 33.82 (4.80–115.25) 44.10 ± 27.52 | NS | p < 0.000001 | p < 0.000001 | p < 0.000001 | p < 0.000001 | NS |
| Total number of spermatozoa (×106) | 69.60 (0.25–672.00) 97.80 ± 96.43 | 29.97 (0.50–293.00) 55.57 ± 65.61 | 31.60 (0.25–412.75) 53.83 ± 69.24 | 117.08 (39.00–475.00) 141.70 ± 87.31 | 106.63 (21.60–672.00) 137.91 ± 122.51 | NS | p < 0.000001 | p < 0.000001 | p < 0.000001 | p < 0.000001 | NS |
| Morphologically normal spermatozoa (%) | 4.00 (0.00–13.00) 3.94 ± 3.39 | 1.00 (0.00–7.00) 1.26 ± 1.63 | 0.00 (0.00–11.00) 1.89 ± 2.79 | 7.00 (4.00–12.00) 6.68 ± 1.99 | 5.00 (0.00–13.00) 5.48 ± 3.24 | NS | p < 0.000001 | p < 0.000001 | p < 0.000001 | p < 0.000001 | NS |
| TZI | 1.68 (1.22–2.52) 1.72 ± 0.28 | 1.67 (1.35–2.50) 1.73 ± 0.24 | 1.94 (1.44–2.52) 1.95 ± 0.27 | 1.48 (1.22–2.12) 1.52 ± 0.18 | 1.70 (1.32–2.25) 1.69 ± 0.19 | p = 0.000017 | p = 0.000001 | NS | p < 0.000001 | p = 0.000003 | p = 0.000029 |
| Progressive motility (%) | 59.00 (0.00–94.00) 53.11 ± 23.07 | 39.00 (0.00–85.00) 36.70 ± 19.92 | 38.00 (00.00–84.00) 39.54 ± 23.53 | 69.00 (34.00–94.00) 68.54 ± 11.24 | 68.50 (22.00–90.00) 66.12 ± 13.99 | NS | p < 0.000001 | p < 0.000001 | p < 0.000001 | p < 0.000001 | NS |
| Nonprogressive motility (%) | 6.00 (0.00–29.00) 6.99 ± 4.63 | 6.00 (0.00–16.00) 6.21 ± 3.83 | 5.00 (0.00–29.00) 5.66 ± 4.09 | 8.00 (0.00–18.00) 8.03 ± 4.15 | 6.00 (1.00–26.00) 8.12 ± 6.10 | NS | p = 0.030324 | NS | p = 0.000087 | NS | NS |
| Total sperm motility (%) | 67.00 (0.00–98.00) 60.10 ± 23.77 | 46,00 (0.00–87.00) 42.91 ± 20.44 | 45.00 (0.00–87.00) 45.21 ± 23.60 | 79.00 (52.00–98.00) 76.58 ± 10.13 | 77.00 (28.00–98.00) 74.25 ± 14.39 | NS | p < 0.000001 | p < 0.000001 | p < 0.000001 | p < 0.000001 | NS |
| Eosin-negative spermatozoa—live cells (%) | 80.00 (3.00–98.00) 76.76 ± 14.38 | 72.00 (3.00–91.00) 68.42 ± 17.19 | 76.00 (23.00–96.00) 72.29 ± 15.26 | 84.00 (60.00–98.00) 82.64 ± 8.95 | 85.00 (48.00–98.00) 83.00 ± 9.11 | NS | p < 0.000001 | p < 0.000001 | p = 0.000001 | p = 0.000006 | NS |
| HOS test-positive spermatozoa—live cells (%) | n = 291 80.00 (13.00–98.00) 76.68 ± 13.39 | n = 49 71.00 (13.00–90.00) 68.51 ± 16.59 | n = 77 73.00 (20.00–92.00) 71.96 ± 15.05 | n = 105 85.00 (58.00–94.00) 81.68 ± 8.81 | n = 60 82.00 (50.00–98.00) 80.66 ± 9.13 | NS | p < 0.000001 | p = 0.000137 | p = 0.000006 | p = 0.003885 | NS |
| Peroxidase-positive cells (mln/mL) | 0.20 (0.00–0.96) 0.23 ± 0.24 | 0.25 (0.00–0.96) 0.32 ± 0.23 | 0.25 (0.00–0.90) 0.29 ± 0.23 | 0.00 (0.00–0.90) 0.13 ± 0.20 | 0.20 (0.00–0.95) 0.22 ± 0.24 | NS | p < 0.000001 | NS | p = 0.000002 | NS | NS |
| SDF (%) | 20.00 (2.00–64.00) 23.29 ± 11.89 | 20.00 (2.00–64.00) 23.29 ± 11.89 | 18.00 (4.00–53.00) 19.35 ± 9.54 | 12.00 (3.00–28.00) 13.33 ± 5.93 | 13.00 (3.00–34.00) 13.85 ± 7.13 | NS | p < 0.000001 | p < 0.000001 | p = 0.000006 | p = 0.000899 | NS |
| ORP (mV/106 sperm/mL) | n = 167 1.69 (0.02–196.50) 14.32 ± 38.17 | n = 41 3.99 (0.28–196.50) 36.10 ± 60.97 | n = 36 7.23 (0.68–169.11) 22.51 ± 40.43 | n = 38 1.29 (0.29–4.98) 1.58 ± 0.91 | n = 52 0.81 (0.02–4.38) 1.00 ± 0.80 | NS | p = 0.0000657 | p < 0.000001 | p = 0.000147 | p < 0.000001 | NS |
| Level of SDF (%) | Total (n = 335) | Varicocele Positive-Infertile Men (1) (n = 71) %(n) | Varicocele Negative-Infertile Men (2) (n = 95) %(n) | Healthy Volunteers with Normozoospermia (3) (n = 105) %(n) | Men with Proven Fertility (4) (n = 64) %(n) | p 1 vs. 2 | p 1 vs. 3 | p 1 vs. 4 | p 2 vs. 3 | p 2 vs. 4 | p 3 vs. 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| >30 | 8.36(28) | 21.13(15) | 35.79(34) | 0(0) | 1.56(1) | p = 0.0411 | p < 0.0001 | p = 0.005 | p < 0.0001 | p < 0.0001 | NS |
| >15–30 | 40.30(135) | 50.70(36) | 51.58(49) | 25.71(27) | 35.94(23) | NS | p = 0.0057 | NS | p < 0.0001 | NS | NS |
| ≤15 | 51.34(172) | 28.17(20) | 12.63(12) | 74.29(78) | 62.50(40) | p = 0.0123 | p < 0.0001 | p = 0.0001 | p < 0.0001 | p < 0.0001 | NS |
| Level of SDF (%) | Varicocele Positive-Infertile Men (n = 71) %(n) | Varicocele Negative-Infertile Men (n = 95) %(n) | Healthy Volunteers with Normozoospermia (n = 105) %(n) | Men with Proven Fertility (n = 64) %(n) | OR1 (95% CI) p | OR2 (95% CI) p | OR3 (95% CI) p | OR4 (95% CI) p | OR5 (95% CI) p | OR6 (95% CI) p |
|---|---|---|---|---|---|---|---|---|---|---|
| >30% | 21.13(15) | 35.79(34) | 0(0) | 1.56(1) | 0.4806 (0.2368–0.9751) p = 0.0424 | 57.8850 (3.40 –985.50) p = 0.0050 | 16.8750 (2.16–131.88) p = 0.0071 | 118.3659 (7.13–1965.11) p = 0.0009 | 35.6721 (4.74–268.72) p = 0.0005 | 0.2006 (0.01–5.00) NS |
| >15–30% | 50.70(36) | 51.58(49) | 25.71(27) | 35.94(23) | 0.9656 (0.52–1.78) NS | 2.9714 (1.57–5.63) p = 0.0008 | 1.8335 (0.92–3.66) NS | 3.0773 (1.70–5.58) p = 0.0002 | 1.8989 (0.99–3.64) NS | 0.6171 (0.32–1.21) NS |
| ≤15% | 28.17(20) | 12.63(12) | 74.29(78) | 62.50(40) | 2.7124 (1.22–6.01) p = 0.0140 | 0.1357 (0.07–0.27) p < 0.0001 | 0.2353 (0.11–0.49) p = 0.0001 | 0.0500 (0.02–0.11) p < 0.0001 | 0.0867 (0.04–0.19) p < 0.0001 | 1.7333 (0.89–3.38) NS |
| ORP | Total (n = 167) | Varicocele Positive-Infertile Men (1) (n = 41) %(n) | Varicocele Negative-Infertile Men (2) (n = 36) %(n) | Healthy Volunteers with Normozoospermia (3) (n = 38) %(n) | Men with Proven Fertility (n = 52) (4) %(n) | p 1 vs. 2 | p 1 vs. 3 | p 1 vs. 4 | p 2 vs. 3 | p 2 vs. 4 | p 3 vs. 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| >1.37 (mV/106 sperm/mL) | 83.05(93) | 82.92(34) | 86.11(31) | 47.37(18) | 19.23(10) | NS | p = 0.0009 | p < 0.0001 | p = 0.0005 | p < 0.0001 | p = 0.0046 |
| ORP | Varicocele Positive-Infertile Men (n = 41) %(n) | Varicocele negative-Infertile Men (n = 36) %(n) | Healthy Volunteers with Normozoospermia (n = 38) %(n) | Men with Proven Fertility (n = 52) %(n) | OR1 (95% CI) p | OR2 (95% CI) p | OR3 (95% CI) p | OR4 (95% CI) p | OR5 (95% CI) p | OR6 (95% CI) p |
|---|---|---|---|---|---|---|---|---|---|---|
| >1.37 (mV/106 sperm/mL) | 82.92(34) | 86.11(31) | 47.37(18) | 19.23(10) | 0.7834 (0.23–2.73) NS | 5.3968 (1.92–15.16) p = 0.0014 | 20.4000 (7.02–59.27) p < 0.0001 | 6.8889 (2.21–21.52) p = 0.0009 | 26.0400 (8.0855–83.8637) p < 0.0001 | 3.7800 (1.48–9.66) p = 0.0055 |
| Parametr | SDF (%) rs(p) (n = 335) | ORP (mV/106 Sperm/mL) rs(p) (n = 167) |
|---|---|---|
| Age (y) | 0.178(0.001025) | 0.015(NS) |
| Semen volume (mL) | 0.064(NS) | 0.041(NS) |
| Sperm concentration (×106/mL) | −0.347(<0.000001) | −0.770(<0.000001) |
| Total number of spermatozoa (×106) | −0.299(<0.000001) | −0.683(<0.000001) |
| Morphologically normal spermatozoa (%) | −0.488(<0.000001) | −0.566(<0.000001) |
| TZI | 0.207(0.0000126) | 0.303(0.000068) |
| Progressive motility (%) | −0.555(<0.000001) | −0.546(0.000001) |
| Nonprogressive motility (%) | 0.040(NS) | −0.275(0.000324) |
| Total sperm motility (%) | −0.524(<0.000001) | −0.588(<0.000001) |
| Eosin-negative spermatozoa—live cells (%) | −0.560(<0.000001) | −0.473(<0.000001) |
| HOS test-positive spermatozoa—live cells (%) | n = 291 −0.483(<0.000001) | n = 142 −0.266(0.001457) |
| SDF (%) | – | 0.364(<0.000001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, K.; Kups, M.; Harasny, P.; Machalowski, T.; Grabowska, M.; Lukaszuk, M.; Matuszewski, M.; Duchnik, E.; Fraczek, M.; Kurpisz, M.; et al. The Negative Impact of Varicocele on Basic Semen Parameters, Sperm Nuclear DNA Dispersion and Oxidation-Reduction Potential in Semen. Int. J. Environ. Res. Public Health 2021, 18, 5977. https://doi.org/10.3390/ijerph18115977
Gill K, Kups M, Harasny P, Machalowski T, Grabowska M, Lukaszuk M, Matuszewski M, Duchnik E, Fraczek M, Kurpisz M, et al. The Negative Impact of Varicocele on Basic Semen Parameters, Sperm Nuclear DNA Dispersion and Oxidation-Reduction Potential in Semen. International Journal of Environmental Research and Public Health. 2021; 18(11):5977. https://doi.org/10.3390/ijerph18115977
Chicago/Turabian StyleGill, Kamil, Michal Kups, Patryk Harasny, Tomasz Machalowski, Marta Grabowska, Mariusz Lukaszuk, Marcin Matuszewski, Ewa Duchnik, Monika Fraczek, Maciej Kurpisz, and et al. 2021. "The Negative Impact of Varicocele on Basic Semen Parameters, Sperm Nuclear DNA Dispersion and Oxidation-Reduction Potential in Semen" International Journal of Environmental Research and Public Health 18, no. 11: 5977. https://doi.org/10.3390/ijerph18115977
APA StyleGill, K., Kups, M., Harasny, P., Machalowski, T., Grabowska, M., Lukaszuk, M., Matuszewski, M., Duchnik, E., Fraczek, M., Kurpisz, M., & Piasecka, M. (2021). The Negative Impact of Varicocele on Basic Semen Parameters, Sperm Nuclear DNA Dispersion and Oxidation-Reduction Potential in Semen. International Journal of Environmental Research and Public Health, 18(11), 5977. https://doi.org/10.3390/ijerph18115977









